Impact of elevated lipoprotein(a) levels on the functional outcomes of ischemic stroke patients: A systematic review and meta-analysis
Huarong Liu and Bo Li contributed equally to this work and share first authorship.
Abstract
Background and purpose
Elevated serum lipoprotein(a) (Lp[a]) levels have been linked to an increased incidence of stroke. This systematic review and meta-analysis aimed to evaluate the impact of serum Lp(a) on the functional outcomes of patients with ischemic stroke (IS).
Methods
We conducted a comprehensive search of the MEDLINE, Web of Science, Embase, Wanfang, and China National Knowledge Infrastructure databases to identify relevant cohort studies. A random effects model was utilized to synthesize the data, accounting for study heterogeneity.
Results
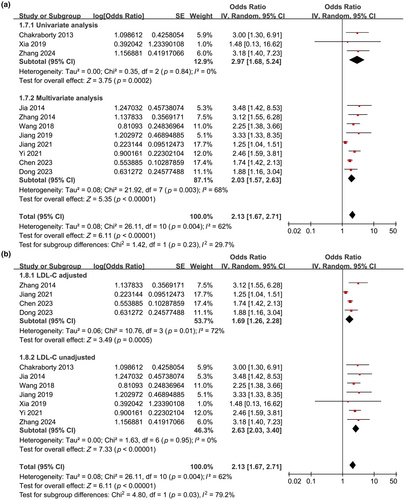
The analysis included 11 cohort studies comprising 11,958 patients with IS. Pooled results indicated that high baseline Lp(a) levels were associated with an increased risk of poor functional outcomes during follow-up (odds ratio [OR] = 2.13, 95% confidence interval = 1.67–2.71, p < 0.001, I2 = 62%). Subgroup analyses revealed that the relationship between high Lp(a) levels and the risk of poor functional outcomes was more pronounced at discharge (OR = 3.25), 3 months (OR = 2.02), and 6 months (OR = 2.11) poststroke, compared to 12 months (OR = 1.25, p for subgroup difference < 0.001). Furthermore, the association was attenuated yet remained significant in studies adjusting for low-density lipoprotein cholesterol (LDL-C) compared to those that did not adjust for LDL-C (OR = 1.69 vs. 2.63, p for subgroup difference = 0.03).
Conclusions
High serum Lp(a) levels at baseline are significantly associated with poor functional outcomes in patients with IS.
INTRODUCTION
Ischemic stroke (IS) is an acute neurological condition where an obstruction in a blood vessel prevents adequate blood flow to the brain, which is responsible for approximately 87% of all stroke cases [1]. Currently, IS remains a significant cause of mortality and long-term disability worldwide, posing substantial challenges to health care systems and affecting millions of individuals annually [1, 2]. Despite advances in management and rehabilitation strategies, the burden of IS remains considerable, with an increasing recognition of the importance of functional outcomes in predicting the overall prognosis and quality of life of stroke survivors [3, 4]. Accurate prediction of functional outcomes after IS is crucial for optimizing treatment strategies, resource allocation, and rehabilitation planning.
In recent years, there has been growing interest in exploring novel lipid biomarkers that may provide insights into the pathophysiology of IS and help refine risk stratification and prognostication [5, 6]. Among these biomarkers, lipoprotein(a) (Lp[a]) has garnered attention due to its established role in atherosclerosis and cardiovascular disease (CVD) development [7]. Lp(a) is a complex lipoprotein particle composed of a low-density lipoprotein (LDL) core covalently bound to apolipoprotein(a) (apo[a]) [8]. Elevated levels of Lp(a) have been implicated in the pathogenesis of atherosclerosis through various mechanisms, including promotion of endothelial dysfunction, inflammation, and thrombosis [9, 10]. Consequently, high serum Lp(a) levels have been linked to an increased risk of CVD events and IS [11].
Given the known link between Lp(a) and atherosclerotic vascular diseases, a clear rationale exists to explore its potential role in predicting functional outcomes following IS [12]. Understanding the influence of Lp(a) on poststroke recovery could offer critical insights into the mechanisms contributing to stroke-related disability and inform strategies for secondary prevention and rehabilitation. Although several pilot studies have examined the relationship between serum Lp(a) levels at admission and poor functional outcomes post-IS [13-23], the results have been inconsistent. Therefore, this systematic review and meta-analysis aims to synthesize the available evidence on the impact of serum Lp(a) levels on functional outcomes among patients with IS.
METHODS
The authors adhered to the guidelines outlined in PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) 2020 [24, 25] and the Cochrane Handbook for Systematic Reviews and Meta-Analyses [26] throughout this meta-analysis, encompassing study design, data collection, statistical analysis, and interpretation of results.
Literature search
To identify studies relevant to the aim of the meta-analysis, we searched MEDLINE, Web of Science, Embase, Wanfang, and China National Knowledge Infrastructure (CNKI) utilizing comprehensive search terms involving (i) “lipoprotein(a)” OR “Lp(a)” OR “Lp[a],” (ii) “stroke” OR “transient ischemic stroke” OR “TIA” OR “cerebral infarction” OR “cerebrovascular infarction,” and (iii) “functional outcome” OR “prognosis” OR “mRS” OR “modified Rankin Scale” OR “function outcome” OR “clinical outcome” OR “cohort” OR “followed” OR “longitudinal” OR “prospective” OR “retrospective” OR “follow-up.” The search terms for Chinese databases Wanfang and CNKI are detailed in Data S1. The search was restricted to clinical research involving human subjects. We only included studies published as full-length articles in English or Chinese in peer-reviewed journals. Additionally, we manually examined the references of relevant original and review articles for potentially pertinent studies. The literature published from the establishment of the databases until 30 March 2024 was reviewed.
Inclusion and exclusion criteria
The inclusion criteria for potential studies were as follows: (i) cohort studies published as full-length articles, encompassing both prospective and retrospective cohorts; (ii) studies involving patients with IS, irrespective of the primary treatment modality; (iii) studies where serum Lp(a) levels were measured at baseline (within 24 h of admission after stroke) and analysed as the exposure factor; (iv) studies reporting the incidence of poor functional outcomes during follow-up, defined as a modified Rankin Scale (mRS) score of ≥2 or 3 [27]; and (v) studies that compared the relative risk of poor functional outcomes after IS between patients with high versus low baseline serum Lp(a) levels.
Exclusion criteria included the following: (i) cross-sectional or case–control studies; (ii) studies including patients with hemorrhagic stroke; (iii) studies that did not measure or report baseline Lp(a) levels or that analysed Lp(a) solely as a continuous variable; (iv) studies that failed to report the incidence of poor functional outcomes as per the mRS score during follow-up; and (v) studies published as conference abstracts, unpublished data, reviews, or editorials. In studies with overlapping populations, the study with the largest sample size was selected for inclusion in the meta-analysis.
Study quality evaluation and data extraction
The literature search, study identification, study quality assessment, and data collection were carried out independently by two authors. In the case of disagreement, the corresponding author was consulted to reach a resolution. To evaluate the quality of the included studies, we utilized the Newcastle–Ottawa Scale (NOS) [28], which assesses three aspects: selection of the population, control of confounders, and outcome measurement and analysis. The NOS scores ranged from 1 to 9, with 9 indicating superior quality. We extracted various data from each study for subsequent analysis, including study information (author, year, country, and design), patient characteristics (sample size, age, sex, and mean body mass index [BMI]), severity of IS as indicated by the mean National Institutes of Health Stroke Scale (NIHSS) at admission, methods for defining the cutoff of Lp(a), follow-up duration, definition of poor functional outcome, number of patients with poor functional outcome during follow-up, and variables adjusted when reporting the association between Lp(a) and poor functional outcome.
Statistics
The relationship between Lp(a) and poor functional outcome after IS was assessed using odds ratio (OR) and corresponding 95% confidence interval (CI), comparing individuals with a high versus a low Lp(a) level at baseline. ORs and their standard errors were computed based on 95% CIs or p-values, followed by logarithmic transformation for variance stabilization. Heterogeneity among studies was evaluated using the Cochrane Q-test and I2 statistics [29], where I2 > 50% indicated significant statistical heterogeneity. The findings were combined utilizing a random effects model that accounted for heterogeneity's influence [26]. Sensitivity analyses involving the exclusion of one dataset at a time were conducted to assess the robustness of the results. Predefined subgroup analyses were also carried out to examine how study characteristics influenced the outcome. The median values of continuous variables were used as cutoffs for defining subgroups. Publication bias in the meta-analysis was assessed through the construction of funnel plots along with visual inspection for plot symmetry [30]; additionally, an Egger regression test was performed [30]. Statistical analysis utilized RevMan (version 5.1; Cochrane Collaboration, Oxford, UK) and Stata software (version 12.0; Stata Corporation, College Station, TX, USA).
RESULTS
Study inclusion
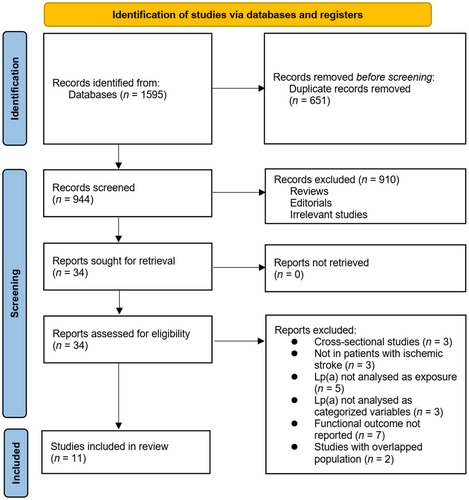
The process of study inclusion is presented in Figure 1. In brief, 1595 potentially relevant records were obtained after a comprehensive search of the three databases, and 651 of them were excluded due to duplication. Subsequently, a screening via titles and abstracts of the remaining records further excluded 910 studies, mostly because they were unrelated to the aim of the meta-analysis. Accordingly, the full texts of the 34 remaining records were read by two independent authors, and a further 23 of them were removed for the reasons listed in Figure 1. Finally, 11 cohort studies were considered suitable for the subsequent quantitative analyses [13-23].
Overview of study characteristics
Table 1 presents the summarized characteristics of the included studies. Overall, 11 cohort studies, involving eight prospective cohorts [13-16, 18-20, 22] and three retrospective cohorts [17, 21, 23], were included in the meta-analysis. These studies were reported from 2013 to 2024 and performed in India [13] and China [14-23]. Overall, 11,958 patients with acute IS were included. The mean ages of the participants were 37.3–69.7 years, and the proportions of men were 53.9%–69.0%. The mean BMI of the included patients [16-19, 21] and the mean NIHSS at admission [14-17, 22] were reported in only five studies each. The cutoff for defining the high Lp(a) level was determined by the median of Lp(a) in two studies [13, 20], by the fourth quartile of Lp(a) in three studies [17, 19, 22], via the upper limit of normal Lp(a) value in three studies [14-16], and via receiver operating characteristic curve analysis in another three studies [18, 21, 23]. The cutoff of serum Lp(a) varied from 259 to 1727 mg/L among these studies. The follow-up duration varied from at discharge to 12 months after IS. A poor functional outcome was defined as mRS ≥ 2 in three studies [13, 21, 23] and mRS ≥ 3 in eight studies [14-20, 22]. A multivariate analysis was performed in eight studies when the association between Lp(a) and poor functional outcome after IS was evaluated [14-17, 19-22], whereas a univariate analysis was performed in three studies [13, 18, 23]. The NOS scores of the included studies were six to nine stars, suggesting overall moderate to good study quality (Table 2).
| Study | Design | Location | Patients, N | Mean age, years | Men, % | Mean BMI, kg/m2 | Mean NIHSS at admission | Methods for defining cutoff of Lp(a) | Cutoff of Lp(a) | Follow-up duration | Definition of poor functional outcome | Patients with poor functional outcome, n | Variables adjusted |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chakraborty 2013 | PC | India | 100 | 54 | 69 | NR | NR | Median | 770 mg/L | 6 months | mRS = 2–6 | 45 | None |
| Jia 2014 | PC | China | 210 | 68 | 62.9 | NR | 9 | UL of normal value | 300 mg/L | At discharge | mRS = 3–6 | 66 | Age, sex, NIHSS at admission, Hcy, and hs-CRP |
| Zhang 2014 | PC | China | 153 | 62 | 57.5 | NR | 5 | UL of normal value | 300 mg/L | At discharge | mRS = 3–6 | 52 | Age, sex, alcohol abuse, smoking, severity of stroke, comorbidities, family history of stroke, TG, TC, HDL-C, LDL-C, D-dimer, fibrinogen, glucose, hs-CRP, and Hcy |
| Wang 2018 | PC | China | 232 | 59 | 53.9 | 26.8 | 6 | UL of normal value | 300 mg/L | 3 months | mRS = 3–6 | 86 | Age, sex, BMI, risk factors, NIHSS, stroke etiology, stroke syndrome, prestroke and acute treatment, duration of diabetes, conventional vascular risk factors, intensive glucose treatment, and serum levels of TC, HDL, SCr, TG, FPG, Hcy, and CRP |
| Jiang 2019 | RC | China | 231 | 69.7 | 65.4 | 24.8 | 6 | Q4 | 340 mg/L | 3 months | mRS = 3–6 | 91 | Age, sex, FPG, SCr, hs-CRP, and NIHSS at admission |
| Xia 2019 | PC | China | 43 | 60.9 | 58.1 | 25.2 | NR | ROC curve analysis derived | 309 mg/L | 3 months | mRS = 3–6 | 8 | None |
| Jiang 2021 | PC | China | 9709 | 63 | 68.9 | 24.5 | NR | Q4 | 358 mg/L | 12 months | mRS = 3–6 | 1331 | Age, sex, BMI, DM, LDL-C, HDL-C, TG, TOAST subtype, and NIHSS score at admission |
| Yi 2021 | PC | China | 100 | 37.3 | 65 | NR | NR | Median | 306 mg/L | 3 months | mRS = 3–6 | 21 | Age, sex, BMI, comorbidities, NIHSS at admission, and AF |
| Chen 2023 | RC | China | 107 | 56.3 | 63.6 | 26.9 | NR | ROC curve analysis derived | 1727 mg/L | 3 months | mRS = 2–6 | 35 | Age, sex, TC, and LDL-C |
| Dong 2023 | PC | China | 973 | 63.4 | 66.8 | NR | 5 | Q4 | 259 mg/L | 6 months | mRS = 3–6 | 201 | Age, sex, LDL-C, smoking and drinking status, time from onset to hospitalization, admission NIHSS score, systolic BP, comorbidities, and concurrent medications |
| Zhang 2024 | RC | China | 100 | 53.8 | 54 | NR | NR | ROC curve analysis derived | 281 mg/L | 3 months | mRS = 2–6 | 30 | None |
- Abbreviations: AF, atrial fibrillation; BMI, body mass index; BP, blood pressure; CRP, C-reactive protein; DM, diabetes mellitus; FPG, fasting plasma glucose; Hcy, homocysteine; HDL, high-density lipoprotein; HDL-C, HDL cholesterol; hs-CRP, high-sensitivity CRP; LDL-C, low-density lipoprotein cholesterol; Lp(a), lipoprotein(a); mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale; NR, not reported; PC, prospective cohort; Q4, fourth quartile; RC, retrospective cohort; ROC, receiver operating characteristic; SCr, serum creatinine; TC, total cholesterol; TG, triglyceride; TOAST, Trial of ORG 10172 in Acute Stroke Treatment; UL, upper limit.
| Study | Representativeness of the exposed cohort | Selection of the nonexposed cohort | Ascertainment of exposure | Outcome not present at baseline | Control for age and sex | Control for other confounding factors | Assessment of outcome | Sufficient follow-up duration | Adequacy of follow-up of cohort | Total |
|---|---|---|---|---|---|---|---|---|---|---|
| Chakraborty 2013 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 7 |
| Jia 2014 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 8 |
| Zhang 2014 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 8 |
| Wang 2018 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Jiang 2019 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Xia 2019 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 7 |
| Jiang 2021 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Yi 2021 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Chen 2023 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Dong 2023 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Zhang 2024 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 6 |
Results of the meta-analysis
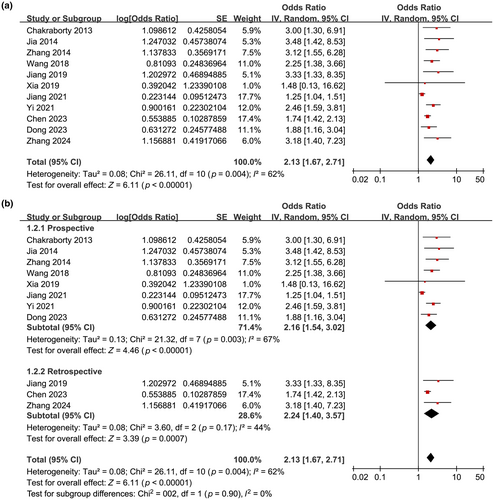
The pooled results of 11 studies with a randomized effects model suggested that compared to IS patients with a low Lp(a), patients with high serum Lp(a) at baseline were associated with a higher incidence of poor functional outcome during follow-up (OR = 2.13, 95% CI = 1.67–2.71, p < 0.001; Figure 2a) with moderate statistical heterogeneity (I2 = 62%).
Results of sensitivity analysis
Further analysis to assess the impact of excluding individual datasets consistently demonstrated similar results (OR = 2.05–2.30, all p < 0.05). In particular, the sensitivity analysis limited to studies with a poor functional outcome defined as mRS ≥ 3 [14-20, 22] showed similar results (OR = 2.17, 95% CI = 1.54–3.06, p < 0.001, I2 = 68%).
Results of subgroup analyses
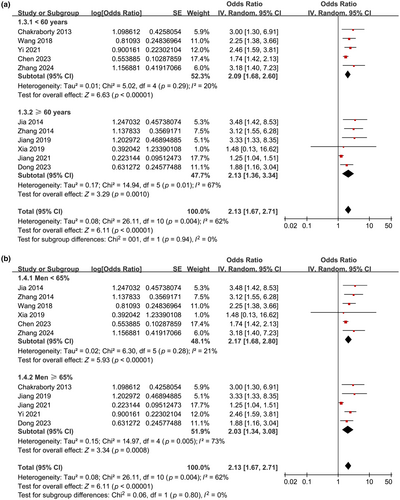
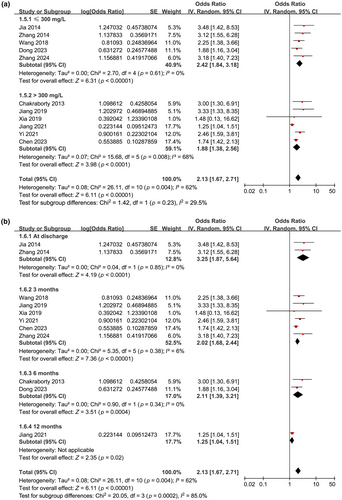
Further subgroup analyses showed similar results in prospective and retrospective cohorts (OR = 2.16 vs. 2.24, p for subgroup difference = 0.90; Figure 2b), in studies with the mean age of the patients < and ≥60 years (OR = 2.09 vs. 2.13, p for subgroup difference = 0.94; Figure 3a), in studies with the proportion of men < and ≥65% (OR = 2.17 vs. 2.03, p for subgroup difference = 0.80; Figure 3b), and in studies with the cutoff for defining high serum Lp(a) ≤ and >300 mg/L (OR = 2.42 vs. 1.88, p for subgroup difference = 0.23; Figure 4a). Interestingly, the subgroup analysis suggested that the association between high serum Lp(a) and the risk of poor functional outcome after stroke was stronger at discharge (OR = 3.25, I2 = 0%), 3 months (OR = 2.02, I2 = 6%), and 6 months (OR = 2.11, I2 = 0%) after stroke onset compared to 12 months (OR = 1.25, p for subgroup difference < 0.001; Figure 4b) after IS, which substantially explained the source of heterogeneity. In addition, consistent results were obtained for studies with univariate and multivariate analysis (OR = 2.97 vs. 2.03, p for subgroup difference = 0.23; Figure 5a). Finally, further subgroup analysis suggested that the association between Lp(a) and poor functional outcome after IS was weakened but still statistically significant in studies with the adjustment of LDL cholesterol (LDL-C) as compared to those without the adjustment of LDL-C (OR = 1.69 vs. 2.63, p for subgroup difference = 0.03; Figure 5b).
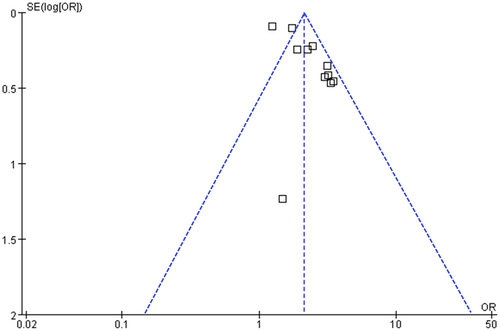
Publication bias evaluation
Upon visual inspection, the funnel plots for the meta-analysis of the relationship between serum Lp(a) and poor functional outcomes after IS appear symmetrical, suggesting a low likelihood of publication bias (Figure 6). Additionally, the results of Egger regression test (p = 0.27) corroborate this finding by indicating a low risk of publication bias.
DISCUSSION
Our meta-analysis provides contemporary evidence that elevated baseline serum Lp(a) levels are significantly associated with poor functional outcomes in patients with IS. Analysing data from 11 cohort studies, which included 11,940 IS patients, revealed that individuals with high Lp(a) levels experienced a 2.13-fold increased risk of poor functional outcomes during follow-up compared to those with lower levels. Subgroup analyses highlighted the temporal dynamics of this association, showing that the impact of elevated Lp(a) on functional outcomes was more pronounced in the acute phase poststroke and gradually diminished over time. Moreover, this association remained significant even in studies that adjusted for LDL-C. These findings support the potential of measuring Lp(a) as a predictor of poor functional outcomes after IS.
This study may be the first meta-analysis evaluating the association between serum Lp(a) and functional outcomes after IS. The association between elevated Lp(a) and poor functional outcomes after IS is likely mediated by multifaceted pathophysiological mechanisms. First, Lp(a) has been implicated in the progression of atherosclerosis, an essential pathological process underlying IS. Through its structural similarity to plasminogen, Lp(a) can interfere with fibrinolysis and promote thrombus formation [31], exacerbating cerebral ischemic injury and impairing neurological recovery. Lp(a) may also exert direct neurotoxic effects through oxidative stress and inflammation-mediated mechanisms [32]. Finally, Lp(a) has been shown to induce endothelial dysfunction and vascular inflammation [33], contributing to microvascular dysfunction and impaired cerebral perfusion, which may further compromise poststroke functional recovery. Besides its association with poor functional outcomes after IS, high Lp(a) has been related to intracranial and extracranial artery stenosis [34], increased risk of deep vein thrombosis [35], and poor cognitive outcome after IS [36], making it a predictor of poor prognosis of patients with IS.
The subgroup analysis suggested potential temporal patterns of the association between Lp(a) levels and poststroke functional outcomes. Specifically, we observed that the association between high Lp(a) levels and poor functional outcomes was stronger at earlier time points after stroke onset (at discharge), progressively diminishing over time (12 months after IS). These findings may suggest a dynamic nature of Lp(a)'s impact on poststroke functional outcomes, with its effects being most pronounced in the acute phase of stroke recovery. The heightened risk observed in the early stages after stroke onset may reflect the acute proinflammatory and prothrombotic effects of elevated Lp(a), exacerbating ischemic brain injury and impeding neurological recovery. Over time, as the acute inflammatory response subsides and the process of neuroplasticity and recovery ensues, the influence of Lp(a) on functional outcomes may gradually decrease, accounting for the observed attenuation in effect size at later time points. Furthermore, the subgroup analysis based on LDL-C adjustment provided additional insights into the potential mechanisms underlying the association between Lp(a) and poststroke functional outcomes. Studies adjusting for LDL-C exhibited a weaker but still statistically significant association between Lp(a) levels and poor functional outcomes compared to those without LDL-C adjustment. This suggests that although Lp(a) may independently influence poststroke recovery, its effects may be partially mediated by LDL-C levels, highlighting the complex interplay between these lipid parameters in shaping IS outcomes.
The strengths of our meta-analysis include a comprehensive literature search across multiple databases and the exclusive inclusion of cohort studies, which emphasize a longitudinal relationship between Lp(a) levels and poor functional outcomes. Additionally, the performance of multiple sensitivity and subgroup analyses enhanced the robustness of our findings. Despite these strengths, several limitations warrant consideration. Notably, significant heterogeneity was observed among the included studies. Subgroup analyses indicated that differences in follow-up duration could substantially contribute to this heterogeneity. Furthermore, variations in study design and potential confounding factors might have influenced the results. The observational nature of the included studies also prevents the establishment of causal relationships. Variations in the cutoff values for Lp(a) across studies may have introduced additional bias. In addition, the influence of BMI and severity of IS as indicated by mean NIHSS at admission on the association between Lp(a) and functional outcome after IS could not be evaluated, because these variables were only reported in five of the included studies. Studies are needed in the future for further investigations. Lastly, the potential modifying effect of statin therapy on the relationship between Lp(a) and functional outcomes after IS remains unclear.
Future research should aim to validate our findings across diverse patient populations and health care settings, employing standardized cutoffs for Lp(a) assessment and consistent methods for outcome ascertainment. Longitudinal studies incorporating serial measurements of Lp(a) levels and comprehensive functional assessments are essential to clarify the dynamic relationship between Lp(a) and stroke recovery trajectories over time. Additionally, mechanistic studies are needed to explore the underlying pathophysiological mechanisms linking Lp(a) to poststroke disability. These studies could identify potential therapeutic targets and contribute to the development of novel interventions aimed at improving functional outcomes in patients with IS.
Furthermore, randomized controlled trials evaluating the efficacy of Lp(a)-lowering therapies, such as antisense oligonucleotide inhibitors or monoclonal antibodies targeting apo(a), are necessary. These trials should determine whether modulation of Lp(a) levels can translate into improved functional outcomes and reduced disability burden in IS patients.
CONCLUSIONS
In conclusion, our meta-analysis highlights the prognostic significance of serum Lp(a) levels in predicting functional outcomes among patients with IS, particularly pronounced in the acute phase after stroke onset. Future research should focus on elucidating the mechanisms linking Lp(a) to poststroke disability and exploring the therapeutic potential of targeting Lp(a) to enhance functional outcomes in IS patients. Advancing our understanding of Lp(a)'s role in IS pathophysiology may lead to personalized therapeutic strategies that optimize poststroke recovery and enhance the quality of life for affected individuals.
AUTHOR CONTRIBUTIONS
Huarong Liu: Formal analysis; methodology; investigation; validation; visualization; writing – original draft. Bo Li: Formal analysis; investigation; methodology; project administration; validation; writing – original draft. Ting Lu: Formal analysis; resources; software; writing – review and editing. Chong Chen: Data curation; resources; software; writing – review and editing. Xi Xiong: Data curation; resources; software; writing – review and editing. Xing Li: Conceptualization; funding acquisition; supervision; writing – review and editing. Rengui Yang: Conceptualization; project administration; supervision; writing – review and editing.
FUNDING INFORMATION
X.L. was the primary recipient of the following funding: Natural Science Foundation of Changsha City (Kq 2209489), Natural Science Foundation of Changsha City Health Commission (KJ-A2023012), Natural Science Foundation of Hunan Province Administration of Traditional Chinese Medicine (A2024048), and Natural Science Foundation of Hunan Province (2024JJ9525).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ETHICS STATEMENT
Institutional review board approval was not required, because this is a meta-analysis.
Open Research
DATA AVAILABILITY STATEMENT
All data generated or analysed during this study are included in this published article.




