Urodynamic study and its correlation with cardiac meta-iodobenzylguanidine (MIBG) in body-first and brain-first subtypes of Parkinson's disease
Min Seung Kim and Jong Keun Kim contributed equally to this work.
Abstract
Background and purpose
Lower urinary tract symptoms (LUTS) are frequently observed in patients with Parkinson's disease (PD), but the underlying mechanism remains elusive. The concept of “body-first” and “brain-first” subtypes in PD has been proposed, but the correlation of PD subtype with LUTS remains unclear. We aimed to investigate the disparities in urological dysfunctions between body-first and brain-first subtypes of PD using urodynamic studies (UDS).
Methods
We reviewed patients with PD (disease duration <3 years) who had undergone UDS and completed urological questionnaires (Overactive Bladder Symptom Score [OABSS] and International Prostate Symptom Score [IPSS]) and a voiding diary. Patients were categorized as having body-first or brain-first PD based on cardiac sympathetic denervation (CSD) using cardiac meta-iodobenzylguanidine (MIBG) uptake and the presence of rapid eye movement sleep behavior disorder (RBD), assessed using a questionnaire (PD with CSD and RBD indicating the body-first subtype).
Results
A total of 55 patients with PD were categorized into body-first PD (n = 37) and brain-first PD (n = 18) groups. The body-first PD group exhibited smaller voiding volume and first desire volume (FDV) than the brain-first PD group (p < 0.05 in both). Also, the body-first PD group had higher OABSS and IPSS scores, and higher prevalence of overactive bladder diagnosed by OABSS, compared to the brain-first PD group. In multiple linear regression, cardiac MIBG uptake was positively correlated with FDV and voiding volume and negatively correlated with OABSS and IPSS (p < 0.05 in all).
Conclusions
Patients with the body-first PD subtype exhibited more pronounced overactive bladder symptoms and impaired storage function in the early stage of disease. Additionally, cardiac MIBG was significantly associated with urological dysfunction.
INTRODUCTION
Parkinson's disease (PD) is the second most common neurodegenerative disorder, characterized by the accumulation of Lewy bodies and loss of dopaminergic neurons within the substantia nigra. Preceding the emergence of motor symptoms, patients experience a spectrum of non-motor symptoms, among which lower urinary tract symptoms (LUTS) are notably prevalent, affecting 27%–85% of PD patients [1]. Typically, LUTS in PD manifest as storage symptoms such as nocturia, frequency, urgency, and incontinence, which significantly disrupt social life, sleep quality, and overall quality of life [2, 3]. Despite their clinical significance, the precise mechanisms and predictive factors of LUTS in PD remain unclear. While dopaminergic dysfunction and frontal disinhibition are proposed as major contributors [4, 5], LUTS likely involves various central and peripheral neuroanatomical structures, requiring comprehensive evaluation.
The Lewy body, composed of misfolded α-synuclein, is a hallmark of PD pathology and its propagation is key to disease progression and clinical manifestations. Recently, the “body-first” versus “brain-first” PD subtypes have been proposed based on the origination and spread of α-synuclein pathology [6-8]. In the body-first subtype it is proposed that there is initial α-synuclein emergence in the enteric nervous system, followed by propagation to the central nervous system via autonomic nerves. Conversely, in the brain-first subtype it is proposed that Lewy pathology originates within the central nervous system, such as the amygdala and olfactory bulb [9]. While people with body-first PD exhibited a higher prevalence of non-motor symptoms including orthostatic hypotension, cognitive impairment, and constipation, differences in LUTS between subtypes remain unclear, as questionnaire-based studies revealed conflicting results [9].
Urodynamic studies (UDS) are a useful method to assess urological function, particularly in patients with neurogenic bladder. Although numerous studies have characterized UDS findings in PD, comparative investigations across PD subtypes are lacking. In this study, we aimed to identify whether the urological functions showed disparities between body-first and brain-first PD subtypes, based on UDS and urological questionnaire assessments.
METHODS
Patient population
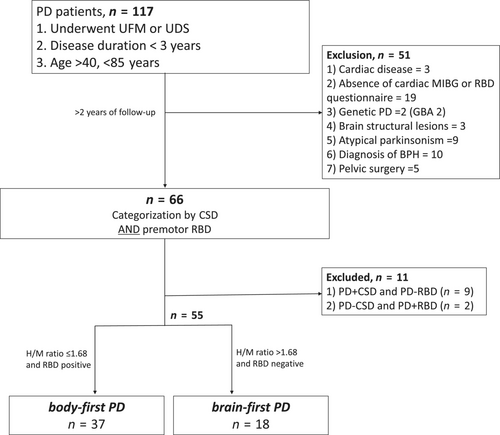
In this retrospective study, participants were enrolled in two university hospitals in South Korea between August 2017 and May 2021. We included patients diagnosed with PD, aged between 40 and 85 years, with a disease duration of less than 3 years, and who had undergone uroflowmetry (UFM) or UDS. The diagnosis of PD was made by movement disorder specialists according to the UK Parkinson's Disease Society Brain Bank criteria, and dopaminergic denervation was confirmed by 18F-N-3-fluoropropyl-2-β-carboxymethoxy-3-β-(4-iodophenyl) nortropane (FP-CIT) positron emission tomography scans [10]. Brain magnetic resonance imaging was also performed to exclude other structural causes. Patients who did not undergo cardiac meta-iodobenzylguanidine (MIBG) or complete a rapid eye movement sleep behavior disorder (RBD) questionnaire were excluded. Participants visited the outpatient clinic regularly and, during the follow-up period of at least 2 years, individuals exhibiting indicative signs of atypical parkinsonian syndromes were excluded. In particular, multiple system atrophy was excluded if participants fulfilled clinically established/probable multiple system atrophy criteria based on recent diagnostic criteria [11]. Patients who had comorbidities possibly influencing cardiac MIBG, such as diabetic or autonomic neuropathy, heart failure or ischemic heart disease, and end-stage renal disease were excluded. Participants with a history of pelvic organ surgery were also excluded. For male participants, benign prostatic hypertrophy was excluded from comprehensive evaluation through clinical history and transrectal ultrasonography by an experienced urologist (J.K.K.) [12]. Patients with prostate-specific antigen levels >4.0 ng/mL were also excluded (Figure 1).
Assessment of urological function
UFM and UDS
All patients underwent UFM or UDS at the time of enrollment. For UFM, individuals were instructed to urinate once their bladder felt comfortably full, allowing for the measurement of maximal flow rate (Qmax), voiding volume and post-void residual volume (PVR). The UDS process encompassed free UFM, bladder filling phase, and voiding phase. Initially, participants performed free UFM. Subsequently, a 6-F double-lumen urethral catheter and rectal tube were inserted. Warm saline was infused into the urethral catheter at a rate of 50 mL/min in the supine position until the patient reported a strong desire to void. The volume at which participants felt the first desire to void (first-desire volume [FDV]) and the maximum volume before voiding were recorded. During the voiding phase, patients were asked to urinate, and detrusor pressure was calculated by subtracting intra-abdominal pressure from intravesical pressure. The bladder contractility index (BCI) was calculated from following formulas: BCI = detrusor pressure at maximum flow rate (PdetQmax) + 5 × Qmax. An experienced urologist (J.K.K.) assessed the presence of detrusor overactivity, detrusor underactivity, bladder compliance, and bladder outlet obstruction.
Urological questionnaires and voiding diary
Participants completed the International Prostate Symptom Score (IPSS) and the Overactive Bladder Symptom Score (OABSS). The IPSS includes seven questions—three addressing storage function (IPSS-s; frequency, nocturia, urgency) and four related to voiding function (IPSS-v; incomplete emptying, intermittency, weak stream, straining), along with an additional question for assessing quality of life. The OABSS assesses bladder storage function through four questions (frequency, nocturia, urgency, urge incontinence). An overactive bladder (OAB) was diagnosed if the OABSS total score was ≥3 and the urgency score was ≥2 [13].
In addition, patients and/or caregivers were asked to complete a voiding diary for 3 days, documenting the volume and timing of each urination, along with the wake-up and bedtime hours. We used the systemized voiding diary recommended by the Korean Urological Association. This diary includes the voiding time, and volume of each voiding for 72 h. Nocturia was defined as the number of voids between bedtime and wake-up time. Mean 24-h frequency, mean number of nocturia episodes, largest volume of all voids, and total voiding volume were obtained.
Clinical assessments
The Hoehn and Yahr (H&Y) stage and motor subscale of the Unified Parkinson's Disease Rating Scale (UPDRS) were evaluated. Cognitive function was measured using the Mini-Mental Status Examination (MMSE) and clinical dementia rating (CDR). To detect RBD, participants were asked to complete the RBD One questionnaire (RBD1Q) [14]. Any secondary RBD potentially caused by alcohol or medication was ruled out through a detailed medical history review. In addition, non-motor symptoms were assessed using the Non-Motor Symptoms Scale (NMSS) [15].
Assessment of cardiac MIBG
Participants underwent 123I-MIBG myocardial scintigraphy. After intravenous injection of 123I-labeled MIBG, planar images of the chest were acquired at 20 and 120 min. Manual regions of interest measuring 5 × 5 pixels were drawn over the left ventricle and upper mediastinum on the anterior planar image. The heart-to-mediastinum (H/M) ratio of MIBG uptake, calculated from the average counts/pixel in the myocardium divided by that in the upper mediastinum, was determined based on the image obtained at 20 (early H/M) and 120 min (delayed H/M). Washout rate was calculated as follows: (early H/M – delayed H/M)/early H/M × 100.
Classification as body-first or brain-first PD
In this study, we classified participants into body-first or brain-first PD subtypes based on premotor RBD and cardiac MIBG uptake. The RBD1Q questionnaire was applied, with additional inquiries regarding the onset of RBD relative to the motor symptoms. As previously suggested, only those who reported dream-enactment behaviors more than 1 year before the onset of motor symptoms were categorized as having premotor RBD [7]. In cardiac MIBG, a delayed H/M ratio of less than 1.68 was considered abnormal and indicative of cardiac sympathetic denervation (CSD) [16]. Participants with premotor RBD and abnormal MIBG uptake were categorized as body-first PD, and participants without RBD and normal MIBG were classified as brain-first PD [7, 17]. Other patients with inconsistent results of RBD and cardiac MIBG were excluded from the analysis.
Statistics
To compare the demographics and clinical presentation between body-first PD and brain-first PD, Student's t-tests and chi-squared tests were used for continuous and categorical variables, respectively. To compare the parameters obtained from UDS and the urological questionnaires, analysis of covariance (ANCOVA) tests were applied, with adjustment for age, sex, and H&Y stage as covariates [18, 19]. Multivariable linear regression was used to assess the association of demographics or MIBG findings with urological parameters. Pearson's correlation and Spearman's rho test were used to evaluate the correlation between clinical characteristics, cardiac MIBG parameters and urological variables. Results were considered significant when p values were < 0.05. All statistical analyses were performed with IBM SPSS Statistics (version 27.0; IBM Corp., Armonk, NY, USA).
RESULTS
Clinical features according to subtype
Between August 2017 and May 2021, a total of 168 de novo PD patients underwent UDS/UFM for baseline evaluation. Among them, 117 patients fulfilled the inclusion criteria and after exclusion, a total of 55 patients were analyzed in our study. Based on cardiac MIBG uptake and RBD presence, 37 patients were assigned to the body-first PD group and 18 to the brain-first PD group (Figure 1).
There were no differences in age, sex, or disease duration between the groups. The UPDRS motor scores, H&Y stage and cognitive status (MMSE, CDR) were also comparable between groups. The prevalence of orthostatic hypotension was higher in the body-first PD group, without statistical significance (Table 1).
| Body-first PD (n = 37) | Brain-first PD (n = 18) | p | |
|---|---|---|---|
| Age, years | 69.8 ± 7.7 | 66.7 ± 10.9 | 0.298 |
| Sex: male, % | 15 (40.5) | 6 (33.3) | 0.606 |
| Disease duration, years | 1.3 ± 1.2 | 1.1 ± 1.1 | 0.556 |
| MIBG early H/M ratio | 1.38 ± 0.17 | 1.95 ± 0.22 | <0.001 * |
| MIBG delayed H/M ratio | 1.27 ± 0.14 | 2.04 ± 0.28 | <0.001 * |
| MIBG washout ratio, % | 7.6 ± 7.2 | −4.8 ± 8.7 | <0.001 * |
| Orthostatic hypotension, n (%) | 18 (72.0) | 6 (46.2) | 0.163 |
| Education years, years | 9.2 ± 5.5 | 7.7 ± 6.0 | 0.348 |
| MMSE | 25.4 ± 3.97 | 25.4 ± 4.5 | 0.999 |
| CDR | 0.5 ± 0.2 | 0.4 ± 0.2 | 0.256 |
| BDI | 14.0 ± 10.3 | 13.9 ± 7.8 | 0.993 |
| H&Y stage | 2.0 [2.0–2.0] | 2.0 [2.0–2.5] | 0.282 |
| UPDRS-I | 2.9 ± 1.9 | 4.7 ± 9.9 | 0.469 |
| UPDRS-II | 10.2 ± 4.4 | 10.0 ± 5.5 | 0.876 |
| UPDRS-III | 34.8 ± 9.1 | 32.6 ± 10.2 | 0.408 |
| NMSS score | 58.5 ± 34.3 | 48.1 ± 36.7 | 0.358 |
- Note: Data are mean ± standard deviation, or number (percentage).
- Abbreviations: BDI, Beck Depression Index; CDR, clinical dementia rating; H&Y, Hoehn and Yahr; H/M, heart-to-mediastinum; MIBG, meta-iodobenzylguanidine; MMSE, Mini-Mental State Examination; NMSS, Non-Motor Symptom Scale; PD, Parkinson's disease; UPDRS, Unified Parkinson's Disease Rating Scale.
- * Bold indicate significant p < 0.05.
Comparison of UDS and urological questionnaires
Urodynamic parameters of voiding volume (from free UFM) and FDV (in the filling phase of UDS) were significantly lower in the body-first PD group. Qmax, PdetQmax and PVR were comparable between the groups. The prevalence of detrusor overactivity was higher in the body-first PD group but did not achieve statistical significance (Figure 2a). There were no significant differences in the prevalence of detrusor underactivity, compliance, or bladder outlet obstruction between the groups (Table 2).

| Body-first PD (n = 37) | Brain-first PD (n = 18) | p | |
|---|---|---|---|
| UFM | |||
| Qmax, cm/s | 15.0 ± 10.3 | 18.7 ± 6.8 | 0.219 |
| Voided volume, mL | 161.4 ± 93.6 | 268.3 ± 122.4 | <0.001 * |
| PVR, mL | 45.1 ± 64.6 | 18.6 ± 21.6 | 0.158 |
| UDS | |||
| MBC, mL | 333.6 ± 111.8 | 358.1 ± 108.2 | 0.315 |
| FDV, mL | 145.3 ± 59.7 | 204.3 ± 90.0 | 0.023 * |
| P det Q max | 34.4 ± 16.1 | 21.7 ± 9.7 | 0.123 |
| BCI | 92.5 ± 32.5 | 72.4 ± 39.9 | 0.109 |
| Detrusor overactivity, n (%) | 12 (50.0) | 2 (22.2) | 0.241 |
| Bladder outlet obstruction, n (%) | 2 (8.3) | 0 (0) | 0.999 |
| Detrusor underactivity, n (%) | 3 (12.5) | 3 (33) | 0.309 |
| IPSS | |||
| Quality of life | 3.1 ± 1.2 | 2.5 ± 2.4 | 0.216 |
| Storage score | 5.8 ± 4.1 | 3.5 ± 3.2 | 0.016 * |
| Voiding score | 5.1 ± 5.0 | 2.6 ± 2.8 | 0.083 |
| Total score | 10.9 ± 7.8 | 6.1 ± 5.6 | 0.023 * |
| OABSS | |||
| Total score | 5.2 ± 3.3 | 2.7 ± 2.1 | 0.010 * |
| OAB, n (%) | 20 (60.6) | 2 (12.5) | 0.001 * |
| Voiding diary | |||
| MVV, mL | 341.9 ± 128.5 | 250.6 ± 68.4 | 0.020 * |
| AVV, mL | 3965 ± 1678 | 3623 ± 1427 | 0.350 |
| Number of voids | 7.9 ± 2.8 | 7.0 ± 3.1 | 0.472 |
| Number of nocturia events | 1.3 ± 0.8 | 1.8 ± 1.4 | 0.251 |
- Note: Mean ± standard deviation or number (percentage).
- Abbreviations: AVV, average voided volume; BCI, bladder compliance index; FDV, first-desire volume; IPSS, International Prostate Symptom Scale; MBC, maximal bladder capacity; MVV, maximum voided volume; OAB, overactive bladder; OABSS, Overactive Bladder Symptom Score; PVR, post-void residual volume; PdetQmax, detrusor pressure at maximum flow rate; Qmax, maximal flow rate; UDS, urodynamic study; UFM, uroflowmetry.
- * Bold indicate significant p < 0.05; p value was adjusted for age, sex, and H&Y stage.
Urological questionnaire scores showed significant differences between groups. The OABSS total score, IPSS total score, and IPSS-s score were significantly higher in the body-first PD group than the brain-first PD group. Voiding diary parameters were comparable between groups, but maximum voided volume was higher in the body-first PD group (Table 2). The prevalence of OAB determined by OABSS was higher in the body-first PD than the brain-first PD group (60.6% vs. 12.5%; p = 0.001 [Figure 2b]). Among questions on LUTS, the “frequency” score from the IPSS questionnaire and “urgency” from the OABSS questionnaire showed higher scores in the body-first PD group. The urinary domain of the NMSS questionnaires did not differ between groups (Table S1).
Correlation between cardiac MIBG scintigraphy and urological parameters
Delayed H/M ratio showed a positive correlation with voiding volume (r = 0.376, p = 0.005), and was negatively correlated with OABSS (Figure 3 and Table S2). Between the urological evaluations, the OABSS score and IPSS-s score exhibited a strong correlation with each other (r = 0.773, p < 0.001).
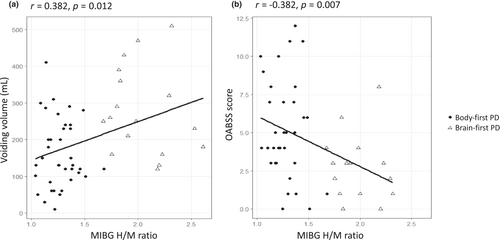
Multiple linear regression analysis identified that delayed H/M ratio was independently associated with urological parameters, including voiding volume (ß = 0.369, p = 0.009) and FDV (ß = 0.390, p = 0.031), and was negatively associated with total OABSS, IPSS, and IPSS-s. PVR and IPSS-v scores did not show a significant association (Table 3).
| Voiding volume | FDV | PVR | OABSS | IPSS | IPSS-s | IPSS-v | |
|---|---|---|---|---|---|---|---|
| Age | |||||||
| β | −0.105 | −0.195 | −0.006 | 0.182 | 0.005 | 0.103 | −0.082 |
| p | 0.520 | 0.389 | 0.975 | 0.291 | 0.977 | 0.552 | 0.685 |
| Disease duration (years) | |||||||
| β | 0.129 | −0.323 | 0.222 | 0.052 | 0.158 | 0.091 | 0.179 |
| p | 0.520 | 0.087 | 0.152 | 0.722 | 0.343 | 0.551 | 0.313 |
| MIBG delayed H/M ratio | |||||||
| β | 0.369 | 0.390 | −0.138 | −0.356 | −0.361 | −0.379 | −0.260 |
| p | 0.009 * | 0.031 * | 0.357 | 0.016 * | 0.035 * | 0.017 * | 0.148 |
| H&Y stage | |||||||
| β | 0.031 | 0.188 | −0.160 | 0.199 | 0.256 | 0.375 | 0.092 |
| p | 0.181 | 0.447 | 0.403 | 0.276 | 0.235 | 0.063 | 0.688 |
| UPDRS-III | |||||||
| β | −0.271 | −0.258 | −0.055 | −0.114 | −0.068 | −0.065 | −0.054 |
| p | 0.079 | 0.198 | 0.741 | 0.369 | 0.709 | 0.697 | 0.779 |
| K-MMSE | |||||||
| β | 0.080 | 0.187 | −0.064 | −0.141 | −0.164 | −0.160 | −0.129 |
| p | 0.618 | 0.409 | 0.721 | 0.406 | 0.382 | 0.355 | 0.520 |
- Abbreviations: FDV, first-desire volume; H&Y, Hoehn and Yarr; H/M, heart-to-mediastinum; IPSS, International Prostate Symptom Score; IPSS-s, IPSS storage subscore; IPSS-v, IPSS voiding subscore; MIBG, meta-iodobenzylguanidine; MMSE, Mini-Mental State Examination; OABSS, Overactive Bladder Symptom Score; PVR, post-void residual volume; UPDRS, Unified Parkinson's Disease Rating Scale; β, standardized regression coefficient.
- * Bold indicate significant p < 0.05.
DISCUSSION
This is the first study to compare urological function between the body-first and brain-first subtypes of PD using comprehensive urodynamic assessments. The major findings of our study were as follows. First, patients with body-first PD had lower FDV and voiding volume, indicative of decreased bladder storage function in the UDS. Second, the body-first PD group had a higher incidence of OAB and exhibited more severe LUTS than the brain-first PD group, based on the questionnaire. Third, cardiac MIBG uptake was significantly associated with urological parameters in early-stage PD.
In our study, the UDS results indicated reduced FDV and voiding volume in the body-first subtype of PD, aligning with findings from previous studies on PD populations at large [20-23]. In addition, the questionnaire results in our study revealed a higher prevalence of urgency and frequency, with relatively rare occurrences of incontinence, consistent with observations in early PD reported in prior studies [22, 23]. These findings suggest that urinary dysfunction in body-first PD follows a similar trajectory to that observed in the general PD population, albeit with more pronounced symptoms, suggesting accelerated pathogenic processes underlying the manifestation of LUTS.
Previous research has indicated that the main pathophysiology underlying OAB in PD patients is related to the degeneration of dopaminergic pathways and disruption of the prefrontal cortex-basal ganglia circuit [1, 5]. This theory is supported by the imaging studies delineating the association between LUTS and dopamine transporter uptake [5, 24-26], as well as the improvement in LUTS symptoms following deep brain stimulation of the subthalamic nucleus or globus pallidus [27-29]. The hypothesis of dopaminergic denervation is also plausible in our findings of severe OAB in body-first PD, as explained by the faster progression of dopaminergic degeneration in this subtype [6].
Furthermore, our study revealed a correlation between CSD, as evidenced by decreased MIBG uptake, and urological dysfunction, particularly in parameters associated with storage dysfunction. These findings implicate sympathetic noradrenergic mechanisms in the development of LUTS, extending beyond the established dopaminergic contributions. The sympathetic nervous system plays a role in urine storage by facilitating the relaxation of the detrusor muscle, hence its dysfunction could contribute to OAB symptoms. Early Lewy pathology has been documented in the sympathetic trunk, including the celiac and stellate ganglia [30, 31], but the presence of α-synuclein pathology in the inferior pelvic plexus, crucial for bladder relaxation, remains underexplored. Additionally, α-synuclein deposition in the sacral dorsal root ganglia, the central hub for bladder filling sensations, may disrupt afferent signaling and contribute to detrusor overactivity [32]. Taken together, our findings raise the possibility that peripheral sympathetic denervation could contribute to LUTS in early PD. Further pathological studies providing α-synuclein pathology in the sympathetic nervous system are warranted.
Accumulating evidence suggests that PD patients with RBD, suggestive of the body-first subtype, experienced more severe autonomic dysfunction [33-37]. Nonetheless, the impact of RBD on urinary function in PD has been subject to debate. While some studies have reported a higher burden of urinary symptoms among patients with PD with RBD [36, 37], others have not observed such associations [33-35]. We hypothesized that the discrepancy might be attributed to confounding factors such as peripheral organ dysfunction or levodopa usage, or variation in RBD diagnostic methods [38]. In our study, we found significant differences in the prevalence of OAB and UDS parameters between PD patients with and without RBD. Interestingly, previous studies identified that patients with urinary dysfunction had a higher prevalence of RBD [23], earlier requirement for levodopa, and a faster disease progression [39]. These findings suggest a possible association between urinary dysfunction and the body-first PD subtype.
Our study identified significant differences in OABSS, IPSS total score and IPSS-s subscore between the body-first PD and brain-first PD groups. In contrast, no significant differences were observed within the urinary domain of the NMSS, a tool commonly applied to assess non-motor symptoms in PD patients. This discrepancy could be associated with differences in questionnaire design: the IPSS and OABSS specifically inquire about the frequency of each symptom, whereas the NMSS evaluates both the severity and frequency of symptoms, with a particular emphasis on disturbance to the patient [15]. Given that urinary symptoms are prevalent but have a lesser impact on quality of life in the early stage of PD [19], additional questionnaires such as the OABSS or IPSS could be beneficial for the assessment of urinary symptoms in early-stage PD.
Our study employed two methods to classify PD into body-first and brain-first subtypes: cardiac MIBG scintigraphy and premotor RBD. However, there are currently no widely validated criteria for distinguishing these subtypes. Although polysomnography (PSG)-proven RBD criteria are valuable for classification purposes [7], the widespread application of PSG is often challenging, and a large proportion of PD patients still rely on RBD questionnaires, which are limited in their accuracy and inter-rater reliability [40, 41]. Cardiac MIBG scintigraphy provided objective measures of CSD, one of the early-affected structures from α-synuclein propagation in the body-first subtype [42]. The relationship between MIBG uptake and RBD was well documented [43-47], supporting the utility of MIBG imaging in subtype classification of body-first and brain-first PD [17]. However, patients with brain-first PD subtypes may also develop CSD eventually after motor symptoms begin. Given that the time interval between motor onset and CSD is uncertain, categorization relying only on cardiac MIBG could lead to misdiagnosis, especially if MIBG is performed at a late stage. Taking these factors into account, we assume that cardiac MIBG is appliable for categorizing PD subtypes only in the early stage of PD, and cardiac MIBG and the RBD questionnaire provide complementary tools for accurate classification.
The main strength of this study lies in its patient selection. We conducted urological evaluations as baseline assessments for all newly diagnosed PD patients, rather than solely in those reporting LUTS, to reflect the urological characteristics of general PD patients. Additionally, we endeavored to minimize the influence of peripheral organ factors by excluding patients with benign prostatic hypertrophy based on diagnosis by a urologist using transrectal ultrasonography.
This study also has several limitations. Firstly, it was a retrospective study, and although we tried to conduct urological assessment at baseline, the potential for selection bias exists, as patients with more pronounced urological symptoms were more likely to undergo urological evaluation. Secondly, the study lacked a comprehensive evaluation linking LUTS with dopaminergic imaging. However, there were similar UPDRS part III scores in the two groups, and we adjusted for H&Y stage in the analysis of urological parameters to minimize confounding effects. Future research to explore the relationship between urodynamic assessments and dopaminergic activity is warranted to validate and expand on our findings. Thirdly, the body-first PD group was older than the brain-first PD group, without statistical significance. Although the disparity was small, we cannot rule out the influence of age in MIBG classification because of the strong correlation between age and MIBG parameters [44]. Fourthly, the body-first PD and brain-first PD groups had similar motor scores and cognitive status, inconsistent with general reports of worse motor and cognitive functions in body-first PD. These discrepancies could be associated with our study populations, which were restricted to early-stage PD. Follow-up motor and cognitive assessment is warranted to identify differences in disease progression between subtypes. Finally, the lack of PSG confirmation in the RBD diagnosis prevented a direct comparison of usefulness between MIBG scintigraphy and PSG-proven RBD, even though RBD based on the RBD1Q showed a high concordance with PSG-proven RBD [14].
In conclusion, our findings identified that individuals with body-first PD exhibited more severe urinary symptoms, and significantly decreased bladder storage function in early-stage PD. In addition, we demonstrated the association between CSD and urological dysfunction. These findings suggest that LUTS could be regarded as a feature of the body-first subtype in PD.
AUTHOR CONTRIBUTIONS
Min Seung Kim: Software; writing – original draft; conceptualization; investigation; data curation; methodology. Jong Keun Kim: Writing – original draft; methodology; data curation; investigation; validation. In Hee Kwak: Data curation; investigation; methodology. Jeongjae Lee: Data curation; investigation. Young Eun Kim: Conceptualization; writing – review and editing; supervision; funding acquisition; data curation; methodology. Hyeo-Il Ma: Methodology; writing – review and editing; formal analysis; resources. Suk Yun Kang: Conceptualization; writing – review and editing; funding acquisition; validation; supervision.
FUNDING INFORMATION
This research was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare (S.Y.K., grant number: RS-2023-00266948 and Y.E.K., grant number: RS-2023-00265159). Y.E.K has received a grant from the Basic Science Research Program through the National Research Foundation of Korea (NRF; grant number: RS-2023-00246655) and the NRF by the Korea government (MSIT; grant number: 2022R1A2C2091254), outside of the submitted work.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ETHICS STATEMENT
This study was approved by the institutional review boards of Dongtan Sacred Heart Hospitals and Hallym University Sacred Heart Hospitals.
Open Research
DATA AVAILABILITY STATEMENT
The data not published within this article will be available from the corresponding author upon reasonable request.




