Elevated serum circulating cell-free mitochondrial DNA in amyotrophic lateral sclerosis
Abstract
Background and Purpose
The substantial role of inflammation in amyotrophic lateral sclerosis (ALS) is gaining support from recent research. Studies indicate that circulating cell-free mitochondrial DNA (ccf-mtDNA) can activate the immune system and is associated with neurodegenerative diseases. This research was designed to quantify ccf-mtDNA levels in the serum of ALS patients.
Methods
The medical records of ALS patients were reviewed. Serum ccf-mtDNA levels of patients with ALS (n = 62) and age-matched healthy controls (n = 46) were measured and compared. Additionally, serum interleukin-6 (IL-6) levels were measured using an enzyme-linked immunosorbent assay in 26 ALS patients. Correlations between variables were analyzed.
Results
Serum ccf-mtDNA was notably higher in the patients with ALS. When stratified by genotype, the superoxide dismutase 1 (SOD1) mutation group showed the greatest increase in ccf-mtDNA levels relative to other ALS patients. Among all 108 individuals, a cut-off set at 1.1 × 105 mtDNA copies on a receiver-operating characteristic curve identified patients with ALS with 80.7% sensitivity and 50.0% specificity; the area under the curve was 0.69 (p < 0.001). Furthermore, serum ccf-mtDNA levels correlated negatively with the progression rate of ALS (ΔFS; rs = −0.26, p = 0.044), but not the ALSFRS-R score (rs = 0.06, p = 0.625). Importantly, the correlation between ccf-mtDNA and ΔFS was more pronounced in the SOD1 mutation group (rs = −0.62, p = 0.018). Lastly, a significant positive association was observed between serum ccf-mtDNA levels and IL-6 levels in ALS (r s= 0.41, p = 0.038).
Conclusion
Our study found increased serum ccf-mtDNA in ALS patients, suggesting a link to inflammatory processes and disease mechanism. Moreover, ccf-mtDNA could be an indicator for ALS progression, especially in those with the SOD1 mutation.
INTRODUCTION
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disease that progressively impairs both upper and lower motor neurons, resulting in muscle atrophy, weakness, and ultimately, respiratory failure, which can be fatal [1]. A critical component in the pathogenesis of ALS is the impairment of mitochondrial function [2], where the mitochondrial DNA (mtDNA) is highly vulnerable to damage caused by oxidative stress owing to the lack of histone protection and an inefficient repair system [3]. This heightened vulnerability may cause mtDNA fragments to enter the circulation, known as circulating cell-free mtDNA (ccf-mtDNA) [4]. ccf-mtDNA can provoke immune and inflammatory responses, a process that parallels mechanisms seen in other neurodegenerative disorders such as Parkinson's disease (PD) and Alzheimer's disease (AD) [5-7]. However, in ALS, there is a lack of corresponding research in patients. In mouse models of ALS, research has shown that the accumulation of abnormal proteins leads to the cytosolic release of mtDNA, thereby triggering the activation of the innate immune response [8, 9]. Furthermore, previous research suggests that elevated interleukin-6 (IL-6) levels in peripheral blood samples from individuals with ALS indicate the activation of neuroinflammation [10]. The aim of this study was to explore serum levels of ccf-mtDNA in ALS patients and investigate their correlation with IL-6, as well as their association with disease progression, aiming to deepen our understanding of the implications of ccf-mtDNA in the pathogenesis of ALS.
MATERIALS AND METHODS
Participant recruitment
We conducted a retrospective analysis of a series of ALS patients admitted to the Department of Neurology at Peking University First Hospital from February 2017 to October 2023. Patients were considered eligible for inclusion in the study if they met the revised El Escorial criteria [11] for probable laboratory-supported, probable, or definite ALS. We categorized the ALS patients into three distinct groups: (1) sporadic ALS (sALS), which included patients lacking the repeat expansion in C9ORF72 or any recognized ALS-associated genetic mutations; (2) SOD1, comprising patients with the SOD1 mutation; and (3) non-SOD1, consisting of patients with mutations other than SOD1. The control group consisted of healthy participants who were matched with the ALS patients in terms of age and gender.
During case selection, we excluded individuals with a history of severe psychiatric disorders, recent antibiotic or anti-inflammatory use, autoimmune or paraneoplastic ALS mimics, unrelated neurological conditions, and non-compliant blood samples. Comprehensive family and personal histories were documented for each participant via a standardized questionnaire. At the onset of the study, participants' functional status was assessed using the ALS Functional Rating Scale-Revised (ALSFRS-R). The progression rate of ALS, denoted as ΔFS, was calculated using the formula ΔFS = (48-ALSFRS-R score at diagnosis) / (duration from symptom onset to diagnosis in months) [12]. The ΔFS value of 0.5 serves as the cut-off for classifying ALS progression into slow (ΔFS <0.5) and fast (ΔFS ≥0.5) [13]. Disease staging in our study was based on the King's College Staging System [14], which defined stage 2A as the time of diagnosis and stage 2B as the point at which a second region became functionally involved. Given that diagnosis was a prerequisite for participation, all ALS patients were classified at stage 2A or beyond, rendering the initial stage 1 unnecessary. Consequently, adopting the approach of Balendra et al. [15], we discarded stage 2A and redefined stage 2 to reflect the functional involvement of a second central nervous system region, corresponding to the previous stage 2B. Patients in King's College stages 1–3 were categorized as having early-stage ALS, with the exclusion of those who had undergone percutaneous endoscopic gastrostomy or were using non-invasive ventilation because these patients represent a more advanced stage of the disease. Subsequent analyses were focused on this subgroup.
Ethics statement
Before initiating the research, written informed consent was obtained from all participants. Subsequently, the research protocol underwent a rigorous review process and received approval from the Ethics Committee at Peking University First Hospital, adhering to the ethical guidelines established by the World Medical Association's Declaration of Helsinki.
Quantification of serum ccf-mtDNA levels
We isolated ccf-mtDNA from 200-μL serum samples using the Serum Free DNA Extraction Kit, which uses magnetic beads (Tiangen, DP709), in accordance with the protocol provided by the manufacturer. We quantified ccf-mtDNA and circulating cell-free nuclear DNA (ccf-nDNA) through quantitative real-time polymerase chain reaction (RT-qPCR), targeting the NADH dehydrogenase 1 (MT-ND1) and beta-actin (ACTB) genes, respectively, as previously described [16, 17]. The PCR procedure involves a 20 μl reaction mixture that includes 2 μL serum-derived cell-free DNA, 0.2 μM MT-ND1 or ACTB primers, 1 × UltraSYBR Mixture (CW2601), and nuclease-free water. The amplification was conducted in sealed 96-well plates with a thermal profile that began with 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min, and a final step of 30 s at 95°C, 30 s at 65°C, and 30 s at 95°C. RT-qPCR data analysis was performed using Agilent Aria software, version 1.71.
- MT-ND1 forward: 5′- CCACCTCTAGCCTAGCCG TTTA-3′;
- MT-ND1 reverse: 5′-GGGTCATGATGGCAGGAG TAAT-3′;
- ACTB forward: 5′-CTCCATCCTGGCCTCGCTGT-3′;
- ACTB reverse: 5′-GCTGTCACCTTCACCGTTCC-3′.
Quantification of serum IL-6 levels
In this cohort, the serum IL-6 concentrations were also determined in the available ALS samples using a quantitative enzyme-linked immunosorbent assay, following the manufacturer's protocols (mlbio, ml058097), to explore potential correlations with ccf-mtDNA levels.
Statistical analyses
Data analysis was performed using SPSS version 26.0. and GraphPad Prism 9.0. Categorical variables, such as sex and bulbar onset, were represented as frequencies and percentages, while continuous variables, including age, disease duration, age at disease onset, ALSFRS-R, and ΔFS, were reported as mean ± standard deviation. To assess inter-group differences in clinical characteristics, one-way analysis of variance was applied to continuous variables, and the chi-squared test was used for categorical variables. Additionally, the Mann–Whitney U-test was utilized to compare the levels of ccf-mtDNA, ccf-nDNA, and the ccf-mtDNA/ccf-nDNA ratio across the four groups. Receiver-operating characteristic (ROC) curve analyses were conducted to distinguish between ALS patients and healthy individuals, as well as between the SOD1 mutation and healthy controls. Additionally, ROC was utilized to identify early-stage ALS patients from healthy controls, thereby offering a thorough evaluation of the diagnostic precision. The optimal cut-off points for each comparison were determined based on the ROC curves, considering both sensitivity and specificity. The area under the curve (AUC) was calculated to quantify the discriminatory ability. Relationships between variables were assessed employing Spearman's correlation coefficient. A threshold of p < 0.05 for both tails was adopted to determine statistical significance.
RESULTS
Clinical characteristics of study cohort
Our study enrolled 62 ALS patients, including 42 with sALS, 14 with SOD1 mutations, four with FUS mutations, one with a NEK1 mutation, and one with a SETX mutation, along with 46 healthy controls matched for age and sex. The mean age for ALS patients was 53.7 ± 14.7 years, compared to 50.41 ± 16.86 years for the healthy controls (p = 0.277). The ALS group consisted of 38 males (61.3%). Detailed clinical characteristics for the sALS, SOD1 and non-SOD1 subgroups are presented in Table 1. Following the revised El Escorial Criteria, our study included 23 (37.1%) probable-laboratory supported cases, 32 (51.6%) probable cases, and seven (11.3%) definite ALS cases at enrollment. The average disease duration from symptom onset to admission was 19.2 ± 22.2 months. The mean ALSFRS-R score at admission was 38.7 ± 8.1, with an average ΔFS of 0.84 ± 0.78 at evaluation. Bulbar onset was observed in seven patients (11.3%). Based on the King's College Staging System, 56 participants were categorized as being at early stage in the patient group.
| Characteristics | sALS (n = 42) | SOD1 (n = 14) | Non-SOD1 (n = 6) | p |
|---|---|---|---|---|
| Male, n (%) | 26 (61.9) | 9 (64.3) | 3 (50) | 0.844 |
| Age, years | 56.7 ± 13.1 | 51.1 ± 16.1 | 39.2 ± 14.4 | 0.015* |
| Age at disease onset, years | 55.2 ± 13.1 | 49.6 ± 15.4 | 37.0 ± 16.4 | 0.012* |
| Disease duration from symptoms onset, months | 18.7 ± 17.0 | 18.5 ± 30.0 | 24.0 ± 35.4 | 0.857 |
| Bulbar onset, n (%) | 6 (14.3) | 1 (7.1) | 0 (0) | 0.843 |
| ALSFRS-R | 38.8 ± 7.9 | 37.9 ± 9.3 | 39.2 ± 7.5 | 0.926 |
| △FS | 0.8 ± 0.7 | 1.0 ± 1.0 | 0.8 ± 0.7 | 0.702 |
- Note: Sex and bulbar onset are expressed as percentages (%) of the cohort. Age, age at onset, disease duration, modified Rankin Scale score, ALSFRS-R scores, and the progression rate of amyotrophic lateral sclerosis (ΔFS) are presented as mean ± SD.
- Abbreviations: ALSFRS-R, Amyotrophic Lateral Sclerosis Functional Rating Scale-Revised; sALS, sporadic amyotrophic lateral sclerosis; SD, standard deviation; SOD1, superoxide dismutase 1.
- * p < 0.05.
Serum ccf-mtDNA levels are elevated in ALS
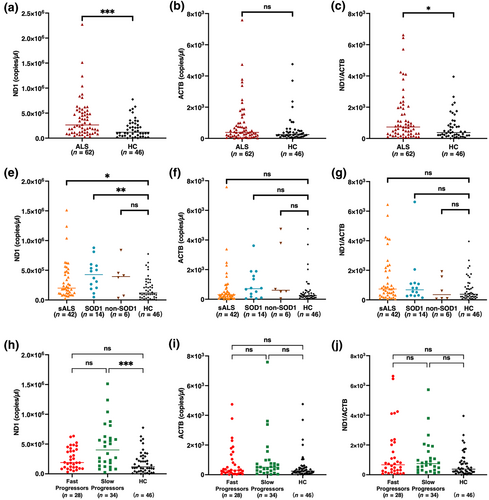
Our initial analysis compared the entire ALS patient population with the control cohort, revealing higher levels of ccf-mtDNA and ccf-mtDNA/ccf-nDNA ratio in ALS patients (Figure 1a,c). In contrast, no significant differences in ccf-nDNA levels were observed between ALS patients and controls (Figure 1b). Upon further stratification of the ALS cohort, we identified significantly elevated ccf-mtDNA levels in both the sALS and SOD1 subgroups (Figure 1d). However, no notable differences in ccf-nDNA levels (Figure 1e) or the ccf-mtDNA/ccf-nDNA ratio (Figure 1f) were found among the sALS, SOD1, and non-SOD1 subgroups compared to healthy controls. In our study, 12 distinct SOD1 genetic variants were identified within the patient cohort. The ccf-mtDNA levels and ΔFS for each variant are presented in Table 2.
| Patient | Sex | Age, years | Age at onset, years | Disease duration, months | Site of onset | ALSFRS-R score | ΔFS | SOD1 variants | ccf-mtDNA, copies/μL |
|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 68 | 58 | 120 | Spinal | 30 | 0.15 | S135T | 5.77 × 105 |
| 2 | F | 65 | 64 | 17 | Spinal | 44 | 0.24 | G11A | 5.05 × 105 |
| 3 | M | 14 | 12 | 20 | Spinal | 43 | 0.25 | G42S | 8.11 × 105 |
| 4 | F | 49 | 49 | 7 | Spinal | 46 | 0.29 | C7W | 2.04 × 105 |
| 5 | M | 68 | 66 | 18 | Spinal | 42 | 0.33 | G62A | 8.78 × 105 |
| 6 | F | 45 | 45 | 6 | Spinal | 46 | 0.33 | G62D | 6.00 × 105 |
| 7 | M | 42 | 41 | 5 | Spinal | 46 | 0.40 | G142A | 5.34 × 105 |
| 8 | M | 51 | 51 | 11 | Spinal | 43 | 0.46 | V48A | 2.66 × 105 |
| 9 | M | 53 | 53 | 2 | Spinal | 46 | 1.00 | G142A | 3.69 × 105 |
| 10 | F | 69 | 67 | 24 | Bulbar | 23 | 1.04 | N87S | 3.09 × 105 |
| 11 | M | 44 | 43 | 3 | Spinal | 42 | 2.00 | A146T | 4.80 × 104 |
| 12 | M | 30 | 30 | 7 | Spinal | 32 | 2.29 | G62A | 4.85 × 105 |
| 13 | M | 68 | 67 | 12 | Spinal | 20 | 2.33 | V6A | 1.87 × 105 |
| 14 | F | 49 | 49 | 7 | Spinal | 28 | 2.86 | H44R | 1.11 × 105 |
- Note: Patient numbers are assigned in ascending order of ΔFS.
- Abbreviations: ALSFRS-R, Amyotrophic Lateral Sclerosis Functional Rating Scale-Revised; ccf-mtDNA, circulating cell-free mitochondrial DNA; SOD1, superoxide dismutase 1; ΔFS, progression rate of amyotrophic lateral sclerosis.
In our progression-stratified analysis, the slow progression ALS subgroup had higher ccf-mtDNA levels compared to healthy controls, while the fast progression subgroup did not (Figure 1g). Furthermore, both ALS subgroups, regardless of progression rate, showed no significant variations in ccf-nDNA levels (Figure 1h) or the ccf-mtDNA to ccf-nDNA ratio (Figure 1i) compared to healthy controls.
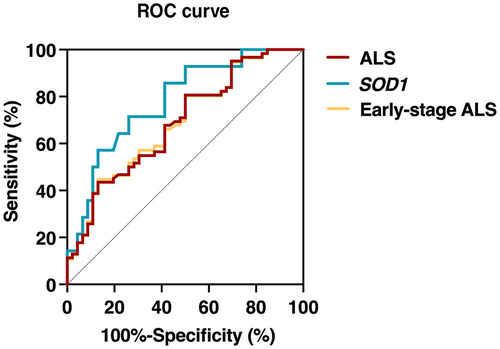
The discriminative potential of ccf-mtDNA for ALS was validated using ROC curve analysis. Serum ccf-mtDNA demonstrated moderate discriminative ability between ALS patients and controls, with an AUC of 0.69 (95% CI 0.59–0.79; p < 0.001), and showed improved efficiency in identifying patients with the SOD1 mutation, with an AUC of 0.86 (95% CI 0.72–1; p < 0.001 [Figure 2]). The sensitivity of ccf-mtDNA for detecting ALS was 80.7%, and its specificity was 50.0%, with the optimal cut-off value established at 1.1 × 105. Moreover, ccf-mtDNA exhibited moderate capability in distinguishing early-stage ALS patients from healthy controls, as indicated by an AUC of 0.69 (95% CI 0.59–0.79; p = 0.001 [Figure 2]).
Correlation with clinical parameters
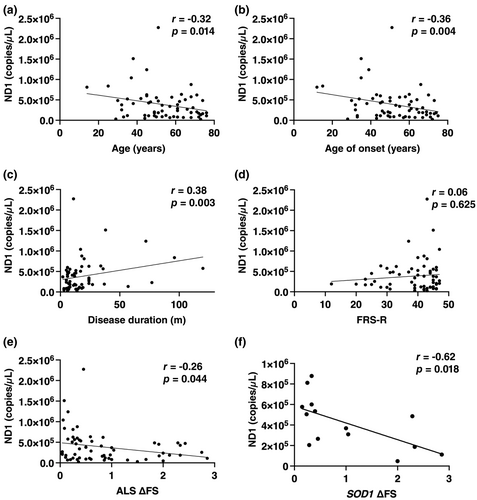
Our analysis revealed correlations between ccf-mtDNA levels and multiple clinical parameters (Figure 3). Specifically, ccf-mtDNA levels in ALS patients were negatively related to both age (rs = −0.32, p = 0.014) and age at symptom onset (rs = −0.36, p = 0.004; Figure 3a,b). The healthy control group also exhibited an inverse correlation between ccf-mtDNA levels and age (rs = −0.34, p = 0.021; Figure S1). No gender-related differences in ccf-mtDNA levels were found among ALS patients and healthy controls (Figure S2). Additionally, a significant positive correlation was observed between ccf-mtDNA levels and disease duration (rs = 0.38, p = 0.003) in ALS patients (Figure 3c). However, no substantial correlations were found between ccf-mtDNA levels and either ALSFRS-R score (rs = 0.06, p = 0.625; Figure 3d) or King's College stage (rs = −0.04, p = 0.781) in ALS patients. Serum ccf-mtDNA levels displayed a negative correlation with ΔFS (rs = −0.26, p = 0.044). Furthermore, a subgroup analysis focusing specifically on patients with the SOD1 mutation (Figure 3f) reinforced the correlation between ccf-mtDNA and ΔFS (rs = −0.62, p = 0.018).
In patients with ALS, ccf-nDNA levels did not show a significant association with clinical parameters such as age, disease onset age, disease duration, disease severity, stage, or progression rate, as detailed in Table S1. Likewise, the ratio of ccf-mtDNA to ccf-nDNA also lacked any notable correlation with these clinical measures, as documented in the same resource.
Correlation with serum IL-6 levels
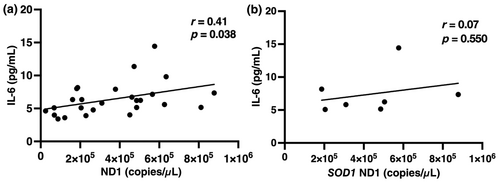
In the 26 available ALS patients evaluated, serum ccf-mtDNA levels correlated positively with IL-6 levels (r = 0.41, p = 0.038; Figure 4a). In the patients with SOD1 mutations, ccf-mtDNA levels showed no significant correlation with IL-6, but a similar trend was noted (r = 0.07, p = 0.550; Figure 4b).
DISCUSSION
It is widely accepted that mitochondrial dysfunction is an early change in patients with ALS [18]. Recent research has indicated that misfolded proteins [8, 9], including SOD1 and TDP-43, can impair cellular mitochondria, leading to the cytoplasmic release of mtDNA and the subsequent activation of the innate immune response in in vivo mice with ALS. Additionally, mtDNA can actively or passively enter the circulatory system and be detected as ccf-mtDNA [3]. Our study contributes novel insights into the potential role of elevated ccf-mtDNA in the serum of ALS patients, which may serve as an indicator for mitochondrial dysfunction related to the disease. Moreover, ccf-mtDNA levels did not show a significant association with ALS severity but were associated with the disease progression. In particular, patients with SOD1 mutations exhibited a more pronounced correlation between ccf-mtDNA levels and the rate of disease progression.
A significant elevation in levels of ccf-mtDNA was noted in our cohort of ALS patients, whereas ccf-nDNA levels remained stable. Our correlation analysis revealed a positive association between ccf-mtDNA and IL-6. This association is particularly noteworthy when considering the characteristics of mitochondria, which autonomously replicate their circular genome and possess a dual-membrane structure similar to bacteria [4]. These features are significant because, upon release, mtDNA fragments can act as damage-associated molecular patterns (DAMPs) that initiate innate immune responses, leading to inflammation [3]. The positive correlation with IL-6, a key inflammatory cytokine, underscored the potential role of ccf-mtDNA in triggering neuroinflammation in ALS.
In our study, we observed that ccf-mtDNA exhibited high diagnostic accuracy in distinguishing between ALS patients and healthy controls. Furthermore, ccf-mtDNA demonstrated even higher accuracy in distinguishing between patients with the SOD1 mutation and healthy controls. Peripheral serum ccf-mtDNA levels, while not a definitive diagnostic biomarker for ALS due to their elevation in other neurological disorders [16, 17, 19], still hold significant interest in identifying novel disease mechanisms and in the characterization of specific patient subgroups. This is particularly true when considering the high ccf-mtDNA levels observed in patients with the SOD1 mutation, which may serve as a useful indicator for ALS subtyping. Furthermore, the capability of ccf-mtDNA to accurately distinguish early-stage ALS patients from healthy controls holds great potential in facilitating the timely initiation of treatment.
Our study documented a significant decrease in ccf-mtDNA levels in both ALS patients and healthy controls over time, contrasting with the age-related increase reported by Pinti et al. [20], who observed an initial decline followed by a rise in individuals aged over 60 years. This discrepancy may stem from our study's younger demographic and notably smaller sample size, with ALS patients averaging 54 years of age and controls 50 years. Echoing our findings, Lazo et al. [21] noted that ccf-mtDNA within plasma extracellular vesicles showed a decline over time among individuals aged 30–64 years. Studies in AD and PD have linked ccf-mtDNA reductions to mtDNA loss and deletions [22, 23], and similar ALS research points to diminished mtDNA and increased deletions in cervical spinal neurons [24]. Collectively, these factors could deplete cellular mtDNA, leading to lowered ccf-mtDNA levels. The dynamics of ccf-mtDNA highlight the necessity for further research with larger patient and control cohorts.
The linear regression analysis demonstrated a negative correlation between ccf-mtDNA levels and ΔFS, which serves as a predictor of ALS survival rates [12]. However, this correlation was not reflected in the relationship with the ALSFRS-R score, which assesses the severity of the disease [25]. Based on this observation, we propose that elevated ccf-mtDNA levels could be a risk factor for the pathogenesis of ALS, suggesting that mitochondrial dysfunction may initiate the disease process and is not merely a secondary effect. Moreover, the negative correlation is particularly pronounced in individuals with the SOD1 mutation, which is biologically plausible given the confirmed co-localization of mutant SOD1 with mitochondria, supported by immunocytochemical and biochemical analyses [26, 27]. The brain and spinal cord, identified as vulnerable sites for the mutant SOD1 accumulation [28], align with the observed mitochondrial dysfunction. Importantly, an increase in mutant SOD1 accumulation within mitochondria has been consistently demonstrated as the disease progresses, reinforcing the link between mitochondrial dysfunction and ALS pathogenesis [26-28]. The observed correlation between ccf-mtDNA levels and ΔFS offers potential therapeutic insights for ALS, suggesting that interventions aimed at enhancing mitochondrial health might slow disease progression.
In addition to the progression rate, our study identified age at onset and disease duration as influential factors for ccf-mtDNA levels. The interactions among these variables—age at onset, disease duration, and progression rate—add a layer of complexity to the ALS pathology, suggesting the potential value of a multivariable analysis for a more nuanced understanding of the disease.
Given the small sample size of our study, we were unable to fully investigate the combined effects of interactions among age of onset, disease duration, and progression rate on ccf-mtDNA levels. Additionally, the retrospective nature of our study, the genetic heterogeneity within the ALS population, and the limitation of collecting samples at a single time point present further challenges for interpreting ccf-mtDNA levels. Nonetheless, this study offers a valuable perspective on the intricate pathophysiology of neuroinflammation and mitochondrial dysfunction in ALS. Therefore, future studies with larger cohorts and longitudinal designs are crucial to delineate the role of ccf-mtDNA in ALS and to enhance our understanding of the disease's pathophysiology.
In summary, our study explores the role of ccf-mtDNA as a prognostic indicator for ALS, particularly in patients with SOD1 mutations, given its correlation with disease progression rate. To fully appreciate the significance of ccf-mtDNA in tracking disease progression and its potential utility in clinical trials, further inclusion in longitudinal follow-up studies is essential.
AUTHOR CONTRIBUTIONS
Jieyu Li: Investigation; methodology; visualization; writing – original draft; formal analysis. Chao Gao: Investigation; data curation. Qingqing Wang: Methodology; investigation; validation; software. Jing Liu: Methodology; investigation; software; validation. Zhiying Xie: Software; resources. Yawen Zhao: Data curation; resources. Meng Yu: Data curation; resources. Yiming Zheng: Data curation; resources. He Lv: Data curation; resources. Wei Zhang: Resources; supervision. Yun Yuan: Resources; supervision. Lingchao Meng: Conceptualization; data curation; project administration; writing – review and editing; funding acquisition. Jianwen Deng: Conceptualization; data curation; project administration; writing – review and editing; funding acquisition. Zhaoxia Wang: Writing – review and editing; funding acquisition; supervision.
ACKNOWLEDGEMENTS
We are grateful for the patients' participation and the support from their families. We would also like to express our sincere gratitude to Qiurong Zhang, Jing Liu and Qingqing Wang for their invaluable assistance in managing the patient specimen bank.
FUNDING INFORMATION
This work was supported by the National Natural Science Foundation of China (grants 82071409, 82171846, 82101469 and U20A20356), Capitals Funds for Health Improvement and Research (2022-4-40716), Beijing Nova Program (20220484017, 20230484403), National High Level Hospital Clinical Research Funding (High Quality Clinical Research Project of Peking University First Hospital [2023HQ03]) & Scientific and Technological Achievements Transformation Incubation Guidance Fund Project of Peking University First Hospital (2023CX05).
CONFLICT OF INTEREST STATEMENT
The authors affirm that there were no financial or commercial affiliations during the research process that could be considered as potential sources of conflict.
Open Research
DATA AVAILABILITY STATEMENT
Due to privacy and ethical considerations, the data supporting the findings of this study are not publicly available. Interested researchers may request access to the data by contacting the corresponding author. All requests will be carefully reviewed, and approval will be granted based on the merits of the proposed research, in compliance with the Institutional Review Board's guidelines.




