Neurofilament light chain levels reflect outcome in a patient with glutamic acid decarboxylase 65 antibody–positive autoimmune encephalitis under immune checkpoint inhibitor therapy
Abstract
Neurological immune-mediated side effects are rare but often severe complications of immune checkpoint inhibitor (ICI) treatment. This report describes a severe case of nivolumab/ipilimumab-associated glutamic acid decarboxylase 65–positive autoimmune encephalitis. It proposes neurofilament light chain levels, a biomarker indicating axonal damage, in the cerebrospinal fluid and serum as a putative novel biomarker for this diagnostically and therapeutically challenging entity with an often unfavorable outcome. Additionally, we provide an overview of previous reports of patients developing autoimmune encephalitis under ICI treatment.
Abbreviations
-
- CNS
-
- central nervous system
-
- CSF
-
- cerebrospinal fluid
-
- GAD65
-
- glutamic acid decarboxylase 65
-
- ICI
-
- immune checkpoint inhibitor
-
- MRI
-
- magnetic resonance imaging
-
- NfL
-
- neurofilament light chain
CASE REPORT
A 52-year-old Caucasian female patient with a history of type 1 diabetes mellitus was diagnosed with BRAF-negative metastatic (lung, liver, and pancreas) melanoma in April 2018, and nivolumab and ipilimumab (1 mg and 3 mg/kg body weight, respectively) were initiated. Computed tomography–based staging revealed significant tumor load reduction under immune checkpoint inhibitor (ICI) treatment 3 months after commencing treatment. Two months later, she developed autoimmune encephalitis with short-term memory loss, cognitive dysfunction, limb ataxia, and epileptic seizures. Cerebrospinal fluid (CSF) analysis revealed pleocytosis and elevated immunoglobulin G antibody index indicating central nervous system (CNS)-localized antibody production throughout the clinical course. Immunohistochemical analysis showed positive CSF and serum staining of monkey cerebellum (Figure 1a). Enzyme-linked immunosorbent assay detected high CSF and serum concentrations of anti-glutamic acid decarboxylase 65 (GAD65) antibodies, whereas commercially available cell-based assays for antibodies to neuronal surface antigens were negative. Cranial magnetic resonance imaging (MRI) showed small alterations such as a gadolinium-enhancing inflammatory lesion of the right superior frontal gyrus in August 2018 and a small new inflammatory lesion without gadolinium enhancement of the left gyrus rectus in December 2018 (Figure 1b). Sequential CSF analyses were negative for tumor cells. Although GAD65-associated encephalopathies usually show significant MRI abnormalities such as temporal lobe involvement, cases with minor MRI alterations (such as in our patient) have also been reported [1, 2. Moreover, a recent study by Vogrig et al., in which ICI-associated CNS side effects were analyzed systematically, showed normal cranial MRI in approximately one-third of patients with ICI-induced autoimmune encephalitis [3. ICI treatment was paused, and immunotherapy was initiated including corticosteroids and B-cell depleting rituximab (375 mg/m2). Although data on diabetes-inducing antibodies to GAD65 before ICI treatment were lacking, we hypothesized the boosting of a preexisting autoimmune condition as the cause for developing autoimmune encephalitis.
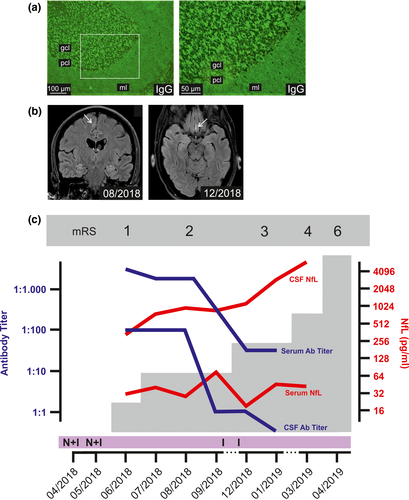
Despite an initial improvement, the encephalitis recurred after reinitiating monotherapy with nivolumab. At this point, immunosuppressive therapy remained ineffective. Following the patient's will, treatment was halted and the patient died under palliative care in April 2019.
A search of the available literature identified 26 reports of patients who developed autoimmune encephalitis while undergoing treatment with ICI. Two patients developed GAD65 autoantibody-positive limbic encephalitis after 3 months of treatment with ICI for a metastatic neuroendocrine tumor of the thymus and progressive lung cancer. In contrast to our patient, both responded well to antibody-depleting therapy consisting of rituximab. As in our case, one patient had a history of type 1 diabetes mellitus. Table S1 summarizes all 26 cases focusing on antibody findings, onset latency of autoimmune encephalitis after initiation of ICI treatment, MRI diagnostics, response to different immunomodulatory treatments, and outcome after onset of autoimmune encephalitis.
ROLE OF CSF MARKERS
Neurofilament light chain (NfL) levels are an emerging biomarker indicating axonal damage in the CNS, especially after recent successful detection in serum using single-molecule array assays [4, 5. NfL levels were assessed in the patient's CSF and serum, showing continuously increasing CSF NfL and constantly elevated serum NfL (mean = 40 pg/ml ± 17.6) compared to serum NfL levels of 62 healthy controls ((mean = 5.7 pg/ml ± 2.8) assessed in our laboratory. NfL reflected the patient's clinical course better than MRI findings or falling GAD65 autoantibody levels (Figure 1c), suggesting that this novel marker for CNS damage might be utilized as an additional monitoring parameter during ICI treatment. We propose that the significant benefit of NfL measurement lies in the possibility of preventing CNS immune-mediated side effects by simple blood tests allowing early cessation of ICI treatment. Moreover, NfL might be utilized as an additional parameter for treatment monitoring of manifest autoimmune encephalitis, especially if patients do not present with a defined syndrome, and paraclinical findings, such as MRI or autoantibody detection assays, show no or minor alterations, as was the case with our patient. In previously published studies, NfL concentrations (i) were found to be elevated and (ii) dropped under sufficient treatment in patients with ICI-independent autoimmune encephalitis 6-8.
ACKNOWLEDGMENTS
The authors thank Cheryl Ernest for proofreading the manuscript. This study was supported by the German Research Foundation. Open access funding enabled and organized by Projekt DEAL. WOA Institution: Johannes Gutenberg Universitat Universitatsmedizin Blended DEAL : Projekt DEAL
CONFLICT OF INTEREST
Frauke Zipp has recently received research grants and/or consultation funds from the DFG, BMBF, PMSA, Genzyme, Janssen, Merck Serono, Roche, Novartis, Celgene, and Sanofi-Aventis. Stefan Bittner has received travel funding and/or speaker honoraria from Biogen, Merck Serono, Novartis, Roche, and Sanofi-Aventis. Carmen Loquai is involved in advisory boards and receives speakers fees and travel reimbursement from MSD, BMS, Merck, Novartis, Pierre Fabre, Roche, Sun Pharma, Biontech, and Kyowa Kirin. All remaining authors have declared no conflicts of interest.
AUTHOR CONTRIBUTIONS
Johannes Piepgras: conceptualization (equal), data curation (equal), investigation (equal), methodology (equal), validation (equal), visualization (equal), writing original draft (lead). Aneka Müller: conceptualization (equal), data curation (equal), formal analysis (equal), investigation (equal), methodology (equal), software (equal), validation (equal), visualization (equal), writing review editing (lead). Falk Steffen: conceptualization (supporting), data curation (equal), formal analysis (equal), investigation (supporting), methodology (equal), software (equal), validation (equal), visualization (supporting), writing review editing (supporting). Johannes Lotz: conceptualization (supporting), data curation (equal), formal analysis (equal), investigation (supporting), methodology (equal), resources (supporting), software (equal), validation (equal), visualization (supporting), writing review editing (supporting). Carmen Loquai: conceptualization (supporting), data curation (supporting), investigation (supporting), methodology (equal), resources (supporting), validation (equal), visualization (supporting), writing review editing (supporting). Frauke Zipp: conceptualization (supporting), funding acquisition (equal), investigation (supporting), project administration (supporting), resources (supporting), supervision (supporting), validation (equal), writing review editing (supporting). Christian Dresel: conceptualization (supporting), investigation (supporting), project administration (supporting), supervision (supporting), validation (equal), visualization (supporting), writing original draft (supporting), writing review editing (supporting). Stefan Bittner: conceptualization (lead), funding acquisition (equal), investigation (equal), methodology (equal), project administration (lead), resources (lead), supervision (lead), validation (equal), visualization (equal), writing original draft (supporting), writing review editing (lead).
PATIENT'S CONSENT
Written informed consent was obtained according to the Declaration of Helsinki.
Open Research
DATA AVAILABILITY STATEMENT
That data that support the findings of this study are available from the corresponding author upon reasonable request.




