Summer and Autumn Movement of Endemic Bartram's Bass, Invasive Alabama Bass and Hybrid Congeners in an Upper Savannah River Tributary
Funding: This study was supported by U.S. Fish and Wildlife Service.
ABSTRACT
Bartram's Bass Micropterus sp. cf. coosae is endemic to the Upper Savannah River Basin of the southeastern United States, and is threatened by hybridization with invasive Alabama Bass M. henshalli. Quantifying movement of these species and their hybrids will improve understanding of how nonnative alleles spread among riverine fish populations. We quantified summer/autumn movement of Bartram's Bass, Alabama Bass and hybrid bass in Eastatoee Creek—a tributary experiencing ongoing invasion from Keowee Reservoir. To do this, we first quantified factors associated with the longitudinal distribution and weekly movement rates of each species, then estimated probabilities of species transitioning among key habitats at the river-reservoir interface. We tagged 291 fish with passive integrated transponder tags, sampling the entire stream length of Eastatoee Creek four times in both 2021 and 2022. We radio-tagged an additional 52 fish and tracked them from early May through mid-October each year. We used mixed effect models and a Bayesian multistate model to quantify movement, river position, movement probability and the effects of abiotic factors thereon. Alabama Bass and hybrid bass moved more than Bartram's Bass and remained in the lower reaches of Eastatoee Creek, apparently restricted by a reach of high-gradient habitat that functioned as a natural barrier. Alabama Bass made greater upstream movements during cooler spring temperatures when higher reservoir levels inundated the creek mouth. Bartram's Bass were distributed throughout Eastatoee Creek, making shorter weekly upstream movements associated with increasing temperature in late spring. Movement of hybrid bass from lower portions of tributaries is likely a primary source of nonnative allele spread in this system.
1 Introduction
Nonnative fish invasions threaten freshwater fish diversity worldwide (Dudgeon 2019). Invasive fishes adversely affect native fishes through a variety of mechanisms, including direct and indirect competition and predation at multiple life stages (Strayer 2010). Freshwater fish invasions can also compromise the genetic integrity of native fish populations, causing fitness reductions that eventually lead to population declines and even extirpation or extinction (Harbicht et al. 2014; Rhymer and Simberloff 1996). Nonnative alleles can spread through freshwater fish populations by both direct hybridization between native and nonnative individuals, as well as through subsequent introgression as hybrid individuals move through a population and reproduce (Largiadèr 2007). As such, understanding movement dynamics of native and nonnative fishes can provide vital information on how aquatic invasions function, including susceptibility of native species to hybridization, potential spread rates of nonnative alleles and environmental correlates of native and nonnative movement/dispersal (Hastings et al. 2005; Kovach et al. 2015). This information can be used to guide management efforts aiming to prevent or remediate hybridization between native and nonnative freshwater fishes (Rahel et al. 2008).
Black basses (Centrarchidae: Micropterus) are among the world's most popular and frequently translocated freshwater sportfishes (Jackson 2002; Peoples and Midway 2018). Several black bass species are cosmopolitan generalists with large native ranges that have been introduced widely outside their native range; these include Largemouth Bass M. nigricans, Smallmouth Bass M. dolomieu, Spotted Bass M. punctulatus and Alabama Bass M. henshalli. However, many other black basses have narrowly restricted ranges, are endemic to isolated sub-basins in upland rivers of the southeastern United States, and are sensitive habitat specialists. These species include members of the Smallmouth Bass M. dolomieu and Redeye Bass M. coosae species complexes (Birdsong et al. 2015; Kim et al. 2022). Because of weak pre- and post-zygotic barriers, intrageneric hybridization is common among black basses (Koppelman 2015). However, hybridization with nonnative generalist congeners is a threat to nearly every species of endemic black bass in the southeastern US (Taylor et al. 2019).
A species of particular conservation need is Bartram's Bass M. sp. cf. coosae (Near and Kim 2021), a member of the Redeye Bass species complex that is endemic to the Upper Savannah River Basin of South Carolina, Georgia and North Carolina, USA. Bartram's Bass is threatened by introgressive hybridization with invasive Alabama Bass, which was introduced into Savannah River reservoirs in the mid-1980s (Leitner and Earley 2015). Upon introduction, Alabama Bass spread rapidly throughout the basin, causing Bartram's Bass to be functionally extirpated from all reservoirs within two decades (Bangs et al. 2018; Oswald et al. 2015). Bartram's Bass is currently restricted to fragmented populations occupying tributaries disconnected by reservoirs, and many populations of pure Bartram's Bass are also experiencing declines due to hybridization (Leitner et al. 2015). Moreover, hybridization rates are associated with poor anthropogenic land use practices, as well as proximity to reservoirs, which serve as a source population for Alabama Bass (Peoples et al. 2021). As such, many populations of Bartram's Bass are currently experiencing active invasion from upstream-dispersing Alabama Bass (Judson et al. 2021). Knowledge of movement dynamics of both invasive Alabama Bass and endemic Bartram's Bass in rivers experiencing active invasion will help managers better anticipate and manage fragmented populations of Bartram's Bass and will also provide baseline history information that may be applicable across the many southeastern black bass species facing essentially the same threats of introgression with nonnative species.
Movement patterns of members of the Redeye Bass complex are grossly understudied, being represented by a single quantitative study from a dam-regulated stretch of the Tallapoosa River, Alabama (Earley 2012) and some anecdotal observations from a small tributary to the Conasauga River, Tennessee (Parsons 1954). Likewise, movement of Alabama Bass has only been studied in larger rivers (Earley 2012; Goclowski et al. 2013) or reservoirs in its native range (Hunter and Maceina 2008). Little is known about movement rates or environmental cues for either of these species in free-flowing streams or their nonnative ranges, or how hybridization affects movement. Accordingly, the goal of the study was to better understand movement dynamics of Bartram's Bass, Alabama Bass and their hybrids from spring through autumn in a Savannah River tributary that is currently experiencing an active invasion. Specifically, our objectives were to (1) characterise the longitudinal distribution of pure Bartram's Bass, Alabama Bass, and hybrid bass, and identify environmental factors associated with their position in the stream (i.e., riverine distance from the reservoir; river kilometres, rkm), (2) quantify weekly movement rates of each species and the environmental correlates associated with movement, (3) quantify probabilities of nonnative bass transitioning among key habitats at the river-reservoir interface—either from the reservoir into the stream or vice versa and (4) conduct a controlled tag retention study to evaluate key assumptions of our tagging approach, namely that survival and tag retention are equal among tagged species.
2 Methods
2.1 Methods Overview
To accomplish our goal and objectives, we tagged Bartram's Bass, Alabama Bass, hybrid bass in Eastatoee Creek, a tributary to Keowee Reservoir in the Upper Savannah River Basin. Depending on their size, individuals were tagged with either passive integrated responder (PIT) tags or radio tags in initial sampling conducted in May–June of 2021 and 2022. The entire length of Eastatoee Creek and its major tributaries were then resampled three times each year. Radio-tagged individuals were tracked manually for the duration of tag life (running through late September each year). We used generalised linear models to estimate effects of discharge and temperature on (1) weekly movement rates of both species and (2) spatial position (river kilometre) of individuals of each species each week. We then used Bayesian multistate models to estimate factors affecting the probability that Alabama Bass would transition from Keowee Reservoir into Eastatoee Creek. Separately from our telemetry efforts, we conducted a lab study to estimate retention of both types of tags by both species, as well as survival rates of tagged and control fish.
2.2 Study Area
We conducted this study in Eastatoee Creek, which flows 25.5 river kilometres (rkm) through the Blue Ridge ecoregion from North Carolina, into South Carolina, USA. Eastatoee Creek flows into Keowee Reservoir, a major impoundment in the Upper Savannah River Basin (Figure 1A,B). Upper reaches of Eastatoee Creek are one of the few remaining strongholds for non-hybridised or introgressed Bartram's Bass populations, also referred to as ‘pure’. However, Keowee Reservoir is an invasion source for Alabama Bass, and Alabama Bass alleles have been detected contemporarily in the lower and middle reaches of Eastatoee Creek (Judson et al. 2021; Leitner et al. 2015). Ongoing monitoring indicates that, compared to nearby streams in the Piedmont ecoregion where pure Bartram's Bass have been extirpated and replaced by Alabama Bass or cosmopolitan Largemouth Bass, Eastatoee Creek is either in earlier stages of invasion or is more resistant to invasion (Cooper et al. 2025; Peoples et al. 2021). Given these conditions, Eastatoee Creek is a valuable system for investigating the mechanisms by which Alabama Bass alleles may disperse in a stream system.
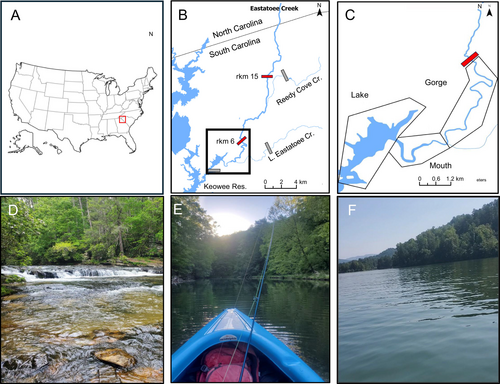
Eastatoee Creek can be partitioned into distinct sections defined by habitat and tributaries (Figure 1B). Natural barriers at approximately rkm 6 and 15 may limit upstream movement of black basses, at least during low-flow conditions (Figure 1B). These natural barriers are steep reaches of swift, shallow habitat that are often dry during low flows. Above the upstream-most barrier, Eastatoee Creek is steep, narrow, and shallow, and sampling throughout the study period indicates this section supports a fish community comprised primarily of minnows (Leuciscinae), darters (Etheostomatinae) and suckers (Catostomidae). Between the two barriers, Eastatoee Creek is a relatively low-gradient stream that meanders through a wide valley and is characterised by alternating pool/riffle sequences with widths between 8 and 20 m and maximum pool depths exceeding 2 m. One major tributary, Reedy Cove Creek, joins Eastatoee Creek in this section (Figure 1B).
The downstream-most section of Eastatoee Creek (below the potential natural barrier at rkm 6) is represented by three sub-sections that vary in flow, depth, habitat complexity, and riparian development, and differ from one another in terms of habitat preferences of our focal species (Figure 1C). For the purposes of this study, the gorge of Eastatoee Creek (hereafter, ‘gorge’), the mouth of Eastatoee Creek (hereafter, ‘mouth’) and the Eastatoee arm of Keowee Reservoir (hereafter, ‘lake’; Figure 1C). These sections represent different ‘spatial states’ that are important for estimating probabilities of fish transition and informing invasion dynamics from the lake into Eastatoee Creek. The furthest upstream state, the gorge, has a densely vegetated riparian zone and is located in steep, mountainous terrain that limits human development (Figure 1D). The gorge also has flows, depths and habitat types similar to other undeveloped, Blue Ridge streams (Leigh 2010; Scott et al. 2002). The mouth section of Eastatoee Creek is the furthest downstream section of Eastatoee Creek and represents a transition zone between the creek and the lake. The hydrology of this section is influenced by both lake level management in Keowee Reservoir and discharge from Eastatoee Creek. Thus, the mouth has low current velocity and experiences drastic depth fluctuations in response to both factors (Figure 1E). Such patterns are very uncharacteristic of other, less-impaired sections of streams in the Blue Ridge Ecoregion (Leigh 2010; Scott et al. 2002). The mouth also experiences moderate channelization and sedimentation from upstream erosion, resulting in very little habitat suitable for lithophilic Bartram's Bass. Another key tributary, Little Eastatoee Creek, enters Eastatoee Creek in the downstream-most portion of the gorge (Figure 1C). The lake spatial state is part of Keowee Reservoir, and has housing development along much of its shoreline. This spatial state represents the invasion source of nonnative bass into the surrounding tributaries (Bangs et al. 2018; Leitner et al. 2015). The lake state has very low current velocity, a large pelagic zone, and is much deeper than the other states, making it well-suited for Alabama Bass (Kerr 2022) (Figure 1F).
2.3 Field Collections
We collected black basses in Eastatoee Creek from early May to late August of 2021 and 2022 such that the entire length downstream of the uppermost barrier (Figure 1B) was sampled four times each year. Sampling was conducted at least 4 days each week, beginning with the most downstream portion of Eastatoee Creek and proceeding upstream each subsequent day. Once the entire length of the stream had been sampled up to the uppermost barrier, this process was repeated three times through the end of August. To account for potential emigration into 2nd order tributaries of Eastatoee Creek, we also sampled Reedy Cove Creek and Little Eastatoee Creek three times each year after the first round of sampling (Figure 1B). We collected black basses using boat electrofishing, backpack electrofishing, barge electrofishing and hook-and-line sampling, where appropriate. We tailored the sampling method to each section of river, specifically to maximise capture given the habitat conditions. We conducted boat electrofishing in the lake state, as well as navigable areas of the mouth. We conducted barge electrofishing in the shallower depths of the mouth that were not navigable by boat, and used backpack electrofishing where possible in wadeable portions of the gorge and tributaries to Eastatoee Creek. We used hook-and-line capture in and above the gorge in reaches that were too wide and deep to electrofish. Hook-and-line sampling is biased, but can be ideal for collecting black basses in remote conditions where traditional sampling is not possible (Hafs et al. 2010; Lewis et al. 2021; Mycko et al. 2018). Hook-and-line sampling was performed using three to five anglers on each sampling visit, each casting different lures that targeted different portions of the water column (benthic, mid-column and surface). Because black basses can exhibit learned behaviour to avoid recapture that affects recapture probability (Hessenauer et al. 2016), we continuously used a combination of both new and previously used lures on each sequential revisit to maximise recapture probability.
For each individual captured, we recorded length (mm), weight (g), release location (decimal degrees) and photographed and collected an anal fin clip to confirm species identification. Captured black basses were implanted with either passive integrated transponder (PIT) tags or radio tags, depending on size. We did not implant radio-tagged individuals with PIT tags in order to reduce tagging stress on individuals. For individuals between 6 and 110 g, we used injection syringes to insert 12 mm PIT tags into the peritoneal cavity. Radio-tagged individuals weighed at least 110 g to adhere to the widely accepted 2% tag weight burden limit (Winter 1996). For 51 individuals weighing > 110 g (27 in 2021 and 25 in 2022) we surgically inserted a 2.2 g F1550 advanced telemetry systems (ATS) radio tag into the peritoneal cavity, following the shielded-needle technique (Ross and Kleiner 1982). We then released tagged individuals at their original capture location after five minutes of recovery (Cooke and Bunt 2001). During all revisits we scanned captured individuals for PIT tags and tagged any newly captured individuals.
We tracked radio-tagged bass starting 10 days after their initial tagging date to give individuals sufficient time to recover from surgery and return to normal behaviour (Earley and Sammons 2015). We used a mobile ATS 3-way Yagi antenna and receiver to search weekly for radio-tagged bass for the entirety of the tags' battery life (~158 days), which occurred from early May to mid-October of 2021 and 2022. This time period is typically when black bass are most active (Earley 2012; Goclowski et al. 2013). Each week, we tracked the entirety of Eastatoee Creek on foot and tracked non-wadeable sections by boat out to the lake's first confluence with the next northern tributary (Figure 1B); we tracked Little Eastatoee Creek and Reedy Cove Creek once a month (Figure 1B). Once we detected a tagged individual, we recorded the tag number, gain setting, signal strength, time stamp, field bearing and location. We recorded two detections for each fish location, then used ArcGIS Pro to bi-angulate fish locations and improve location accuracy and precision (Simpkins and Hubert 1998). To assess location accuracy, we also conducted 12 ‘tag hunts’ throughout different areas of the stream using un-implanted tags hidden at locations that were known by one member of the crew but were unknown to the crew members conducting telemetry. Tag hunts were done under the full range of conditions in which radio telemetry occurred; they were distributed evenly in all three states of Lower Eastatoee Creek and transition to Keowee Reservoir (Figure 1C), in the mainstem of Eastatoee Creek about the barrier at rkm 6, as well as in Little Eastatoee and Reedy Cove creeks (Figure 1B). As a result, mean tracking error was 8.6 m ± 1.6 standard error (SE) in the upper part of the stream and 30.5 m ± 9.2 SE near the mouth and Eastatoee arm of Keowee Reservoir.
2.4 Abiotic Factors
We obtained environmental data on daily water temperature (°C), daily discharge (cubic meters per second) and reservoir depth (local datum meters). To collect daily temperature data, we deployed 10 ONSET UA-001-64 hobo loggers along spatially separated locations along mainstem Eastatoee Creek, Keowee Reservoir, Little Eastatoee Creek and Reedy Cove Creek. Loggers recorded hourly temperature, which we averaged to obtain a daily average temperature for each tributary. We obtained daily discharge data from the U.S. Geological Survey stream gauge #02185010 located on Eastatoee Creek (USGS 2025). We obtained daily reservoir depth data for Keowee Reservoir from the Duke Energy dam gauge (Duke Power, unpublished data).
2.5 Genetic Analyses
Genetic species identification was conducted based on a microsatellite panel developed by Tringali et al. (2015) and according to protocols used in previous analyses for Bartram's Bass (Judson et al. 2021; Peoples et al. 2021; Cooper et al. 2025). Fin clips were genetically processed at the Hollings Marine Laboratory in Charleston, South Carolina, in the South Carolina Department of Natural Resources (SCDNR) Population Genetics Laboratory. Unknown field sample genotypes were assigned to species by comparison to a reference set of known species genotypes using an optimised STRUCTURE assignment protocol (Judson et al. 2021). All samples genotyped at 10 or more microsatellite loci were included in STRUCTURE analysis for species assignment using the optimised analysis protocol at K = 9 for 20 iterations to assess variability in q-value assignment scores. Final q-value assignments were obtained from a representative iteration and were used to establish definitions for species and hybrid assignments. Following hybridisation thresholds identified by previous studies, individual samples with a maximum q-value ≥ 0.87 were designated as ‘pure’ and were otherwise categorised as hybrid bass. Assignments across STRUCTURE runs (i.e., resulting q-value-based species and hybrid assignment) for individual samples were consistent (mean standard deviation = 0.001).
2.6 Data Analysis
We recognise that this metric can be biased because it does not account for movement between detection intervals. However, it represents the finest resolution possible for characterising movement, given the logistical constraints of our study. We did not use PIT-tagged individuals in calculations of weekly movement due to the low number of recaptures.
To quantify river position, we used data on both radio-tagged individuals and PIT-tagged individuals captured via hook-and-line. We calculated the rkm location for each individual detection using Nearest Facility Analysis in ArcGIS Pro. We then paired each detection with the daily average temperature and discharge corresponding to the day of each detection for both response variables. For all models, we treated species, temperature and discharge as fixed effects and individual fish as a random intercept. We used interaction effects between species and the other fixed effects to quantify differences among species. All covariates were scaled and centered to mean = 0 and standard deviation = 1 prior to analyses. We also screened fixed effects for collinearity based on a variance inflation factor less than 4 in R software version 4.3.2 (R Development Core Team 2023), with a threshold value of 5.
To meet our third objective of quantifying the probabilities of upstream movement by nonnative bass into Eastatoee Creek, we used Bayesian inference to estimate the parameters of a multistate Cormack–Jolly–Seber model fit to weekly capture histories of 35 radio-tagged individuals that were genetically assigned as either pure Alabama Bass or hybrid bass, hereafter collectively referred to as nonnative bass. No pure Bartram's bass were included in the Bayesian multistate model, as this model focused on nonnative bass movement probabilities in lower sections of the study area. For this analysis, we lumped pure Alabama Bass with hybrid bass because (1) it improved model stability by increasing sample size and (2) our main concern with this analysis was whether nonnative alleles may be passed upstream, regardless of whether they are from a hybrid or pure Alabama Bass. Nonnative bass from each year (2021: n = 17, 2022: n = 19) were analysed in separate models. Overall, the dataset contained imperfect detection data from week to week and relatively low sample sizes, but such issues are common in field-based telemetry studies (Cooke et al. 2012). Flexible Bayesian approaches are well suited for handling such imperfections and uncertainty (Kéry and Schaub 2011). We used the model by Bunch et al. (2022) adapted from (Kéry and Schaub 2011) as a reference and fit the Cormack–Jolly–Seber model to 19 weeks of tracking data from each year using Markov chain Monte Carlo (MCMC) simulation to estimate parameters in JAGS (Plummer 2013) called from R with the ‘jagsUI’ package (Kellner 2015). The model estimated overall transition or movement probabilities (Ψi,t) among three spatial states (i) for each week (t). Spatial states included Keowee Reservoir (i.e., ‘lake’, state A), Eastatoee Creek mouth (state B) and Eastatoee Creek gorge (state C: Figure 1C). Transition probability from state A to C was constrained to 0. Because transition probabilities for state A and state C were complementary (e.g., ΨAB = 1 − ΨAA), it was only necessary to estimate parameters of the reservoir depth model for one of the complements in order to predict the complete set of Ψi,t. Three transition probabilities were estimated for state B (remain in the state: ΨBB; move to an adjacent state: ΨBA and ΨBC) For each state, the model also estimated overall detection probability (pi) and apparent survival (Φi).
For each of the 19 tracking weeks within each sampling year, we assigned each radio-tagged individual to a spatial state (either the lake [state A], mouth [state B], or gorge [state C]), giving us two capture history matrices, one for each year. Individuals that went undetected in a given week but were detected later were assigned a ‘0’ in the capture history matrix. These ‘0’ assignments were used to estimate detection probability and apparent survival for each state. In particular, individuals detected again after being assigned a ‘0’ were used to estimate detection probability. When individuals were not detected again after a ‘0’ assignment, they could have either: (i) shed their tags, (ii) died or (iii) permanently emigrated from the study area. As there were limited observations to distinguish between these fates, our apparent survival estimates (Φ) represent these combined effects. In the rare instances that individuals were confirmed dead (e.g., tags recovered in scat of birds or terrestrial mammals), we used their weekly location data up until the point of confirmed mortality, then censored all future weekly state observations from the model.
Uninformative distributions were used for all priors in the Bayesian multistate model and prior distributions for all parameters followed Bunch et al. (2022). Posterior distributions were sampled using a thinning rate of every other sample among 10,000 iterations in 3 MCMC chains after burning in 2000 iterations of each chain. We checked model convergence by examining trace plots of all parameters and ensuring all values were below 1.1 (Gelman and Hill 2007).
2.7 Tag Retention Study
As a fourth objective, separate from our capture and telemetry efforts, we conducted a tag retention study to assess the effects of radio- and PIT tagging on pure Bartram's Bass and Alabama Bass tag shedding and overall survival. To do so, we captured 59 Alabama Bass and 69 Bartram's Bass in tributaries outside of Eastatoee Creek in fall 2022. We then placed captured individuals in 108-L aerated tanks at the Aquatic Research Laboratory at Clemson University. Tanks were arranged in a flow-through system (approx. 10 L/min) fed by untreated water supplied directly from Hartwell Reservoir, which is approximately 100 m from the facility. During the tag retention study, pH, ammonia and dissolved oxygen were monitored daily and the natural photoperiod was recreated using overhead fluorescent lights adjusted weekly (Cannaday and Farmer 2022). Individuals were stocked in tanks at a low density to minimise stress-induced mortality (Cooke et al. 2013). This amounted to one radio-taggable fish (i.e., > 110 g) per tank, or two individuals below that size per tank. We held individuals for 14 days prior to experimental tagging to ensure handling stress did not affect results of the tag retention study. We assigned each individual to an experimental tag group, including PIT, radio and control. After the 14-day acclimation period, we proceeded to tag individuals using the same capture techniques described above. For radio-tagged individuals, we used dummy tags of equal size and weight of ATS tags. We did not tag the control group and simply monitored their survival throughout the study period. For the tagged individuals, we monitored individual survival and tag retention for 30 days post-tagging. After the 30-day period, individuals were euthanized and scanned for tags to confirm tag retention/shedding. All tanks were also scanned for lost tags. Following this procedure, all individuals were frozen following procedures outlined in Clemson University IACUC Protocol AUP2022-0136.
Upon completion of the tag retention study, we used mixed effect logistic regression models to quantify survival and tag retention for each species and tag type. We treated species and experimental group as fixed effects in each model and treated tank as a random intercept. We used interaction effects between species and experimental groups to quantify differences among species and experimental groups.
3 Results
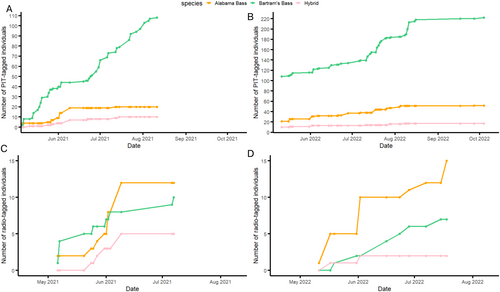
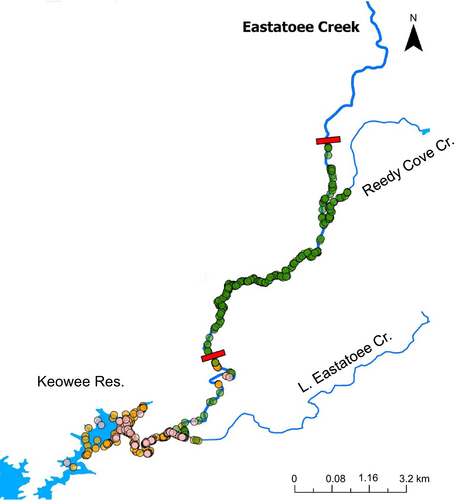
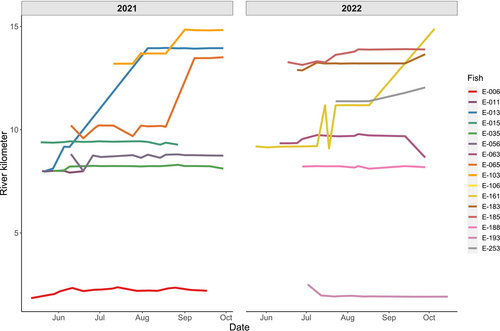
We captured a total of 342 taggable-sized black basses (> 6 g) including 239 pure Bartram's Bass, 79 pure Alabama Bass and 24 hybrid bass. PIT-tagged individuals included 222 Bartram's Bass (108 in 2021 and 114 in 2022), 52 Alabama Bass (20 in 2021 and 32 in 2022) and 17 hybrid bass (10 in 2021 and 7 in 2022). The remaining 51 individuals were radio-tagged. These include 17 Bartram's Bass (10 in 2021 and 7 in 2022), 27 Alabama Bass (12 in 2021 and 15 in 2022) and 7 hybrid bass (5 in 2021 and 2 in 2022) (Figure 2). The subsequent 5–6 months of radio tracking in each year yielded 221 detections in 2021 and 234 in 2022 (average of 9 detections/individual). Eight PIT-tagged individuals were recaptured, with 3 recaptured in 2021 and 5 recaptured in 2022. With the capture/recapture detections, a total of 805 distinct river positions were collected among both years (Figure 3).
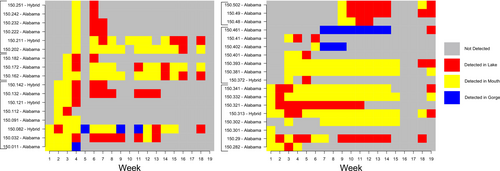
Most black bass individuals we captured and classified as hybrid bass were backcrossed progeny, with microsatellite assignments indicating majority assignment clusters over 75% to one species or another. Only one individual approached an even split in species assignment between the two species (62% Alabama, 38% Bartram's Bass), indicating that few of the hybrid bass we detected were first generation resulting from reproduction between pure Bartram's Bass and pure Alabama Bass.
Addressing our first objective, we found that pure Bartram's Bass were distributed significantly farther upstream and were mostly spatially segregated from Alabama Bass and hybrid bass above the first natural barrier near rkm 6 (Figures 3 and 4A,B). In fact, we observed only seven detections of Alabama Bass between rkm 6 and 6.3, and detected no nonnative bass upstream of the barrier. This suggests the transition to shallow, swift lotic habitat functions as a natural barrier for upstream movement of Alabama Bass and hybrid bass. Bartram's Bass had a much wider longitudinal range than Alabama Bass and hybrid bass, ranging from rkm 3 to 15 (Figures 3 and 4A). This suggests that the natural barrier near rkm 15 prevented upstream movement of Bartram's Bass during our study period, and also that they generally avoided the most downstream portions of Eastatoee Creek near the reservoir (Figures 3 and 4A). Alabama Bass and hybrid bass had longitudinal ranges similar to one another, ranging from rkm 0 to 6 (Figure 4A).
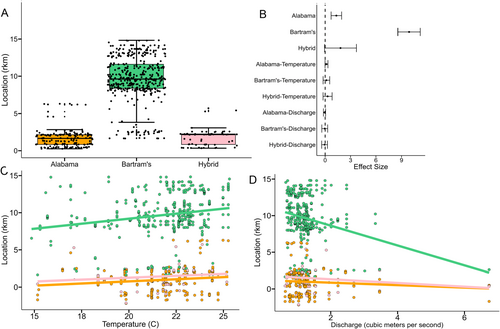
Across all species, most individuals were captured at temperatures ranging from 19.7°C to 23.9°C and discharges of 0.1–1.4 cubic meters/s (Figure 4C,D). Main- and interaction effects of temperature and discharge in linear mixed models of river position had 95% confidence intervals that bounded zero. However, the main effect of temperature was positive and had non-bounding confidence intervals at the 90% level (0.02 < 1.70 < 0.31), indicating a weak positive effect of association with river position (Figure 4C). This suggests that either bass generally moved upstream throughout the summer, or that we were more effective at capturing bass in upstream habitats as temperatures increased. This is difficult to parse out because of the low number of recaptured PIT-tagged fish. However, examining the spatial position of radio-tagged Bartram's Bass reveals that individuals either (a) remained at a fairly constant rkm throughout the lifetime of the tag, or (b) progressed upstream throughout the summer and autumn. All but one radio-tagged Bartram's Bass that moved downstream at any point after initial capture subsequently progressed back upstream, at least to their point of initial capture (Figure 5).
Addressing our second objective, we found that movement rates were variable among species and hybrid status, with hybrids moving an average of 550 m/week (95% CI: 240–860), Alabama Bass averaging 502 m/week (95% CI: 371–632) and Bartram's Bass averaging a much lower 151 m/week (95% CI: 107–195). Large movements (≥ 1000 m/week) were observed for both species and hybrid bass, but occurred at a greater magnitude and frequency for nonnative bass than Bartram's Bass (Figure 6A). The largest movement rate recorded for any individual across species was 6902 m/week by a hybrid bass. Weekly movement rates of Bartram's Bass were significantly lower than Alabama Bass and hybrid bass, and movement rates of Alabama Bass and hybrid bass did not differ from one another (Figure 6A,B).
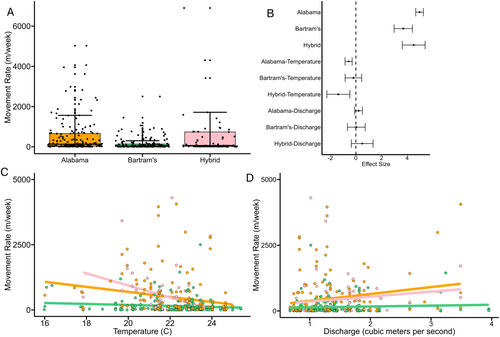
Temperature had a significant negative effect on weekly movement rates of both Alabama Bass and hybrid bass; this effect was stronger for hybrid bass than Alabama Bass. These results suggest Alabama Bass and hybrid bass make larger weekly movements during spring and early summer than during the heat of summer and early fall. Temperature was not associated with weekly movement of Bartram's Bass (Figure 6B,C), suggesting that although it had some positive effect on where Bartram's Bass were captured as temperatures warmed, it had no observable effect on how far they moved each week. Discharge was not associated with weekly movement rate of the two species or hybrid bass (Figure 6B,D). Alabama Bass and hybrid bass showed indications of increased movement at higher discharges, but few periods of high discharge rates occurred during tracking (Figure 6D).
In examining radio detections of each species among key habitat states below the first natural barrier near rkm 6 (the lake, mouth and gorge), we found that Bartram's Bass was detected more frequently in the gorge compared to Alabama Bass or hybrid bass, and were also the most abundant black bass in the gorge (Table 1). Alabama Bass were detected most frequently in the mouth and were the most abundant bass in samples from both the mouth and the lake. Differences in Alabama Bass detection between mouth and lake are likely due to the difference in sampling effort between the two states and the fact that the pelagic tendencies of Alabama Bass in lentic systems makes them difficult to sample during much of the year (Kerr 2022). Relative to the other spatial states, radio-tagged Alabama Bass were detected most frequently in the mouth and were the most abundant black bass in samples from both the mouth and the lake. Hybrid bass were the most abundant in sampling in the mouth and were detected more frequently in the mouth (Table 1).
| (A) Eastatoee arm of Lake Keowee | (B) Mouth | (C) Gorge | Total | |
|---|---|---|---|---|
| # Of individuals | ||||
| Bartram's Bass | 0 | 4 | 11 | 15 |
| Alabama Bass | 20 | 45 | 9 | 74 |
| Hybrid Bass | 7 | 14 | 3 | 24 |
| # Of detections | ||||
| Bartram's Bass | 0 | 12 | 18 | 30 |
| Alabama Bass | 65 | 139 | 15 | 219 |
| Hybrid Bass | 13 | 67 | 3 | 83 |
| Species composition by state | ||||
| Bartram's Bass | 0.0% | 40.0% | 60.0% | |
| Alabama Bass | 29.7% | 63.5% | 6.8% | |
| Hybrid Bass | 15.7% | 80.7% | 3.6% | |
| State composition by species | ||||
| Bartram's Bass | 0.0% | 5.5% | 50.0% | |
| Alabama Bass | 83.3% | 63.8% | 41.7% | |
| Hybrid Bass | 16.7% | 30.7% | 8.3% | |
- Note: Species include Bartram's Bass Micropterus sp. cf. coosae, Alabama Bass M. henshalli, and their hybrids. Individuals captured include those PIT tagged and radio-tagged in Eastatoee Creek from May to September of 2021 and 2022.
The multistate model addressing objective three effectively estimated nonnative bass movement probability, detection probability and apparent survival across all three spatial states, with slightly more uncertainty for the gorge state. All parameters converged successfully ( < 1.1), and visual assessment of MCMC chains indicated proper mixing. Unfortunately, in 2021, 10 of 24 fish were lost by the midpoint of the study period, as verified by tag recovery in scat of terrestrial predators. As such, we were unable to estimate model parameters with as much precision as for 2022, when a greater proportion of tagged fish were detected after the midpoint of the study period (Tables 1 and 2). Despite these confirmed mortalities, weekly survival probability (Φ) estimates were high in both years in the mouth (> 90%) and mean survival probability was > 70% in the lake and gorge. Mean weekly detection probability was variable across states and years, ranging from 63% to 94% (Table 2).
| Parameter | 2021 | 2022 | ||
|---|---|---|---|---|
| Estimate | 95% Credible interval | Estimate | 95% Credible interval | |
| p A | 0.753 | 0.502–0.994 | 0.600 | 0.475–0.746 |
| p B | 0.755 | 0.552–0.976 | 0.936 | 0.804–0.994 |
| p C | 0.632 | 0.243–0.996 | 0.881 | 0.601–0.997 |
| ΦA | 0.763 | 0.595–0.887 | 0.904 | 0.822–0.963 |
| ΦB | 0.982 | 0.938–0.999 | 0.975 | 0.931–0.999 |
| ΦC | 0.721 | 0.349–0.964 | 0.851 | 0.621–0.980 |
In general, the probability that a tagged fish stayed in the same state from week to week was higher than transitioning to another state regardless of year or state (Table 3, Figure 7). When nonnative bass did move out of the mouth (state B), movement downstream into the lake was more likely than upstream movement into the gorge in both years (Table 3, Figure 7). Across years, weekly upstream transition probabilities into the gorge typically remained below 10%. In fact, this movement was only documented four times in three nonnative bass. The only other nonnative bass detected in the gorge was released there and was last detected downstream in the creek mouth (see Appendix for full capture histories).
| Effect | 2021 | 2022 | ||
|---|---|---|---|---|
| Estimate | 95% Credible interval | Estimate | 95% Credible interval | |
| β AA | 0.467a | 0.0297–16.98 | 0.656a | 0.0639–15.82 |
| β BA | 1.065 | 0.0377–33.60 | 2.10a | 0.116–14.18 |
| β BB | 0.908 | 0.0316–32.16 | 0.845 | 0.048–17.31 |
| β BC | 1.116 | 0.0353–26.36 | 0.636 | 0.0403–18.34 |
| β CC | 1.024 | 0.0295–30.32 | 0.652 | 0.0322–18.69 |
- a Indicator variable (w) weight was above 0.5, indicating significance of β covariates.
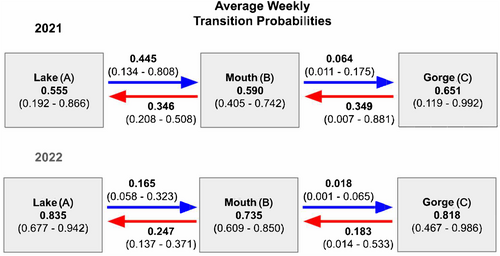
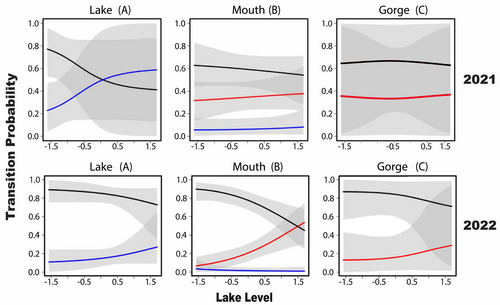
Lake level was associated with several movement transitions between states. Upstream movement transitions into the creek mouth from the lake state became more likely with increasing lake level in both years, although the effect of lake level varied across years and was less certain for 2021 (Figure 8). Downstream movement transitions from the mouth to the lake were also predicted to increase with increasing lake levels during 2022 (Figure 8). However, staying in the mouth was almost always more likely than moving downstream to the lake during 2022. Only at the highest lake levels in 2022 was the probability of moving downstream to the lake similar to the probability of remaining in the mouth. Overall, although increasing lake levels were positively correlated with some movement transitions between the lake and mouth, lake level had no effect on transitions between the mouth and gorge.
In the tag retention study addressing our fourth objective, survival was high for tagged Alabama Bass and Bartram's Bass (Table 4). Survival was not significantly different between PIT-tagged and control Alabama Bass (F2,31 = 5.10; p = 0.99) or radio-tagged and control Alabama Bass (F2,33 = 2.13; p = 0.87). Similar patterns were observed between PIT-tagged and control Bartram's Bass (F2,39 = 5.04; p = 0.99) and radio and control Bartram's Bass (F2,32 = 5.03; p = 0.99). Tag retention was also high for PIT tags and radio tags across both species (Table 4). Based on these results, we were confident that tag shedding did not cause major violations to the assumptions of our study design.
| Species | Experimental group | Sample size (n) | Estimate (%) | 95% Confidence interval |
|---|---|---|---|---|
| Alabama Bass | Control survival | 17 | 70.5 | 47.8–93.2 |
| Radio survival | 17 | 87.1 | 66.7–100.0 | |
| PIT survival | 15 | 80.3 | 57.8–100.0 | |
| Radio retention | 17 | 88.2 | 72.9–100.0 | |
| PIT retention | 15 | 100.0 | 99.8–100.0 | |
| Bartram's Bass | Control survival | 21 | 96.0 | 87.2–100.0 |
| Radio survival | 12 | 100.0 | 99.8–100.0 | |
| PIT survival | 19 | 92.1 | 81.5–100.0 | |
| Radio retention | 12 | 93.3 | 80.8–100.0 | |
| PIT retention | 19 | 100.0 | 99.8–100.0 |
4 Discussion
We studied the movement of endemic Bartram's Bass, invasive Alabama Bass, hybrid bass from the headwaters of Eastatoee Creek to its confluence with Keowee Reservoir in northwestern South Carolina, USA. To our knowledge, this is the first study explicitly quantifying the movement of hybrid Micropterus, and represents the first study of any member of the Redeye Bass complex in an unregulated river. Direct observations of captured individuals, radio telemetry detections and associated statistical models indicate that Alabama Bass and hybrid bass move extensively upstream from the reservoir, through the stream's mouth, and into Eastatoee Creek. However, the movement of these individuals appears to have been inhibited by a stretch of high-gradient, swift and shallow habitat (the gorge) because no nonnative alleles were detected upstream of this spatial state, where several hundred pure Bartram's Bass were detected and tagged. Given the persistence of pure Bartram's Bass in upper Eastatoee Creek, the duration since the introduction of Alabama Bass in Keowee Reservoir (nearly three decades), and the prolific movement rate of nonnatives, this section of upland stream habitat may function as a crucial barrier preventing colonisation of Alabama Bass and allowing pure Bartram's Bass to persist while other populations in streams with better connectivity to reservoirs have been extirpated (Cooper et al. 2025).
Spatial position of all species was associated with increasing temperatures from early to late spring, although this estimate is accompanied by some uncertainty. Likewise, radio telemetry showed that Alabama Bass and hybrid bass move considerably more each week than do Bartram's Bass, and that they move more during cooler periods of late spring and early summer. Increasing lake levels had a significant influence on upstream and downstream movement transition probabilities of Alabama Bass and hybrids between the lake and the mouth of Eastatoee Creek. Elevated lake levels likely made the mouth of Eastatoee Creek more accessible and suitable for the nonnative habitat generalist by providing better connectivity through the heavily silted and often very shallow stream mouth.
4.1 Longitudinal Bass Position and Movement
Our results suggest Bartram's Bass in Eastatoee Creek generally did not make large weekly movements during the warm season. However, weekly movement was variable, as was the relationship between temperature and spatial position. Although 69% (124 of 181) of all weekly observations for pure Bartram's Bass demonstrated less than 100 m of movement, 6% (12) of observations showed weekly movements of Bartram's Bass between 0.5 and 1 km, 3 weekly observations were between 1 and 1.5 km, and one observation was over 2.5 km in a week. These larger movements were restricted to the same three individuals. More impressively, these largest upstream movements occurred during periods of higher discharge, in sections with steep gradients and fast-flowing chutes. Similar movements through high-gradient headwater conditions have been recorded in Redeye Bass by Parsons (1954). Bartram's Bass are apparently well adapted to the heterogeneity in flow that many streams and smaller rivers exhibit. More broadly, our results generally track with the common finding of a leptokurtic distribution of organismal movement caused by many individuals that move very little (often termed ‘stayers’), and punctuated by a few individuals that make large movements (‘movers’) (Fraser et al. 2001).
Bartram's Bass movement rates were comparable to those observed for Redeye Bass in the Tallapoosa River (Earley and Sammons 2015), but lower than many other black basses in lotic systems (Goclowski et al. 2013; McClure et al. 2020; Schall et al. 2019; Yeager et al. 2024). Like ours, other studies of fluvial black bass species have also shown no statistical effect of river discharge on movement rates, suggesting that this adaptation may be consistent throughout the Redeye Bass clade (Earley 2012). It is also possible that discharge was not sufficiently variable (aside from one considerable flood event in 2021) across our two study periods to elicit movement events if they indeed were associated with flow. Moreover, difficulty and safety issues associated with manually tracking fish during high water events complicates data collection. However, this only happened once over the course of the study. Further, the limited lifespan of radio tags makes long-term tracking difficult, and telemetry studies focusing on movements through the entire year would provide a more comprehensive understanding of black bass movement dynamics in rivers and their relationships with temperature, flow and other factors.
Spatial position of Bartram's Bass was positively, but weakly, associated with increasing temperature. This, in addition to the fact that most non-stationary radio-tagged Bartram's Bass were found increasingly upstream throughout the summer and into the autumn, suggests that at least some individuals may be moving upstream to access cooler waters throughout summer. However, their upstream movement would be ultimately limited by the natural barrier at rkm 15. Many other stream fishes have shown similar behaviours, making substantial upstream movements to access cooler temperatures during summer months (Dobos et al. 2016; Muhlfeld and Marotz 2005). Moreover, during periods of highest temperatures, multiple Bartram's Bass were detected directly below this barrier, suggesting they were likely as far upstream as they could physically go during that time. Extensive sampling and tracking yielded no captures or detections of Bartram's Bass above this barrier in either 2021 or 2022. As such, Bartram's Bass may be utilising close to all of their optimal thermal habitat in Eastatoee Creek. However, given the variable relationship between river position and temperature, as well as our limited ability to track basses through the fall and winter, we are unable to differentiate between whether summer upstream movements were truly a response to increasing temperatures, or were related to other factors. Future studies with longer tracking periods will be better suited to answering this question, and coupled lab and field studies investigating the thermal tolerances of Bartram's Bass will provide important insight onto the thermal requirements of this species.
Alabama Bass showed the highest overall movement rates in this study, but stayed near Keowee Reservoir in response to increasing temperatures and shallow depths near the mouth of Eastatoee Creek. Alabama Bass movement rates exceeded 4000 m/week in some instances. Their mean movement rate was between those observed for Alabama Bass in the Tallapoosa River, AL and Lake Martin (an impoundment of the Tallapoosa River) (Earley and Sammons 2015), and was lower than those recorded in Hartwell Reservoir (GA/SC; Kerr 2022). Although we tracked Alabama Bass in both lotic and lentic environments, most of the larger movements made by Alabama Bass in this study were in Keowee Reservoir and rarely within Eastatoee Creek. Therefore, it is possible that the larger movement events exhibited by Alabama Bass and hybrid bass were due to the differences in the habitats they occupied. Moreover, our study also differed from those because we were focused mostly on movement within the stream and not the reservoir. Alabama Bass in Eastatoee Creek also shifted downstream to Keowee Reservoir as temperatures increased, rather than staying in the stream or continuing to move upstream. These results suggest Alabama Bass may not be well adapted for smaller, colder Blue Ridge streams. Moreover, the section Alabama Bass inhabited in Eastatoee Creek, rkm 0–3, almost completely dried up as temperatures increased. Dispersal by Alabama Bass to deeper habitat at higher temperatures has also been observed within their native range (Earley and Sammons 2015; Hunter and Maceina 2008).
4.2 Implications for Gene Flow
This study provides insight into the factors influencing the vectors of nonnative gene flow. Our results suggest that introgressed individuals are likely the primary vector of nonnative genes in Eastatoee Creek, as most hybrids were not clear F1 offspring of pure Bartram's Bass and Alabama Bass. Hybrid bass showed high movement rates comparable to Alabama Bass, but also had more distributional overlap with Bartram's Bass in the lower reaches of Eastatoee Creek. Moreover, hybrid bass seemed to express movement patterns that may represent a mixture between Bartram's Bass and Alabama Bass behaviours. For example, Alabama Bass tended to move downstream toward the reservoir with increasing temperature as summers progressed. However, hybrid bass shifted upstream during this time and remained there, more similarly to Bartram's Bass. As such, it is likely that in Eastatoee Creek, upstream movement and successful spawning of hybrid bass with pure Bartram's Bass is responsible for the upstream spread of nonnative alleles. It is also possible that hybrid bass would generally have more success in producing viable offspring with pure Bartram's Bass (Koppelman 2015). Such mechanisms of nonnative gene spreading have been shown in other stream fishes, with hybrid congeners showing increased mobility and fitness relative to pure individuals (Fukui and Koizumi 2020; Kovach et al. 2015).
According to the 2022 multistate model, once nonnative bass moved from the lake to the mouth state, they were more likely to stay within the mouth than to transition upstream to the gorge or back downstream to the lake. Similar trends were present during 2021, but 95% credible intervals overlapped for remaining in the mouth and transitioning downstream to the lake. Increased residence of nonnative bass in upstream sections reveals how nonnative genes may be gradually spreading further into tributaries. Our results also show how gene flow may be influenced by factors that vary on the reach scale. For example, upstream transition probabilities from the mouth into the gorge were low across years and typically < 10% on any given week. In contrast, upstream transition probabilities from the lake to the mouth spanned a much greater range and were frequently > 10% on any given week. Additionally, weekly changes in lake level significantly affected transition probabilities between the lake and mouth, but had no effect on transition probabilities between the mouth and the gorge. This reach-specific variation in movement probability is consistent with other invasion fronts of Micropterus around the world. For example, in South Africa where nonnative Largemouth Bass are a growing concern, studies revealed dispersal and colonisation in new stream sections also varied by reach-specific environmental conditions (Howell et al. 2015). As in our study, individuals that dispersed from their tagging location into low salinity habitat were also more likely to continue to reside in their new locations for long periods (Murray et al. 2015). These patterns indicate that vectors of invasive gene flow may be predictable if associated with certain habitat conditions.
The relationships we observed between nonnative bass movement and temperature indicate that they make more upstream movements during springtime. Such environmental conditions are characteristic of springtime conditions and suggest higher levels of stream activity during this period. Other studies have also reported spring as a higher movement period for Alabama Bass (Earley and Sammons 2015; Hunter and Maceina 2008). Spring is also a high movement period for many other riverine black basses, often associated with upstream spawning movements (Earley and Sammons 2015; Goclowski et al. 2013; VanArnum et al. 2004). Our multistate model showed increased bi-directional movement between Keowee Reservoir and Eastatoee Creek at elevated lake levels and high residency probabilities across all three spatial states, including the gorge state where Bartram's Bass were also detected. Together with the seasonal factors, increased reservoir depth may increase the chances of upstream movement of nonnative individuals. However, it will be important to determine if and how these factors affect the interaction dynamics between Bartram's and Alabama Bass in other geophysically different streams in the nearby Piedmont ecoregion, where instream habitat and connectivity to reservoirs is quite different.
5 Conclusions
Endemic Bartram's Bass are restricted by negative interactions with invasive Alabama Bass at the downstream end of Eastatoee Creek, and by habitat availability at the upstream end. The natural barrier near rkm 6 appears to have limited access of Alabama Bass and hybrid bass to upstream portions of Eastatoee Creek, as no nonnative alleles were detected above this barrier. This indicates that previous detection of nonnative alleles far upstream of this barrier (Peoples et al. 2021) may have been due to human introduction and not natural dispersal by nonnative individuals. Eastatoee Creek is a renowned fishery for heavily stocked nonnative trouts (Salmonidae) and receives heavy fishing pressure, which is a common predictor of nonnative species establishment and richness (Davis and Darling 2017). Additionally, the absence of nonnative alleles upstream of the barrier in the present study is encouraging as it may suggest that the less disturbed conditions found in most of Eastatoee Creek disfavour hybridization, as has been demonstrated for several lotic specialist species (e.g., Muhlfeld et al. 2009), including Bartram's Bass (Judson et al. 2021). Moreover, if summer upstream movement is indeed intended to seek thermal refugia during summer months, it would make Bartram's Bass potentially vulnerable to climate warming because upstream thermal refugia will become increasingly rare. The dual upstream-downstream threat of limited habitat availability and invasive species, respectively, presents a particular challenge for the persistence of Bartram's Bass in a rapidly changing environment.
Although Eastatoee Creek is unique in terms of its physical habitat and connectivity to the reservoir, it can also be representative of the plight of Bartram's Bass throughout the Savannah River Basin, as well as endemic Southeastern black basses more broadly. Outside of its native Mobile River basin, Alabama Bass has widely colonised southeastern US reservoirs of Atlantic Slope river basins (Sammons et al. 2023) and threaten endemic black basses through negative biotic interactions and hybridization. Although Bartram's Bass in Eastatoee Creek appears to be protected from upstream-dispersing Alabama Bass and hybrid bass by a natural barrier and less disturbed habitat, most other populations of southeastern endemic black basses are unlikely to be protected by such distinct barriers. Few natural barriers separate tributaries from large reservoirs in the generally less steep Piedmont ecoregion that encompasses most of these species' distribution. Moreover, much of this region is affected by large amounts of human development, which degrades instream habitat and makes it less suitable for endemic basses and more suitable for nonnatives and Largemouth Bass—a native but tolerant cosmopolitan species. As such, endemic black basses in many of these streams have been completely extirpated. Although intensive, this study represents a small step toward understanding how movement dynamics of an endemic and invasive freshwater fish respond to environmental conditions and establish the context for observed distributions of hybridization. However, much more work is needed over a larger study extent and time period to better understand this increasingly pressing issue.
Author Contributions
T.R.Z. and B.K.P. designed the study. T.R.Z. conducted and oversaw fieldwork. D.J.F. and T.L.D. conducted and oversaw all aspects of the molecular work. T.M.F. and H.J.H. assisted with the Bayesian analyses. M.C.S. and B.K.P. secured funding for this work. T.R.Z. led analysis and manuscript preparation with support from all co-authors.
Acknowledgements
This project was funded by the U.S. Fish and Wildlife Service Competitive State Wildlife Grant program in partnership with the South Carolina Department of Natural Resources (SCDNR), Georgia Department of Natural Resources, University of Georgia and Clemson University. We thank Adam Smallridge, Drew Kanes, Andrew Peel, Eric Stamm, Alex Hall, Kevin Stoner, Preston Finley, Kathryn Lusk, Daniel St. Amand, Andrew Jameson, Colby Denison, Jessie Kriner and Deon Kerr for their endless help with lab and field efforts, as well as other undergraduate students who assisted through Clemson University Creative Inquiry courses. We also would like to thank the Clemson SCDNR staff, including Kevin Kubach, Drew Gelder, Phil Carson and Megan Limehouse, for assistance in the field and for general project guidance, as well as SCDNR staff at Hollings Marine Laboratory for genetic processing of all project fin samples. We also would like to extend huge appreciation to all of the landowners along Eastatoee Creek for your gracious hospitality and access to the stream that made this project possible. This work was performed under protocols presented in Clemson University Institutional Animal Care and Use Committee AUP-2020-028 and AUP-2022-0136. This material is based upon work supported by the NIFA/USDA, under project number SC-1700599, and represents Technical Contribution Number 7436 of the Clemson University Experiment Station, and publication number 901 from the SCDNR Marine Resources Research Institute. Comments from two anonymous reviewers greatly improved earlier drafts of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.