Metabolic predictors of pain, fatigue, depression and quality of life in people with long-term type 1 diabetes—the Dialong study
Abstract
Aim
To examine associations of metabolic parameters (mean 30 years' time-weighted HbA1c and low-density lipoprotein-cholesterol [LDL-c], current methionine sulfoxide [MetSO], advanced glycation end products [AGEs], inflammatory markers and hypoglycaemia) with pain, fatigue, depression and quality of life (QoL) in people with long-term type 1 diabetes.
Methods
A total of 104 persons with type 1 diabetes ≥45 years duration were included. Participants completed questionnaires measuring bodily pain (RAND-36 bodily pain domain with lower scores indicate higher levels of bodily pain), fatigue (Fatigue Questionnaire), depression (Patient Health Questionnaire), overall QoL (World Health Organization Quality of Life—BREF) and diabetes-related QoL (Audit of Diabetes-Dependent Quality of Life). In this observational study, mean time-weighted HbA1c and LDL-c were calculated based on longitudinal measures obtained from medical records of up to 34 years, while current HbA1c, LDL-c and inflammatory markers were analysed in blood samples and collagen MetSO and AGEs in skin biopsies. History of hypoglycaemia was self reported. Associations between metabolic parameters and questionnaire scores were analysed using linear regression analyses and are reported as standardized regression coefficients (beta).
Results
Of the metabolic variables, higher mean time-weighted HbA1c was associated with higher levels of bodily pain and total fatigue (beta [p-value]) −0.3 (<0.001) and 0.2 (0.001).
Conclusions
Long-term chronic hyperglycaemia may have a negative influence on pain and fatigue in people with type 1 diabetes. These results may assist health care workers in emphasizing the importance of strict glycaemic control in people with diabetes and identifying and treating type 1 diabetes-related pain and fatigue.
What's new?
- People with long-term type 1 diabetes report high levels of bodily pain, fatigue, depressive symptoms and reduced quality of life compared to people without diabetes. The potential role of metabolic parameters in these symptoms is scarcely studied.
- This study is the first to show associations of long-term mean time-weighted HbA1c with pain and fatigue in people with long-term type 1 diabetes.
- The findings of this study may assist health care workers in emphasizing the importance of strict glycaemic control to people with diabetes. Further, it is important to identify and treat type 1 diabetes-related pain and fatigue.
1 INTRODUCTION
People with long-term type 1 diabetes of more than 45-years duration report higher levels of bodily pain and fatigue, more depressive symptoms and worse quality of life (QoL) than people without diabetes.1 However, the aetiology of these patient-reported outcomes is not well known. Metabolic parameters such as HbA1c, low-density lipoprotein-cholesterol (LDL-c) and tissue advanced glycation end products (AGEs) are risk factors for vascular complications in type 1 diabetes,2-5 while the effect of low grade inflammation is disputed.6, 7 However, their role in peoples' pain, fatigue, depression and QoL is not well understood.
Prior studies on long-term glycaemic control and its association with pain in long-term type 1 diabetes has been limited to hand, shoulder and neuropathic foot pain.5, 8, 9 However, long-term glycaemic control and its association with total bodily pain have not yet been studied. Further, except for one study with 4 years follow-up of HbA1c,10 there are no studies on chronic hyperglycaemia and its association with fatigue in people with type 1 diabetes of very long duration. AGEs have been associated with pain both in people with and without diabetes,5, 11, 12 and with depressive symptoms in people with type 2 diabetes and without diabetes,13 but no studies have assessed the associations of AGEs with total bodily pain, fatigue, depression or QoL in long-term type 1 diabetes. C-reactive protein (CRP) has been associated with depression in people with type 2 diabetes, but not type 1 diabetes with diabetes duration of 16 years,14 but the role of inflammation with total bodily pain, fatigue, depression and QoL in long-term type 1 diabetes has not yet been studied. In the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) study, poor glycaemic control and incidence of hypoglycaemic events requiring assistance were associated with lower QoL scores, while total cholesterol was not.15
Therefore, we aimed to examine associations of metabolic parameters with pain, fatigue, depression and QoL in people with long-term type 1 diabetes. There are common features, interactions and associations between these outcomes. Hence, metabolic parameters were selected based on reported association with at least one outcome. Access to 14–34 years of HbA1c and LDL-c measurements enabled us to conduct an observational study of long-term exposure to these parameters in people with type 1 diabetes.
2 PARTICIPANTS AND METHODS
This paper is based on the Dialong study, an observational study with long-term exposures conducted in 2015. All persons diagnosed with type 1 diabetes before 1970 (n = 136) who attended the Norwegian Diabetes Centre (NDC) in Oslo, Norway, were invited to participate by post (Figure 1). The Norwegian Regional Committee for Medical and Health Research Ethics South-East approved the study (project no. 2014/851). We obtained written informed consent from each participant.
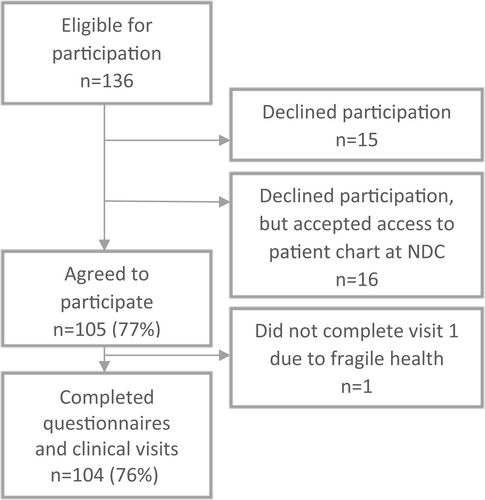
In total, 104 persons (76%) with long-term type 1 diabetes participated in the study. There were no difference in demographic data or presence of vascular complications between those who did not participate, but allowed a chart review (n = 16) and those who agreed to participate (n = 105)16 suggesting that our sample should be representative of those with diabetes invited to participate (Figure 1). At the NDC, the participants' charts were reviewed for information on complication status, co-morbidities and medications. At the first visit, participants answered questionnaires and underwent interviews and clinical examinations. Within 3 weeks, participants attended Oslo University Hospital to provide urine samples (albuminuria), fasting blood tests (eGFR, HbA1c and inflammatory markers), retina photos and questionnaires. Detailed descriptions of the data collection methods, except inflammatory markers, have been published elsewhere.1, 17
3 PATIENT-REPORTED MEASURES
Participants completed Norwegian-translated versions of all instruments, which were scored according to published algorithms.18-23
3.1 Pain
Participants' total bodily pain was measured by the bodily pain domain of the RAND-36 questionnaire, which includes two items: ‘How much bodily pain have you had during the past 4 weeks?’, and ‘During the past 4 weeks, how much did pain interfere with your normal work (including both work outside the home and housework)?’. Higher scores indicate lower levels of bodily pain (range 0–100).22
3.2 Fatigue
Extent and severity of fatigue was assessed by the Fatigue Questionnaire (FQ) where the 11 items are rated from 0 (better than usual) to 3 (much worse than usual). Higher scores represent higher levels of fatigue (range 0–33).19, 20
3.3 Depression
Depressive symptoms were measured by the Patient Health Questionnaire (PHQ-9) based on nine items rated from 0 (not at all) to 3 (nearly every day). Higher scores represent higher symptom levels (range 0–27).21
3.4 Quality of Life
Overall QoL was assessed by the World Health Organization Quality of Life—BREF (WHOQOL-BREF) first overall item: during the previous 2 weeks: ‘How would you rate your quality of life?’, ratings from 1 (very poor) to 5 (very good).23 Diabetes-specific QoL was assessed by the Audit of Diabetes-Dependent Quality of Life (ADDQoL-18), measuring how diabetes affects QoL. Respondents rate how their diabetes (negative to positive, range −3 to +3) affects the 18 diabetes-specific domains and rate the importance (range +3 to 0) of each domain for their QoL. The latter two scores were multiplied for each of the 18 domains generating a weighted score and summing the weighted impact scores for each of the 18 domains and dividing by the number of applicable domains this generate a weighted impact score (range −9 to +9).18 For both instruments, higher scores represent better QoL.
4 METABOLIC PARAMETERS
4.1 Current and mean time-weighted HbA1c and LDL-c
Current HbA1c and LDL-c were obtained from fasting morning blood samples. We collected HbA1/HbA1c (displayed in mmol/mol and %) and LDL-c available in medical records from the early 1980s up until 2015. The mean number of HbA1c and LDL-c measurements available for each participant was 73 and 7. A mean time-weighted (mtw) value of respectively HbA1/HbA1c (displayed in mmol/mol and %) and LDL-c was calculated from the actual measurements available from the last 14–34 years, by calculating the mean HbA1c for each year, further the mean of these values. Both variables and the transformation of HbA1 to HbA1c have been described previously.17, 24
4.2 Current levels of skin collagen methionine sulfoxide (MetSO) and AGEs
For the collagen-linked oxidation product MetSO and the collagen AGEs methylglyoxal-hydroimidazolone (MG-H1) and glucuronidine/LW1, we obtained a 3-mm skin punch biopsy from each participant. MetSO and AGEs were analysed by liquid chromatography–mass spectrometry. Details of these procedures have been published elsewhere.17, 25
4.3 Current levels of inflammatory markers
Fasting blood samples without additives were separated within 1 h by centrifugation at 2500×g for 10 min, and serum was kept frozen at −80°C until analysis. CRP was measured by high sensitive (Hs) ELISA (DRG Instruments GmbH), and interleukin-6 and TNF-α with Quantikine Hs ELISAs (R&D Systems). The inter-assay coefficients of variation were 8.1%, 4.2% and 7.4% respectively.
4.4 Hypoglycaemia and severe hypoglycaemia
For each participant, we recorded the number of self reported episodes of hypoglycaemia (capillary blood glucose <4.0 mmol/L) during the past month and severe hypoglycaemic events, that is, requiring assistance from another person for treatment, during the past year.
5 STATISTICAL ANALYSIS
Data are presented as mean ± standard deviation, median (quartiles), median (min-max) or n (%).
We examined associations of the metabolic parameters and self reported history of hypoglycaemia and severe hypoglycaemia with the outcomes using bivariate and adjusted linear regression analyses. Multivariable analyses were adjusted for three known possible confounders: age, sex and education. To explore if current HbA1c mediated the effect of mtw-HbA1c, we added current HbA1c as an adjustment variable in analyses where both current and mtw-HbA1c were significantly associated with the same outcomes. To further explore mediations, we added complications as an adjustment variable in analyses where there were significant associations after adjustments of age, sex and education. To compare the strength of the associations of different outcomes with different predictors, we report standardized regression coefficients. To examine gender interactions, we stratified by gender, and where an association was significant in one strata but not the other, we added an interaction term with gender and reported the p-value of the interaction term.
A p-value <0.05 was considered statistically significant. Since the study is exploratory, we did not adjust for multiple comparisons. All analyses were performed using STATA/MP 16.1.
6 RESULTS
6.1 Baseline characteristics
Characteristics of the study population includes baseline characteristics, questionnaire scores and metabolic parameters (Table 1). Participants were older (61 ± 7.3 years), male (50%), college educated (62%), living alone (26%) and working (44%). Current HbA1c was 58 ± 8.6 mmol/mol/7.4 ± 0.8%, while mtw-HbA1c was 63 ± 8.3 mmol/mol/7.9 ± 0.8%. All study measures were available for at least 100 respondents.
| N | ||
|---|---|---|
| Sociodemographic | ||
| Age, years | ||
| Mean ± SD | 61 ± 7.3 | 104 |
| Min–max | 48–81 | 104 |
| Sex male, n (%) | 52 (50) | 104 |
| Education college, n (%) | 64 (62) | 104 |
| Living alone, n (%) | 26 (25) | 104 |
| Working, n (%) | 44 (42) | 104 |
| Lifestyle factor | ||
| Currently smoking, n (%) | 5 (4.8) | 104 |
| Diabetes-specific variable | ||
| Diabetes duration, years, median (min–max) | 49 (45–67) | 104 |
| Morbidity | ||
| Blood pressure | ||
| Systolic, mean ± SD | 146 ± 19.6 | 104 |
| Diastolic, mean ± SD | 75 ± 8.2 | 104 |
| Retinopathya | ||
| None, n (%) | 5 (4.8) | 104 |
| Background, n (%) | 54 (52) | 104 |
| Proliferative, n (%) | 45 (43) | 104 |
| Neuropathyb, n (%) | 66 (64) | 104 |
| Persistent albuminuriac, n (%) | 17 (16) | 104 |
| Cardiovascular disease inclusive total obstructive CADd, n (%) | 41 (39) | 104 |
| Questionnaire scores | ||
| RAND-36 Bodily pain domain score [0–100]e, mean ± SD | 66 ± 28.2 | 104 |
| FQ Total fatigue [0–33]e, mean ± SD | 15.2 ± 6.1 | 104 |
| PHQ-9 Depression [0–27]e, mean ± SD | 6.3 ± 4.9 | 104 |
| WHOQOL-BREF Overall quality of life (QoL) [1–5]e, mean ± SD | 3.4 ± 1.1 | 102 |
| ADDQoL-18 Diabetes-specific QoL [−9 to +9]e, median (IQR) | −1.3 (−2.3, 0.7) | 100 |
| Metabolic parameters | ||
| HbA1c, mmol/mol, mean ± SD | 58 ± 8.6 | 102 |
| HbA1c, %, mean ± SD | 7.4 ± 0.8 | 102 |
| HbA1c—mean time-weighted, mmol/mol, mean ± SD | 63 ± 8.3 | 104 |
| HbA1c—mean time-weighted, %, mean ± SD | 7.9 ± 0.8 | 104 |
| LDL-cholesterol, mmol/L, mean ± SD | 2.7 ± 0.8 | 102 |
| LDL-cholesterol—mean time-weighted, mmol/L, mean ± SD | 2.9 ± 0.6 | 104 |
| Skin collagen MetSO, nmol/mg, mean ± SD | 61 ± 8.1 | 101 |
| Skin collagen Advanced Glycation Endv products (AGEs) | ||
| Methylglyoxal-Hydroimidazolone (MG-H1), pmol/mg, mean ± SD | 447 ± 192 | 101 |
| Glucuronidine/LW1, pmol/mg, mean ± SD | 1061 ± 652 | 101 |
| Inflammatory markers | ||
| CRP, mg/L, median (IQR) | 1.55 (0.70–3.15) | 100 |
| Interleukin-6 (IL-6), pg/ml, median (IQR) | 2.65 (1.57–4.15) | 102 |
| TNF-α, pg/ml, mean ± SD | 1.41 ± 0.85 | 102 |
| Hypoglycaemia, number last month, median (IQR) | 12 (5.5–20) | 104 |
| Severe hypoglycaemia last year (requiring assistance), yes, n (%) | 16 (15) | 104 |
| Number of times, median (min–max) | 2 (1–10) | 16 |
| Body mass index (BMI), kg/m2, mean ± SD | 26.1 ± 4.0 | 104 |
- Abbreviations: ADDQoL-18, the Audit of Diabetes-Dependent Quality of Life; CAD, Coronary Artery Disease; CRP, C-reactive protein; FQ, the Fatigue questionnaire; PHQ-9, the Patient Health Questionnaire; TNF-α, Tumour necrosis factor-alpha; WHOQOL-BREF, the World Health Organization Quality of Life-BREF.
- a Retinopathy was categorized as either none, background or proliferative (pan-retinal photocoagulation scars or proliferative findings) retinopathy based on retina photos.
- b Neuropathy was defined based on the presence of both typical symptoms in the lower extremities (numbness, unsteadiness, aching or burning pain or pins and needles) and symmetrical signs in both lower extremities using standard monofilament and vibration tests.
- c Persistent albuminuria was defined as an albumin/creatinine ratio of >2.9 mg/mmol on two consecutive samples.
- d Cardiovascular disease included a diagnosis of TIA/stroke, previous coronary heart disease (acute coronary syndrome, angina pectoris diagnosed by a cardiologist, or previous revascularization procedure), obstructive CAD on computed tomography coronary angiography (CTCA) in the Dialong study, and peripheral vascular disease (claudication intermittent [CI], peripheral angiopathy, peripheral bypass).
- e For RAND-26 bodily pain domain: the higher score, the less influence of bodily pain on quality of life. For FQ, PHQ-9: higher score indicates more depressive symptoms or fatigue. For WHOQOL-BREF, ADDQoL-18: higher score is better.
6.2 Regression of HbA1c an LDL-c with the outcomes
Higher current and mtw-HbA1c values were associated with higher levels of bodily pain (standardized regression coefficient and p-value: −0.3, 0.05 and −0.3, <0.001) (lower bodily pain score indicates higher levels of pain) after adjustments of age, sex and education (Table 2). The association of current HbA1c with pain remained significant after further adjustments for complications (−0.2, p = 0.022). The association of mtw-HbA1c with pain remained significant after further adjustment for current HbA1c
| Adjusted age, sex, education | Adjusted age, sex, education, current HbA1c | Adjusted age, sex, education, complicationsa | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Regression coefficient B (95% CI) | Standardized regression coefficient | p-value | Regression coefficient B (95% CI) | Standardized regression coefficient | p-value | Regression coefficient B (95% CI) | Standardized regression coefficient | p-value | |
| Pain (RAND-36 bodily pain domain) | |||||||||
| Current HbA1c, mmol/mol | −0.8 (−1.4, −0.3) | −0.3 | 0.005 | −0.7 (−1.3, −0.1) | −0.2 | 0.022 | |||
| % | −9.2 (−15.5, −2.9) | −7.9 (−14.7, −1.2) | |||||||
| mtw-HbA1c, mmol/mol | −1.1 (−1.8, −0.5) | −0.3 | <0.001 | −0.9 (−1.7, −0.01) | −0.3 | 0.048 | −1.04 (−1.7, −0.4) | −0.3 | 0.003 |
| % | −12.5 (−19.2, −5.8) | −9.5 (−19.0, −0.1) | −11.3 (−18.7, −3.97) | ||||||
| Current LDL-cholesterol, mmol/L | −0.7 (−7.1, 5.6) | −0.02 | 0.821 | ||||||
| mtw LDL-cholesterol, mmol/L | −1.9 (−10.03, 6.3) | −0.04 | 0.654 | ||||||
| Fatigue, total (FQ) | |||||||||
| Current HbA1c, mmol/mol | 0.1 (−0.02, 0.2) | 0.2 | 0.091 | ||||||
| % | 1.2 (−0.2, 2.7) | ||||||||
| mtw-HbA1c, mmol/mol | 0.2 (0.04, 0.3) | 0.2 | 0.011 | 0.2 (0.02, 0.3) | 0.2 | 0.024 | |||
| % | 1.98 (0.5, 3.5) | 1.97 (0.3, 3.7) | |||||||
| Current LDL-cholesterol, mmol/L | −1.1 (−2.5, 0.2) | −0.2 | 0.106 | ||||||
| mtw LDL-cholesterol, mmol/L | −1.2 (−3.0, 0.6) | −0.1 | 0.179 | ||||||
| Depression (PHQ-9) | |||||||||
| Current HbA1c, mmol/mol | 0.1 (−0.1, 0.2) | 0.1 | 0.293 | ||||||
| % | 0.6 (−0.6, 1.8) | ||||||||
| mtw-HbA1c, mmol/mol | 0.1 (−0.04, 0.2) | 0.1 | 0.222 | ||||||
| % | 0.8 (−0.5, 2.1) | ||||||||
| Current LDL-cholesterol, mmol/L | −0.8 (−2.0, 0.3) | −0.1 | 0.160 | ||||||
| mtw LDL-cholesterol, mmol/L | −0.8 (−2.2, 0.7) | −0.1 | 0.312 | ||||||
| Overall QoL (WHOQOL-BREF) | |||||||||
| Current HbA1c, mmol/mol | −0.003, (−0.03, 0.02) | −0.02 | 0.816 | ||||||
| % | −0.03 (−0.3, 0.2) | ||||||||
| mtw-HbA1c, mmol/mol | −0.01 (−0.04, 0.01) | −0.1 | 0.282 | ||||||
| % | −0.2 (−0.4, 0.1) | ||||||||
| Current LDL-cholesterol, mmol/L | 0.2 (−0.1, 0.4) | 0.1 | 0.237 | ||||||
| mtw LDL-cholesterol, mmol/L | 0.2 (−0.2, 0.5) | 0.1 | 0.289 | ||||||
| Diabetes-specific QoL (ADDQoL-18) | |||||||||
| Current HbA1c, mmol/mol | −0.01 (−0.04, 0.02) | −0.1 | 0.518 | ||||||
| % | −0.1 (−0.4, 0.2) | ||||||||
| mtw-HbA1c, mmol/mol | −0.04 (−0.07, 0.001) | −0.2 | 0.056 | ||||||
| % | −0.4 (−0.8, 0.01) | ||||||||
| Current LDL-cholesterol, mmol/L | 0.1 (−0.3, 0.4) | 0.04 | 0.729 | ||||||
| mtw LDL-cholesterol, mmol/L | 0.1 (−0.3, 0.6) | 0.1 | 0.546 | ||||||
- Note: Respondents with missing values on any of the explanatory variables were excluded from the regression analyses, giving a sample size ranging from 99 to 104. For RAND-36 bodily pain domain: the higher score, the less influence of bodily pain on QoL. For FQ, PHQ-9: higher score indicates more depressive symptoms or fatigue. For WHOQOL-BREF, ADDQoL-18: higher score is better. Abbreviations: ADDQoL-18, the Audit of Diabetes-Dependent Quality of Life; FQ, the Fatigue questionnaire; mtw, mean time-weighted; PHQ-9, the Patient Health Questionnaire; WHOQOL-BREF, the World Health Organization Quality of Life-BREF.
- a Complications included a diagnoses of retinopathy, neuropathy, nephropathy or cardiovascular disease (TIA/stroke, previous coronary heart disease [acute coronary syndrome], angina pectoris diagnosed by a cardiologist, or previous revascularization procedure), obstructive coronary artery disease on computed tomography coronary angiography (CTCA) in the Dialong study and peripheral vascular disease (claudication intermittent [CI], peripheral angiopathy, peripheral bypass).
(−0.3, 0.048), and after further adjustments for complications (−0.3, 0.003). Higher mtw-HbA1c was associated with higher total fatigue (0.2, 0.011) after adjustment of age, sex and education, and after further adjustments for complications (0.2, 0.024). Current HbA1c was not associated with fatigue after adjustments. Neither overall QoL nor diabetes-specific QoL was associated significantly with HbA1c. Nor were current or mtw LDL-c associated with pain, fatigue, depression or QoL.
6.3 Other metabolic parameters
In unadjusted, but not adjusted analyses, higher levels of skin collagen AGE MG-H1 were associated with lower levels of fatigue (−0.2, 0.048) and depression (−0.3, 0.007), and higher levels of glucuronidine/LW1 was associated with lower levels of depression (Table 3). Lastly, increased number of hypoglycaemic events was associated with higher levels of depression (0.2, 0.011) in unadjusted, but not adjusted analyses. When stratified by gender, increased hypoglycaemic events were associated with depression in men (unstandardized regression coefficient 0.1 [0.03, 0.21, p = 0.007]), but not in women, after adjustments. There was no significant interaction between gender and depression (p = 0.259). Moreover, also stratified by gender, the association of severe hypoglycaemia with pain (men and women) and fatigue (men), were clinically relevant as the size of the regression coefficients were more than 10% of the average total questionnaire scores after adjustments, however, not statistically significant, neither were the interaction terms. No inflammatory variables were significantly associated with the outcomes, either unadjusted or adjusted.
| Unadjusted | Adjusted age, sex, education | |||||
|---|---|---|---|---|---|---|
| Regression coefficient B (95% CI) | Standardized regression coefficient | p-value | Regression coefficient B (95% CI) | Standardized regression coefficient | p-value | |
| Pain (RAND-36 bodily pain domain) | ||||||
| Skin collagen MetSO | 0.2 (−0.5, 0.9) | 0.1 | 0.613 | −0.1 (−0.7, 0.6) | −0.02 | 0.824 |
| Skin collagen AGEs | ||||||
| MG-H1 | 0.01 (−0.02, 0.04) | 0.1 | 0.446 | −0.01 (−0.04, 0.02) | −0.1 | 0.555 |
| Glucuronidine/LW1 | 0.002 (−0.01, 0.01) | 0.1 | 0.579 | −0.001 (−0.01, 0.01) | −0.02 | 0.810 |
| Inflammatory markers | ||||||
| CRP below 15 mg/L | −1.7 (−3.8, 0.5) | −0.2 | 0.123 | −1.1 (−3.1, 1.003) | −0.1 | 0.312 |
| IL-6 | −1.2 (−2.9, 0.5) | −0.1 | 0.163 | −1.2 (−2.8, 0.5) | −0.1 | 0.158 |
| TNF-α | −1.8 (−8.3, 4.7) | −0.1 | 0.577 | −1.8 (−8.0, 4.5) | −0.1 | 0.573 |
| Hypoglycaemia, number last month | −0.04 (−0.4, 0.4) | −0.02 | 0.862 | 0.1 (−0.3, 0.5) | 0.04 | 0.662 |
| Severe hypoglycaemia last year (requiring assistance), yes | −4.97 (−20.2, 10.3) | −0.1 | 0.520 | −2.3 (−16.5, 11.9) | −0.03 | 0.750 |
| Fatigue, total (FQ) | ||||||
| Skin collagen MetSO | −0.1 (−0.2, 0.1) | −0.1 | 0.253 | −0.04 (−0.2, 0.1) | −0.1 | 0.564 |
| Skin collagen AGEs | ||||||
| MG-H1 | −0.01 (−0.01, −0.000048) | −0.2 | 0.048 | −0.002 (−0.01, 0.005) | −0.1 | 0.577 |
| Glucuronidine/LW1 | −0.001 (−0.003, 0.001) | −0.1 | 0.196 | −0.001 (−0.002, 0.001) | −0.1 | 0.517 |
| Inflammatory markers | ||||||
| CRP below 15 mg/L | 0.2 (−0.2, 0.7) | 0.1 | 0.345 | 0.1 (−0.3, 0.6) | 0.1 | 0.594 |
| IL-6 | 0.1 (−0.3, 0.5) | 0.1 | 0.510 | 0.1 (−0.2, 0.5) | 0.1 | 0.495 |
| TNF-α | 0.2 (−1.2, 1.6) | 0.02 | 0.811 | 0.2 (−1.2, 1.6) | 0.03 | 0.772 |
| Hypoglycaemia, number last month | 0.02 (−0.1, 0.1) | 0.04 | 0.715 | −0.02 (−0.1, 0.1) | −0.04 | 0.691 |
| Severe hypoglycaemia last year (requiring assistance), yes | 2.9 (−0.4, 6.1) | 0.2 | 0.080 | 2.1 (−1.0, 5.2) | 0.1 | 0.189 |
| Depression (PHQ-9) | ||||||
| Skin collagen MetSO | −0.1 (−0.2, 0.04) | −0.1 | 0.197 | −0.03 (−0.2, 0.1) | −0.1 | 0.577 |
| Skin collagen AGEs | ||||||
| MG-H1 | −0.01 (−0.01, −0.002) | −0.3 | 0.007 | 0.004 (−0.01, 0.001) | −0.1 | 0.151 |
| Glucuronidine/LW1 | −0.002 (−0.003, −0.0002) | −0.2 | 0.027 | −0.001 (−0.003, 0.0003) | −0.1 | 0.128 |
| Inflammatory markers | ||||||
| CRP below 15 mg/L | 0.1 (−0.3, 0.4) | 0.03 | 0.771 | −0.03 (−0.4, 0.4) | −0.02 | 0.877 |
| IL-6 | −0.1 (−0.4, 0.2) | −0.1 | 0.435 | −0.1 (−0.4, 0.2) | −0.1 | 0.469 |
| TNF-α | −0.3 (−1.4, 0.9) | −0.05 | 0.630 | −0.1 (−1.3, 1.0) | −0.02 | 0.803 |
| Hypoglycaemia, number last month | 0.1 (0.02, 0.2) | 0.2 | 0.011 | 0.1 (−0.005, 0.1) | 0.2 | 0.068 |
| Severe hypoglycaemia last year (requiring assistance), yes | 1.2 (−1.5, 3.8) | 0.1 | 0.385 | 0.7 (−1.8, 3.3) | 0.1 | 0.579 |
| Overall QoL (WHOQOL-BREF) | ||||||
| Skin collagen MetSO | 0.02 (−0.01, 0.04) | 0.1 | 0.154 | 0.02 (−0.01, 0.04) | 0.1 | 0.194 |
| Skin collagen AGEs | ||||||
| MG-H1 | 0.00049 (−0.001, 0.002) | 0.1 | 0.380 | 0.0002 (−0.001, 0.001) | 0.03 | 0.759 |
| Glucuronidine/LW1 | 0.00002 (−0.0003, 0.0003) | 0.01 | 0.887 | −0.00001 (−0.0003, 0.0003) | −0.01 | 0.950 |
| Inflammatory markers | ||||||
| CRP below 15 mg/L | 0.01 (−0.1, 0.1) | 0.02 | 0.822 | 0.02 (−0.1, 0.1) | 0.04 | 0.677 |
| IL-6 | −0.1 (−0.1, 0.001) | −0.2 | 0.055 | −0.1 (−0.1, 0.004) | −0.2 | 0.067 |
| TNF-α | −0.2 (−0.4, 0.1) | −0.1 | 0.143 | −0.2 (−0.4, 0.1) | −0.1 | 0.187 |
| Hypoglycaemia, number last month | −0.01 (−0.02, 0.01) | −0.1 | 0.513 | −0.01 (−0.02, 0.01) | −0.1 | 0.536 |
| Severe hypoglycaemia last year (requiring assistance), yes | −0.1 (−07, 0.5) | −0.03 | 0.787 | 0.04 (−0.6, 0.6) | 0.01 | 0.892 |
| Diabetes-specific QoL (ADDQoL-18) | ||||||
| Skin collagen MetSO | 0.001 (−0.03, 0.03) | 0.01 | 0.944 | −0.001 (−0.04, 0.03) | −0.004 | 0.969 |
| Skin collagen AGEs | ||||||
| MG-H1 | −0.0004 (−0.002, 0.001) | −0.1 | 0.585 | −0.001 (−0.002, 0.001) | −0.1 | 0.477 |
| Glucuronidine/LW1 | 0.0001 (−0.0003, 0.001) | 0.1 | 0.568 | 0.0001 (−0.0003, 0.001) | 0.1 | 0.637 |
| Inflammatory markers | ||||||
| CRP below 15 mg/L | −0.02 (−0.1, 0.1) | −0.04 | 0.734 | −0.02 (−0.1, 0.1) | −0.03 | 0.786 |
| IL-6 | −0.1 (−0.1, 0.03) | −0.1 | 0.191 | −0.1 (−0.1, 0.03) | −0.1 | 0.165 |
| TNF-α | −0.1 (−0.4, 0.2) | −0.1 | 0.543 | −0.1 (−0.4, 0.2) | −0.1 | 0.443 |
| Hypoglycaemia, number last month | −0.01 (−0.03, 0.01) | −0.1 | 0.495 | −0.01 (−0.03, 0.02) | −0.1 | 0.643 |
| Severe hypoglycaemia last year (requiring assistance), yes | 0.5 (−0.3, 1.3) | 0.1 | 0.254 | 0.6 (−0.3, 1.4) | 0.1 | 0.177 |
- Note: Respondents with missing values on any of the explanatory variables were excluded from the regression analyses, giving a sample size ranging from 95 to 104. For RAND-36 bodily pain domain: higher the score, the less influence of bodily pain on QoL. For FQ, PHQ-9: higher score indicates more depressive symptoms or fatigue. For WHOQOL-BREF, ADDQoL-18: higher score is better. Abbreviations: ADDQoL-18, the Audit of Diabetes-Dependent Quality of Life; AGEs—Advanced Glycation End products; MG-H1—Methylglyoxal-hydroimidazolone; CRP—C-reactive protein; IL-6—Interleukin-6; FQ, the Fatigue questionnaire; PHQ-9, the Patient Health Questionnaire; TNF-α—Tumor necrosis factor-alpha; WHOQOL-BREF, the World Health Organization Quality of Life-BREF.
7 DISCUSSION
This is the first study to explore associations of mean time-weighted HbA1c and LDL-c over the past 14–34 years with bodily pain, fatigue, depression and QoL in people with long-term type 1 diabetes. Our main findings were that mtw-HbA1c was associated with higher levels of pain and fatigue in analyses adjusted for age, sex and education.
Long-term mtw-HbA1c over approximately 30 years is associated with total pain burden in long-term type 1 diabetes. The unstandardized regression coefficients did not change markedly after further adjustment for current HbA1c or complications, indicating that the observed association was mostly independent of current HbA1c, or complications. The association between higher mtw-HbA1c and more total bodily pain is concordant with the association between mean HbA1c over 27 years and neuropathic heat pain thresholds in the feet of people with type 1 diabetes duration of 40 years,5 as well as cross sectional associations between HbA1c and musculoskeletal complications often causing pain.26, 27 Landmark studies have shown that increased HbA1c is a major risk factor for several vascular complications in diabetes4 and as HbA1c is associated with pain, such complications could have mediated our results. However, the lack of mediation is concordant with the previously reported association between mtw-HbA1c and shoulder and hand pain.8, 9 Moreover, in the Joslin 50-year Medalist Study, current and long-term (15 years) glycaemic control were unrelated to vascular complications.28 As the proportion of people with long-term type 1 diabetes increases, there is a need to investigate other long-term sequelae and co-morbidities. Bodily pain, chronic fatigue and musculoskeletal complications of shoulders and hands are important because they may cause difficulties performing routine daily activities and thus QoL.
In this study, higher mtw-HbA1c was associated with more fatigue. A prospective study found a weak association between a single follow-up HbA1c after 43 months and fatigue versus non-severe fatigue in people with type 1 diabetes.10 In a cross sectional study, current HbA1c was not associated with chronic fatigue.29 Comparing results between these studies is difficult due to different study designs and study limitations. It is likely that the glycaemic burden during half a lifetime reflects a mechanism associated with fatigue other than glycaemic control over a shorter time period. However, the association was not large enough to be clinically relevant.
We found no associations between LDL-c and the outcomes. Yet 54% of the participants, most likely individuals considered having a high risk for cardiovascular disease were on cholesterol-lowering medications (statins). However, there were no associations between the outcomes and either current or mtw LDL-c among the 46% not taking statins.
Levels of inflammatory variables measured were low, which may explain the lack of associations with any outcomes. This may be supported by longitudinal results in people aged 41 years with type 1 and type 2 diabetes duration of 16 and 13 years, where IL-6 was even lower than what we report, while CRP in the type 1 diabetes group was similar, and no association with depressive symptoms was reported. Interestingly, in the type 2 diabetes group where CRP was higher, reduction in CRP was associated with reduction in depressive symptoms.14 The lack of association may be explained by the small sample size in our study.
No AGEs measured in skin collagen were associated with pain, in contrast to other studies.5, 11, 12 This might be explained by the small sample size. Contrary to our findings, a previous study found an association of AGEs measured by skin autofluorescence with depressive symptoms in people with type 2 or without diabetes with mean age 60 years.13 This might be explained by the different measurement methods used; skin autofluorescence measurements of AGEs versus skin collagen AGEs in our study. To our knowledge, no other studies have described associations of AGEs with fatigue and depression in people with diabetes.
Neither number of hypoglycaemic events nor severe hypoglycaemia were associated with any of the outcomes in adjusted analyses. However, number of hypoglycaemic events were associated with depression in men. We were not able to find other studies reporting similar gender-stratified data. Contrary to our findings, a previous study found a bidirectional association of severe hypoglycaemia resulting in emergency room care with depression in people with type 1 diabetes, mean age of 56 years, with diabetes of unknown duration.30 We defined severe hypoglycaemia as an event requiring assistance, but not necessarily requiring emergency room care. Thus, our self reported hypoglycaemic events may have been less severe and may account for the lack of association in our study. Another explanation might be type 2 error as only 16 of our participants reported severe hypoglycaemia, while Gilsanz et al.30 found severe hypoglycaemia in the medical records of 641 of 3742 persons with type 1 diabetes.
We found no significant association of any of the metabolic predictors with overall and diabetes-specific QoL. For LDL-c, the lack of association with QoL corresponds to the lack of association between total cholesterol and QoL in the DCCT/EDIC study.15 For mtw-HbA1c and severe hypoglycaemia, our findings did not correspond to the increased risk observed in the DCCT/EDIC, possibly due to the low statistical power in the present study. But the lack of correspondence between our results and the DCCT/EDIC also may be explained by differences in study design. For example, the DCCT was a randomized controlled trial comparing strict versus non-strict glycaemic control, in order to investigate whether this was associated with incidence of diabetes complications, whereas all respondents in our study presumably were encouraged to control their blood glucose to avoid diabetes complications.
Strengths of this study include the phenotypically well characterized population with long-term type 1 diabetes of more than 45 years, with a high response rate on the PROM questionnaires. Moreover, the average HbA1c in our diabetes group was comparable to that of the Norwegian population with type 1 diabetes for more than 45 years.16 By using all available HbA1c and LDL-c measurements from the last 14–34 years as a mean time-weighted value, the problem of participants with a missing value was avoided. Nevertheless, it is a limitation as one single measure of mtw-HbA1c cannot distinguish individuals with different trajectories over time. However, since most participants had a slightly decreasing trend in HbA1c over time resulting in very little variation in longitudinal trajectories, trajectory analyses would not be meaningful. Moreover, some statistically significant regression coefficients may not be clinically relevant as they were small in absolute size. Finally, this study is limited by the cross sectional nature of all concurrent measures, preventing assessment of causal relationships.
8 CONCLUSION
This is the first-time significant associations of higher mean time-weighted HbA1c values during the past 30 years with higher levels of bodily pain and fatigue are reported in people with type 1 diabetes of more than 45 years duration. Long-term chronic hyperglycaemia may have a negative influence on the burden of pain and fatigue. Thus, improving suboptimal long-term blood glucose control may be important for preventing bodily pain and fatigue in type 1 diabetes. Our results highlight the importance of health care workers' emphasizing strict glycaemic control to people with diabetes and identifying and treating type 1 diabetes-related pain and fatigue.
AUTHOR CONTRIBUTIONS
Tore Julsrud Berg, Anne Karin Molvær, Marjolein M. Iversen, Jannicke Igland, Mark Peyrot and Grethe S. Tell made substantial contributions to conception and design, Tore Julsrud Berg, Anne Karin Molvær and Kristine Bech Holte, were responsible for the data collection. Tore Julsrud Berg, Anne Karin Molvær, Marjolein M. Iversen, Jannicke Igland, Mark Peyrot, Grethe S. Tell, Vincent M. Monnier and Ingebjørg Seljeflot made substantial contributions to analysis and interpretation of data. Tore Julsrud Berg, Anne Karin Molvær, Marjolein M. Iversen, Jannicke Igland, Mark Peyrot and Grethe S. Tell made substantial contributions in drafting the manuscript. All authors (Tore Julsrud Berg, Anne Karin Molvær, Marjolein M. Iversen, Jannicke Igland, Mark Peyrot, Grethe S. Tell, Kristine Bech Holte, Vincent M. Monnier and Ingebjørg Seljeflot) were involved in revising the manuscript critically for important intellectual content, reviewed and approved the manuscript. The manuscript submission is approved by all authors. Tore Julsrud Berg, Anne Karin Molvær and Marjolein M. Iversen applied for funding. Tore Julsrud Berg is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
ACKNOWLEDGEMENTS
We thank the participants in the study and the staff at the Norwegian Diabetes Centre and Centre for Clinical Heart Research, Oslo University Hospital Ullevål, Oslo, Norway.
FUNDING INFORMATION
This work was supported by The Western Norway Regional Health Authority and the Norwegian Diabetes Centre research fund and Stein Erik Hagen's Foundation for clinical research, Oslo Norway. The funders were not involved in designing the study, data collection, data analyses, preparation of the manuscript and/or publication decisions.
CONFLICT OF INTEREST
None declared.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author.




