Postpartum uptake of diabetes screening tests in women with gestational diabetes: The PANDORA study
Abstract
Aims
To determine rates and predictors of postpartum diabetes screening among Aboriginal and/or Torres Strait Islander and non-Indigenous women with gestational diabetes mellitus (GDM).
Methods
PANDORA is a prospective longitudinal cohort of women recruited in pregnancy. Postpartum diabetes screening rates at 12 weeks (75-g oral glucose tolerance test (OGTT)) and 6, 12 and 18 months (OGTT, glycated haemoglobin [HbA1C] or fasting plasma glucose) were assessed for women with GDM (n = 712). Associations between antenatal factors and screening with any test (OGTT, HbA1C, fasting plasma glucose) by 6 months postpartum were examined using Cox proportional hazards regression.
Results
Postpartum screening rates with an OGTT by 12 weeks and 6 months postpartum were lower among Aboriginal and/or Torres Strait Islander women than non-Indigenous women (18% vs. 30% at 12 weeks, and 23% vs. 37% at 6 months, p < 0.001). Aboriginal and/or Torres Strait Islander women were more likely to have completed a 6-month HbA1C compared to non-Indigenous women (16% vs. 2%, p < 0.001). Screening by 6 months postpartum with any test was 41% for Aboriginal and/or Torres Strait Islander women and 45% for non-Indigenous women (p = 0.304). Characteristics associated with higher screening rates with any test by 6 months postpartum included, insulin use in pregnancy, first pregnancy, not smoking and lower BMI.
Conclusions
Given very high rates of type 2 diabetes among Aboriginal and Torres Strait Islander women, early postpartum screening with the most feasible test should be prioritised to detect prediabetes and diabetes for intervention.
What Is Already Known?
- Women with gestational diabetes mellitus (GDM) are at risk of developing type 2 diabetes after pregnancy, with First Nations women at particularly high risk.
- Screening for postpartum diabetes is low, with very few studies involving First Nations participants.
What this Study Has Found?
- Aboriginal and/or Torres Strait Islander women with GDM had similar rates of screening as non-Indigenous women when screening included a 75-g oral glucose tolerance test (OGTT), HbA1C, or fasting plasma glucose.
What Are the Implications of the Study?
- A pragmatic approach, whereby as many women as possible are targeted for HbA1C testing after GDM, may be preferable to performing a postpartum OGTT.
1 INTRODUCTION
First Nations women experience high rates of pre-existing type 2 diabetes in pregnancy and gestational diabetes mellitus (GDM)1 and are at high risk of developing type 2 diabetes after GDM.2 Many women do not present for pre-conception care,3 and the postpartum period therefore presents a unique opportunity to screen and optimally manage prediabetes, type 2 diabetes and other cardiovascular risk factors, with benefits for women and their future pregnancies.
Best practice guidelines recommend a 6–12-week postpartum 75-g oral glucose tolerance test (OGTT) to identify those at highest risk for type 2 diabetes and cardiovascular conditions for pre-pregnancy planning and lifestyle modification.4 However, uptake of postpartum OGTTs has been universally low, ranging from 19 to 61% in various studies around the world.5, 6
Most data on uptake of, and associations with, postpartum diabetes screening come from retrospective databases with few studies involving First Nations populations who experience high rates of type 2 diabetes and cardiovascular disease.7 In this context, the Pregnancy and Neonatal Diabetes Outcomes in Remote Australia (PANDORA) study, with a high proportion of Australian First Nations participants, was established. This is a prospective study of women with and without hyperglycaemia in pregnancy across the Northern Territory (NT), Australia. Aboriginal and/or Torres Strait Islander people is the preferred collective term nationally for Australia's First Nations Peoples.8 This study aimed to report rates of postpartum diabetes screening with an OGTT within 12 weeks postpartum and rates of any screening (OGTT, HbA1C or fasting plasma glucose) within 6, 12 and 18 months postpartum among women in the PANDORA cohort. The secondary aim was to determine antenatal factors associated with 6 months postpartum screening, to identify whether certain groups should be prioritised for improved screening. The 6-month time frame was chosen because it is important to detect type 2 diabetes before the next pregnancy and by 12 months postpartum several women may be pregnancy planning or pregnant again.
2 METHODS
2.1 Participants
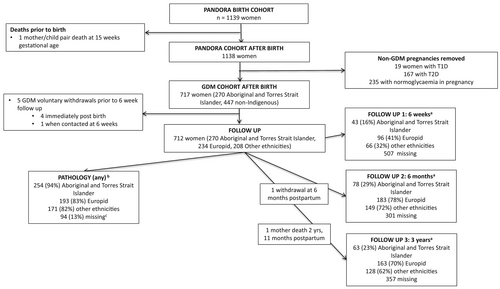
Details of the PANDORA study have been previously published.9 Participants were Aboriginal and/or Torres Strait Islander and non-Indigenous women (n = 1139) with (i) hyperglycaemia in pregnancy (n = 904: pre-existing type 1, type 2 diabetes, GDM) recruited from the NT Diabetes in Pregnancy Register during pregnancy and (ii) normoglycaemia recruited from antenatal clinics (n = 235). As the focus in this analysis was on postpartum screening among women with GDM, 717 women with GDM were included (including 96 women meeting glucose or HbA1C values diagnostic of type 2 diabetes outside of pregnancy, termed ‘diabetes in pregnancy’ (DIP)).10 After pregnancy, women were contacted at 6 weeks, 6 months and 3 years postpartum (information collected from the 3-year questionnaire relating to the time frame 0–18 months postpartum was included). Five women withdrew from the study prior to first follow-up, resulting in 712 women included for most analyses. Of these 712 women, 262 (37%) identified as Aboriginal, 2 as Torres Strait Islander (0.3%), 6 as Aboriginal and Torres Strait Islander (0.7%) and 442 (62%) as non-Indigenous. Among non-Indigenous women, 234 (53%) identified as Europid (European ancestry) and 208 (47%) as other ethnicities (40% Indian subcontinent, 19% Filipino, 41% other ethnicities). One woman withdrew at 6 months postpartum and was not included in 6-, 12- and 18-month analyses (Figure 1). The study was approved by the Human Research Ethics Committee of the NT Department of Health and Menzies School of Health Research, and the Central Australian Human Research Ethics Committee. All aspects of this study were guided and supported by an Aboriginal and Torres Strait Islander Advisory Group, including Aboriginal and Torres Strait Islander women across the NT and represented here by SC.
2.2 Data measurements
During pregnancy, data were obtained from medical records and questionnaires. The GDM screening and diagnostic guidelines changed during the study. Women with GDM were diagnosed by either the 1998 Australasian Diabetes in Pregnancy Society (ADIPS)11 guidelines or a universal 75 g OGTT and revised glucose cut-points as recommended by the International Association of the Diabetes and Pregnancy Study Groups (IADPSG)12 and the WHO.10 DIP was defined as a fasting plasma glucose ≥7.0 mmol/L, and/or 2-h plasma glucose ≥11.1 mmol/L, or HbA1C ≥ 48 mmol/mol (6.5%)10 and women meeting these diagnostic criteria were included in the GDM group.
Postpartum information on diabetes screening and detail of subsequent pregnancies were collected via three approaches. (1) Directly from two large pathology companies, with a third minor company not accessed due to feasibility, (2) extracted from primary health records and hospital medical records (in the NT, most women have postpartum testing in primary care, and hospital medical records were checked for an additional level of completeness) and (3) women were contacted by telephone or emailed a questionnaire at 6 weeks, 6 months and 3 years postpartum.
If self-reported data could not be verified with pathology, these women were included in the not-screened group. Only primary care records for women known to Aboriginal medical services were accessed, including all remote dwelling women (71% of Aboriginal and/or Torres Strait Islander women) and most urban dwelling Aboriginal and/or Torres Strait Islander women. Primary care in remote areas send all pathology specimens to one of the two major pathology companies accessed for data collection. Medical records for all four public hospitals and the only private hospital in the NT were accessed. The most recent complete collection of pathology results was at 18 months postpartum; hence this time frame was used as the cut-off for postpartum screening.
2.3 Outcome definitions
The outcomes were (i) rates of screening with an OGTT within 12 weeks postpartum and (ii) cumulative rates of any postpartum screening (OGTT, HbA1C, fasting plasma glucose) within 6, 12 and 18 months postpartum. Only the first screening test performed postpartum was included for analysis.
2.4 Statistical analysis
Statistical analysis was conducted using Stata v15 (Stata Corporation). Differences in rates of those who did and did not have postpartum screening by ethnicity were determined using Pearson's chi-square tests. Differences in maternal characteristics between those who did and did not complete any screening (OGTT, HbA1C, fasting blood glucose) within 6 months postpartum were determined using Pearson's chi-square tests for categorical variables and Student's t-test or Wilcoxon rank sum tests as appropriate for continuous variables. Results are presented separated by ethnicity due to differences in clinical characteristics between the two cohorts, with Aboriginal and/or Torres Strait Islander women known to experience socio-economic disadvantage and a higher burden of metabolic risk.13 BMI in pregnancy was presented as means adjusted for gestational age using linear regression.
Univariable and multivariable Cox proportional hazards regression models were used to estimate crude and adjusted incidence rate ratio (IRR) and corresponding 95% confidence interval (CI) of completion of any screening (OGTT, HbA1C, fasting plasma glucose) within 6 months postpartum for each maternal characteristic. Interactions between ethnicity and all other variables were assessed using interaction terms in Cox regression models. Variables selected for the multivariable regression model were those described in the literature to be associated with postpartum screening for diabetes.14, 15 Insulin use in pregnancy was selected, being the marker of higher risk for diabetes as the most clinically relevant marker. As education and employment were correlated, the effect of these variables was assessed in separate multivariable models. An alternate model was explored that selected variables for the multivariable regression with a p value ≤0.2 on univariable analysis.
3 RESULTS
3.1 General characteristics of women with GDM
Characteristics of this cohort have been previously reported16; compared to non-Indigenous women, Aboriginal and/or Torres Strait Islander women were younger (29.4 vs. 31.7 years, p < 0.001) and more likely to have smoked during pregnancy (46 vs. 11%, p < 0.001), live in a remote area (71 vs. 6%, p < 0.001), and less likely to have completed year 12 or equivalent schooling (11 vs. 53%, p < 0.001). In terms of markers of severity of hyperglycaemia in pregnancy, Aboriginal and/or Torres Strait Islander women were also more likely to have an early diagnosis of GDM (less than 20 weeks) (26 vs. 12%, p < 0.001), a higher fasting plasma glucose in pregnancy (5.1 vs. 4.8 mmol/L, p < 0.001) and meet criteria for DIP (23 vs. 8%, p < 0.001) compared to non-Indigenous women. There was no difference in rates of insulin use in pregnancy between ethnicities (36 vs. 39%, p = 0.317).
3.2 Postpartum data collection
Two hundred and seventy-nine women self-reported postpartum glucose testing (not related to a subsequent pregnancy) which could not be verified with pathology company data for 24 women (2 Aboriginal, 8 Europid and 14 other ethnicity women) who were included in the not-screened group. Aboriginal and/or Torres Strait Islander women (42%, n = 113) were less likely to have completed questionnaires than non-Indigenous women (91%, n = 403) (Figure 1). Aboriginal and/or Torres Strait Islander women (94%, n = 254) were more likely to have postpartum pathology data (any form of blood tests) than non-Indigenous women (84%, n = 372) (Figure 1).
3.3 Postpartum diabetes screening among women with GDM
Stratified by ethnicity: 18% (48/270) of Aboriginal and/or Torres Strait Islander women, 27% (62/234) of Europid women, and 33% (69/208) of other ethnicity women completed a 12-week postpartum OGTT. Most of these women had an OGTT between 6 and 12 weeks as per clinical guidelines with only eight women having an OGTT prior to 6 weeks (all non-Indigenous women). Screening with an HbA1C was more common for Aboriginal and/or Torres Strait Islander women than non-Indigenous women (16% vs. 2%, p < 0.001) by 6 months postpartum. When either an OGTT, HbA1C or fasting plasma glucose level was included, by 6 months postpartum, 43% (307/711) had completed screening, with no differences between ethnic groups (Table 1).
| All women n = 712a | Aboriginal and/or Torres Strait Islander women n = 270 | Non-ndigenous womenb n = 442 | p value for ethnicity | |
|---|---|---|---|---|
| Completion of OGTT postpartum | ||||
| Within 12 weeks, n (%) | 182 (26) | 48 (18) | 134 (30) | <0.001 |
| Within 6 months, n (%) | 227 (32) | 63 (23) | 164 (37) | <0.001 |
| Within 12 months, n (%) | 253 (36) | 70 (26) | 183 (42) | <0.001 |
| Within 18 months, n (%) | 258 (36) | 72 (27) | 186 (42) | <0.001 |
| Completion of HbA1Cc | ||||
| Within 6 months n (%) | 53 (8) | 43 (16) | 10 (2) | <0.001 |
| Within 12 months, n (%) | 72 (10) | 60 (22) | 12 (3) | <0.001 |
| Within 18 months, n (%) | 87 (12) | 74 (27) | 13 (3) | <0.001 |
| Completion of FPG | ||||
| Within 6 months, n (%) | 27 (4) | 4 (2) | 23 (5) | 0.011 |
| Within 12 months, n (%) | 45 (6) | 6 (2) | 39 (9) | <0.001 |
| Within 18 months, n (%) | 53 (8) | 6 (2) | 47 (11) | <0.001 |
| Completion of any testd | ||||
| Within 6 months, n (%) | 307 (43) | 110 (41) | 197 (45) | 0.304 |
| Within 12 months, n (%) | 370 (52) | 136 (50) | 234 (53) | 0.486 |
| Within 18 months, n (%) | 398 (56) | 152 (56) | 246 (56) | 0.893 |
- Note: Data are n (%).
- Screening tests reported are the first screening test performed postpartum.
- Random plasma glucose was not included in the main analysis due to its limitations in diagnosing prediabetes or diabetes.
- Abbreviations: FPG, fasting plasma glucose; GDM, gestational diabetes; OGTT, 75 g oral glucose tolerance test.
- a n = 711 all women and n = 441 for non-Indigenous women for data collected 6 months and later because one non-Indigenous women withdrew from the study when contacted at 6 months.
- b Includes Europid and other ethnicity women.
- c Of the 74 women who had an HbA1C within 6 months—55 had before 4 months (median 10.5 weeks [0–25]) with five women having an HbA1C on the baby's date of birth.
- d OGTT or HbA1C or fasting plasma glucose.
3.4 Maternal antenatal factors associated with postpartum diabetes screening (OGTT, HbA1C, fasting plasma glucose)
Among Aboriginal and/or Torres Strait Islander women, those who had a higher fasting glucose, were using insulin during pregnancy or had a diagnosis of DIP were more likely to have completed 6 months postpartum screening. For non-Indigenous women, those who were older, employed, did not smoke during pregnancy and had a lower BMI in pregnancy were more likely to have completed screening (Table 2).
| All women n = 711 | Aboriginal and/or Torres Strait Islander women n = 270 | Non-Indigenous women n = 441 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Screened n = 308 | Not screened n = 403 | p value | Screened n = 111 | Not screened n = 159 | p value | Screened n = 197 | Not screened n = 244 | p value | |
| Demographic | |||||||||
| Aboriginal and/or Torres Strait Islander ethnicity, n (%) | 111 (36) | 159 (39) | 0.352 | ||||||
| Age at baby's DOB, years | 31.2 (5.6) | 30.6 (5.7) | 0.153 | 29.1 (6.2) | 29.6 (6.2) | 0.504 | 32.4 (4.9) | 31.2 (5.3) | 0.013 |
| Urban residence, n (%) | 209 (68) | 282 (70) | 0.545 | 27 (24) | 51 (32) | 0.167 | 182 (92) | 231 (95) | 0.328 |
| Year 12 education, n (%) | 225 (75) | 280 (72) | 0.310 | 48 (45) | 73 (48) | 0.664 | 177 (92) | 207 (87) | 0.117 |
| Employed full or part time, n (%) | 157 (53) | 180 (46) | 0.105 | 23 (22) | 38 (25) | 0.550 | 134 (70) | 142 (6) | 0.044 |
| Markers of risk for diabetes | |||||||||
| Pregnancy OGTT | |||||||||
| Fasting glucose, mmol/L | 4.9 (1.0) | 4.8 (1.0) | 0.396 | 5.2 (1.3) | 4.9 (0.9) | 0.049 | 4.8 (0.8) | 4.8 (1.1) | 0.673 |
| 1-h glucose, mmol/L | 10.0 (2.0) | 9.7 (1.9) | 0.110 | 10.1 (2.1) | 9.7 (2.1) | 0.105 | 9.9 (1.9) | 9.8 (1.8) | 0.446 |
| 2-h glucose, mmol/L | 8.7 (1.9) | 8.5 (1.8) | 0.342 | 8.3 (2.1) | 8.4 (2.0) | 0.542 | 8.8 (1.7) | 8.7 (1.6) | 0.560 |
| Gestational age at OGTT, weeks | 27.0 [22.7, 28.6] | 27.3 [23.1, 8.9] | 0.151 | 24.8 [17.7, 28] | 26.7 [17.6, 29.1] | 0.034 | 27.4 [25, 29] | 27.4 [25, 28.9] | 0.913 |
| Use of insulin during pregnancy, n (%) | 133 (43) | 139 (34) | 0.018 | 52 (47) | 45 (28) | 0.001 | 81 (41) | 94 (39) | 0.580 |
| Early diagnosis GDM, <20 weeks, n (%) | 51 (17) | 68 (17) | 0.992 | 29 (28) | 36 (24) | 0.564 | 22 (12) | 32 (13) | 0.412 |
| DIP, n (%) | 47 (16) | 49 (12) | 0.231 | 34 (31) | 29 (18) | 0.018 | 13 (7) | 20 (8) | 0.526 |
| Family history diabetes, n (%) | 151 (51) | 183 (49) | 0.553 | 64 (62) | 85 (58) | 0.494 | 87 (45) | 98 (43) | 0.644 |
| Previous history of GDM | 62 (34) | 77 (30) | 0.315 | 28 (33) | 36 (28) | 0.525 | 34 (35) | 41 (31) | 0.440 |
| Clinical | |||||||||
| Nulliparity, n (%) | 127 (41) | 144 (37) | 0.134 | 27 (24) | 32 (20) | 0.411 | 100 (51) | 112 (46) | 0.310 |
| Smoking in pregnancy, n (%) | 57 (19) | 114 (28) | 0.003 | 47 (43) | 75 (48) | 0.426 | 10 (5) | 39 (16) | <0.001 |
| Pregnancy characteristics | |||||||||
| First recorded BMI in pregnancy, kg/m2 | 28.0 (6.1) | 28.8 (7.2) | 0.132 | 28.8 (7.7) | 29.7 (6.7) | 0.336 | 27.1 (5.5) | 28.8 (6.9) | 0.005 |
| First BMI adjusted gestational age, kg/m2 | 28.0 (6.9) | 28.8 (6.9) | 0.118 | 29.8 (7.3) | 28.8 (7.4) | 0.259 | 27.1 (6.4) | 28.7 (6.5) | 0.009 |
- Note: Data are mean (standard deviation), median [interquartile range] or n (%).
- For women who were screened: education, n = 299; employment, n = 299; family history, n = 297; first recorded BMI, n = 296; fasting glucose, n = 296; 1-h glucose, n = 269; 2-h glucose n = 298; previous history of GDM among multiparous women only n = 181; smoking, n = 305.
- For women who were not screened: education, n = 390; employment, n = 389; family history, n = 377; first recorded BMI, n = 378; fasting glucose, n = 394; 1-h glucose, n = 348; 2-h glucose n = 395; previous history of GDM among multiparous women only n = 259; smoking, n = 400.
- Abbreviations: BMI, body mass index; DIP, diabetes in pregnancy (diagnosed in pregnancy but meeting the diagnostic glucose/HbA1C values for type 2 diabetes outside of pregnancy); DOB, date of birth; GDM, gestational diabetes; OGTT, 75 g oral glucose tolerance test.
3.4.1 Regression analysis
Among the full cohort, on univariable regression, insulin use and not smoking in pregnancy were associated with postpartum diabetes screening (Table 3). Insulin use in pregnancy, first pregnancy, not smoking in pregnancy and lower BMI remained significantly associated with postpartum screening after adjusting for ethnicity, age, locality (urban vs. remote) and employment status (Table 3, Model 1). When insulin use was substituted for other indicators of diabetes risk (higher OGTT results, early diagnosis of GDM, a diagnosis of DIP in pregnancy, family history of diabetes or personal history of previous GDM), a lower BMI was no longer associated with increased screening for each of these models (results not shown). When variables with p value ≤0.2 on univariable analysis were included in the model, results were similar (Table 3, Model 2). There were no clinically significant interactions between ethnicity and other independent variables (data not shown).
| UNADJUSTED | Model 1 | Model 2 | ||||
|---|---|---|---|---|---|---|
| IRR (95% CI) | p value | IRR (95% CI) | p value | IRR (95% CI) | p value | |
| Demographics | ||||||
| Fist Nations Ethnicitya | 0.84 (0.67–1.06) | 0.143 | 1.05 (0.72–1.53) | 0.808 | 1.06 (0.71–1.51) | 0.782 |
| Maternal age at baby's DOB, years | 1.08 (0.98–1.20) | 0.113 | 1.11 (0.99–1.24) | 0.064 | 1.11 (0.996–1.25) | 0.018 |
| Urban residenceb | 0.99 (0.78–1.26) | 0.953 | 0.83 (0.57–1.20) | 0.318 | 0.78 (0.53–1.16) | 0.225 |
| Completed year 12 educationc | 1.15 (0.89–1.50) | 0.285 | ||||
| Employed full or part timed | 1.25 (0.99–1.56) | 0.058 | 1.08 (0.83–1.40) | 0.586 | 1.20 (0.91–1.59) | 0.204 |
| Markers of risk for diabetes | ||||||
| Pregnancy OGTT | ||||||
| Fasting glucose, mmol/L | 1.05 (0.95–1.16) | 0.325 | ||||
| OGTT 1 h, mmol/L | 1.05 (0.99–1.12) | 0.110 | 1.02 (0.95–1.09) | 0.575 | ||
| OGTT 2 h, mmol/L | 1.03 (0.97–1.10) | 0.306 | ||||
| Insulin during pregnancye | 1.32 (1.05–1.66) | 0.015 | 1.39 (1.09–1.79) | 0.009 | 1.26 (0.96–1.66) | 0.095 |
| Early diagnosis GDM (<20 weeks)f | 1.01 (0.75–1.37) | 0.937 | ||||
| DIPg | 1.20 (0.88–1.64) | 0.258 | ||||
| Family history of diabetesh | 1.04 (0.83–1.31) | 0.738 | ||||
| Personal history of GDMi | 1.07 (0.81–1.41) | 0.633 | ||||
| Clinical | ||||||
| Nulliparousj | 1.19 (0.95–1.49) | 0.132 | 1.33 (1.02–1.75) | 0.038 | 1.35 (1.01–1.80) | 0.039 |
| Smoking in pregnancyk | 0.62 (0.47–0.83) | 0.001 | 0.61 (0.44–0.84) | 0.003 | 0.53 (0.37–0.76) | 0.001 |
| Anthropometric | ||||||
| First BMI in pregnancy, kg/m2 | 0.97 (0.92–1.02) | 0.185 | ||||
| First BMI adjusted gestational age, kg/m2 | 0.96 (0.91–1.01) | 0.147 | 0.94 (0.89–1.0) | 0.050 | 0.95 (0.89–1.00) | 0.060 |
- Note: The incidence rate ratio for age represents a 5-year increase and for BMI a 3 kg/m2 increase.
- Model 1: Variables selected for the multivariable model were those described in the literature to be associated with postpartum screening for diabetes. Insulin use in pregnancy was selected as the marker of higher risk for diabetes as the most clinically relevant marker. As education and employment were correlated, the effect of these variables were assessed by including them in two separate multivariable models. When employment status was substituted for education, associations remained similar (data not shown).
- Model 2: Variables selected for the multivariable regression model were those with a p value ≤0.2 on univariable analysis.
- Abbreviations: BMI, body mass index; DIP, diabetes in pregnancy (diagnosed in pregnancy but meeting the diagnostic glucose/HbA1C values for type 2 diabetes outside of pregnancy); DOB, date of birth; GDM, gestational diabetes; IRR, incidence rate ratio; OGTT, 75 g oral glucose tolerance test.
- a Compared to non-Indigenous.
- b Compared to regional/remote residence.
- c Compared to less than 12 years of school or equivalent.
- d Compared to unemployed.
- e Compared to not taking insulin in pregnancy.
- f Compared to diagnosis ≥20 weeks.
- g Compared to not meeting diagnostic criteria for DIP.
- h In parent or sibling, compared to no family history.
- i Compared to no previous GDM.
- j Compared to any parity.
- k Compared to not smoking in pregnancy.
4 DISCUSSION
We report four key findings. First, uptake of postpartum screening with an OGTT was low, with 18% and 30% of Aboriginal and/or Torres Strait Islander and non-Indigenous women respectively completing a 12-week OGTT. Second, screening with an HbA1C was more common for Aboriginal and/or Torres Strait Islander women than non-Indigenous women (16% vs. 2%) by 6 months postpartum. Third, when screening was broadened to include an OGTT, HbA1C or fasting plasma glucose, uptake was higher in all women, with no differences between ethnic groups. Fourth, insulin use, first pregnancy, not smoking and lower BMI in pregnancy were associated with increased 6 months postpartum screening.
Low uptake of postpartum screening (19% to 61%) is widely reported in systematic reviews incorporating studies around the world.5, 6 The range in screening rates observed is likely due to heterogeneity in data sources, study design, diagnosis of GDM and reminder systems in place. There are few studies on the rates of postpartum diabetes screening among First Nations women. In the Australian context, a retrospective cohort study in Far North Queensland reported 14% of Aboriginal and/or Torres Strait Islander women with GDM had postpartum OGTT screening by 6 months from 2004 to 2010.17 An audit of mostly Aboriginal women with GDM in remote communities in the NT (2013–2014), reported 31% of women had an OGTT by 12 months postpartum,18 similar to our reported rate (26% for Aboriginal and/or Torres Strait Islander women) at 12 months.
Barriers to uptake of postpartum OGTT testing include health systems factors such as inadequate communication between hospitals and primary care health centres and lack of clarity around who is responsible for postpartum follow-up.19 At an individual level, barriers include the need to fast, time-consuming nature and the unpleasantness of the OGTT, caregiver responsibilities, and low perception of the long-term risk associated with GDM.20
It is important to consider that providing individuals with motivation to have postpartum screening cannot be effective if the environment makes it difficult or impossible to make these decisions. Hence, a multilevel approach—targeting the individual and health systems barriers—is needed in overcoming barriers to postpartum screening, which include finding alternative effective methods for postpartum screening.
While Aboriginal and/or Torres Strait Islander women in our study were less likely to have completed a postpartum OGTT than non-Indigenous women, they were more likely to have an HbA1C and just as likely to have had some form of postpartum diabetes screening by 6 months postpartum, with 41% of women completing screening. In contrast, the above-mentioned Queensland study reported a lower proportion of Aboriginal and/or Torres Strait Islander women had completed postpartum screening by 6 months, compared to non-Indigenous women (17% vs. 33%).17 The investigators also reported lower overall uptake of any postpartum screening by 6 months than our study (including OGTT, HbA1C, fasting or random plasma glucose). In contrast to that study, the improved uptake reported in our cohort may reflect increased awareness in recent years of the importance of postpartum screening,21 as well as changes to government reimbursement for HbA1C testing. As expected, given the relative ease of the test, when an HbA1C is included in postpartum screening, rates markedly improve. A New Zealand study, with a large proportion of Māori women, reported that 30% of women had an OGTT at 6 weeks postpartum which improved to 74% with the inclusion of an HbA1C.22
In our study, 71% of Aboriginal and/or Torres Strait Islander women lived in remote locations (compared to 6% of non-Indigenous women). This is reflective of the general population in the NT where 80% of Aboriginal and/or Torres Strait Islander people live in remote communities.23 There are challenges for women living remotely, including accessible and appropriately coordinated healthcare, the high turnover and shortage of health professionals and socio-economic disadvantage.24 We report no differences in diabetes screening rates (with an OGTT, HbA1C or fasting plasma glucose) based on location. Given the known health disparities for people living remotely,25 this lack of difference in screening rates based on locality, as well as by ethnicity, points to the strengths of community-based screening in remote locations. Remote primary health centres in Australia are not likely to charge fees for postpartum visits, and, in communities with low staff turnover, women are more likely to be personally known to health professionals. Our study supports the effectiveness of community-based screening in the remote NT.
Whether an HbA1C is an appropriate alternate postpartum screening option is an important consideration. Indeed, guidelines in regional and remote NT now (after this study period) recommend an HbA1C 4 months postpartum if an OGTT is not able to be performed.26 While prediabetes and diabetes can be identified by either an OGTT, HbA1C or fasting plasma glucose, each test identifies a different group of individuals, such that there may be discordance between each.27 Randomised controlled trials have provided strong evidence that several interventions, including lifestyle and pharmacotherapy can delay or prevent type 2 diabetes in women with impaired glucose tolerance diagnosed by an OGTT.28, 29 Further studies are needed to determine whether interventions based on an HbA1C diagnosis of prediabetes leads to improved outcomes. In addition to detecting prediabetes and diabetes in that early postpartum period, it is important to continue to monitor women for diabetes progression long term, which most practically can be performed with an HbA1C. This is recommended annually by our local guidelines26 and New Zealand Guidelines30 and at least 3 yearly by the Australian Diabetes Society depending on risk.31
Characteristics independently associated with increased screening by 6 months postpartum included, older age, insulin use in pregnancy, first pregnancy, not smoking in pregnancy and a lower BMI in pregnancy. The association between insulin use and higher postpartum screening potentially reflects beliefs regarding insulin use as a marker of increased diabetes risk. The association between lower parity and higher postpartum screening is attributed to the difficulty in performing screening with responsibilities of other children.5 These findings highlight a need for improved awareness of diabetes risk among all women with GDM, not just those on insulin, and to consider the ease of testing for women with competing priorities. Not smoking in pregnancy and lower BMI are associated with higher socio-economic status. While studies have reported associations with proxy measures of higher socio-economic status and increased screening (such as older age, ‘white’ ethnicity, non-smoking and higher education),5, 6 the Far North Queensland study did not find these same associations among Aboriginal and/or Torres Strait Islander women and postulated that this was due to Aboriginal and/or Torres Strait Islander status being such a strong marker of social disadvantage.32 Indeed, when stratified by ethnicity we also did not observe smoking, BMI, remoteness, education or employment levels to be associated with screening rates for Aboriginal and/or Torres Strait Islander women.
This is the first longitudinal prospective study of Aboriginal and/or Torres Strait Islander women with GDM. The following limitations are related to postpartum data collection: (i) Of the 712 women in the cohort, 17 women had no follow-up data. These women have been included in the not-screened group although they may have had screening out of the NT, leading to an underestimation of screening. (ii) Questionnaires asked whether women had been screened for diabetes, potentially serving as a reminder to be screened (particularly for non-Indigenous women who were more likely to complete questionnaires). (iii) Non-Indigenous women were more likely to attend private general practice clinics and while we accessed two of the three pathology companies in the NT, a minor third pathology company was not accessed. Another limitation is the possibility that participants were not representative of the wider NT population. However, women with hyperglycaemia in PANDORA were similar to women with hyperglycaemia on the NT Diabetes in Pregnancy Register (for age, ethnicity and remoteness).33 Results may not be applicable to Torres Strait islander women as few were included. No postpartum data were collected on diet and exercise advice which may have strengthened this paper. Finally, this study was not powered to detect characteristics associated with screening for postpartum diabetes and some factors may have been missed. Nevertheless, identified factors may be useful for determining which women to target for postpartum screening.
With high rates of type 2 diabetes among Aboriginal and/or Torres Strait Islander populations, early screening and intervention is critical to preventing diabetes and its complications. A pragmatic approach of seeking to test as many women with a history of GDM as possible with an HbA1C (which can be done as a point of care test) within the first 6 months postpartum and an emphasis on the importance of annual HbA1C monitoring may be preferable to focusing on postpartum OGTTs.34 Initiatives to improve postpartum screening rates for Aboriginal and/or Torres Strait Islander women need to simultaneously address social and economic disadvantages alongside initiatives focused on individuals.
AUTHOR CONTRIBUTIONS
A. J.W and F.B analysed the data. A.J.W wrote the manuscript. I-L.L, J.A.B, E.L.M.B, A.T, C.C, E.M, J.J.N.O, H.D.M, P.Z.Z, A.D.H.B and J.E.S. provided intellectual input and reviewed the manuscript. S.C provided intellectual input as representative of the Aboriginal and Torres Strait Islander Advisory Group. I-L.L and A.S. were responsible for collecting the data. L.J.M.-B. conceptualised the project and led the funding application, ethics application, led supervision of data collection, analysis and interpretation and led supervision of manuscript development. All authors have read and agreed to the published version of the manuscript.
ACKNOWLEDGEMENTS
The authors acknowledge the participants of the study, The Diabetes across the Lifecourse Northern Australia Partnership investigators, partners, staff, Clinical Reference Group, Aboriginal and Torres Strait Islander Advisory Group, NT health professionals from NT Department of Health hospitals and remote primary health care and Aboriginal Community Controlled Health Organisations who have supported this work. Investigators of The Partnership in addition to those named as authors are: S Campbell, K O’Dea, B Davis, A Hanley, R McDermott, A McLean, J Mein, A Sinha and M Wenitong. Open access publishing facilitated by Charles Darwin University, as part of the Wiley - Charles Darwin University agreement via the Council of Australian University Librarians.
FUNDING INFORMATION
This work was supported by the National Health and Medical Research Council of Australia (NHMRC Partnership Project Grant #1032116, NHMRC #1078333). AW was supported by an NHMRC scholarship (#1151049) and LJMB was supported by an NHMRC fellowship (#605837) and NHMRC Practitioner fellowship (#1078477). ELMB was supported by a National Heart Foundation, Australia post-doctoral fellowship (#101291). ADHB was supported by an NHMRC Senior Research fellowship (#1137563) and a Sylvia and Charles Viertel Senior Medical Research fellowship, Australia. JES was supported by an NHMRC fellowship (#1079438). This paper reflects the views of the authors and not the NHMRC.
CONFLICT OF INTEREST
None declared.
Open Research
DATA AVAILABILITY STATEMENT
Data are available on request to the Partnership Steering Committee. They are not available on an online repository.




