Antenatal diagnosis, neonatal brain volumes, and neurodevelopment in transposition of the great arteries
Abstract
Aim
To examine whether antenatal diagnosis modifies relationships between neonatal brain volumes and 18-month neurodevelopmental outcomes in children with transposition of the great arteries (TGA).
Method
In a retrospective cohort of 139 children with TGA (77 antenatally diagnosed), we obtained total brain volumes (TBVs) on pre- (n = 102) and postoperative (n = 112) magnetic resonance imaging. Eighteen-month neurodevelopmental outcomes were assessed using the Bayley Scales of Infant and Toddler Development, Third Edition. Generalized estimating equations with interaction terms were used to determine whether antenatal diagnosis modified associations between TBVs and neurodevelopmental outcomes accounting for postmenstrual age at scan, brain injury, and ventricular septal defect.
Results
Infants with postnatal diagnosis had more preoperative hypotension (35% vs 14%, p = 0.004). The interactions between antenatal diagnosis and TBVs were significantly related to cognitive (p = 0.003) outcomes. Specifically, smaller TBVs were associated with lower cognitive scores in infants diagnosed postnatally; this association was attenuated in those diagnosed antenatally.
Interpretation
Antenatal diagnosis modifies associations between neonatal brain volume and 18-month cognitive outcome in infants with TGA. These findings suggest that antenatal diagnosis may be neuroprotective, possibly through improved preoperative clinical status. These data highlight the need to improve antenatal diagnosis rates.
What this paper adds
- Antenatal diagnosis of transposition of the great arteries modified relationships between neonatal brain volume and neurodevelopment.
- Smaller brain volumes related to poorer cognitive scores with postnatal diagnosis only.
- There was more preoperative hypotension in the postnatal diagnosis group.
What this paper adds
- Antenatal diagnosis of transposition of the great arteries modified relationships between neonatal brain volume and neurodevelopment.
- Smaller brain volumes related to poorer cognitive scores with postnatal diagnosis only.
- There was more preoperative hypotension in the postnatal diagnosis group.
We examined whether antenatal diagnosis modifies relationships between neonatal brain volumes and neurodevelopment in a clinical cohort of children with transposition of the great arteries. In infants with postnatal diagnosis, smaller neonatal brain volumes were associated with lower cognitive scores; these associations were not present in the antenatal diagnosis group. These findings suggest that antenatal diagnosis may be neuroprotective, possibly mediated through improved hemodynamic state in the preoperative period.
Abbreviations
-
- CHD
-
- congenital heart disease
-
- PMA
-
- postmenstrual age
-
- TGA
-
- transposition of the great arteries
-
- TBV
-
- total brain volume
-
- VSD
-
- ventricular septal defect
Antenatal diagnosis of complex congenital heart disease (CHD), including transposition of the great arteries (TGA), has been inconsistently associated with decreased neonatal mortality.1-3 Antenatal diagnosis may also be associated with decreased rates of preoperative risk factors for cardiac surgery,4 reduced adverse neurological complications after surgery,5 and improved neurodevelopmental outcomes, although inconsistently.6, 7 In a prospective study of children with TGA, antenatal diagnosis was associated with better scores on tests of executive function and cognition;6 however, the factors mediating improved neurodevelopmental outcomes in infants with antenatal diagnosis are unknown. Antenatal diagnosis has been associated with reduced brain injury and more advanced microstructural and metabolic brain maturation across the pre- and postoperative periods in infants with CHD, including those with TGA.8 However, how antenatal diagnosis and brain maturation in infancy intersect to predict neurodevelopmental outcomes is unknown.
In this study, we sought to understand how timing of TGA diagnosis and neonatal total brain volume (TBV), a marker of brain maturation,9 interact and are related to neurodevelopment in a clinical cohort of children with TGA who underwent pre- and postoperative magnetic resonance imaging (MRI) as infants. We investigated whether pre- and postoperative TBV, for a given postmenstrual age (PMA), differed between infants with antenatal and postnatal TGA diagnosis. We then examined whether the relationship between pre- and postoperative brain volumes with 18-month neurodevelopmental outcomes is modified by timing of TGA diagnosis.
METHOD
Study population
This was a retrospective cohort of 139 children with TGA born between 2013 and 2020 who underwent cardiac surgery in infancy at the Hospital for Sick Children in Toronto, Canada. Children with TGA with an intact ventricular septum or ventricular septal defect (VSD) were included; those with double outlet right ventricle were excluded. This study was approved by the Research Ethics Board. Parental informed written consent was obtained for use of clinically obtained data for research purposes. Detailed data of pre-, intra-, and postoperative clinical course were collected retrospectively by reviewing medical records. We also obtained continuous preoperative oxygen saturation data at 5-second intervals as previously described which was available for a subset of patients (n = 115).10 These data were used to obtain the percentage of preoperative time with oxygen saturations less than 75% and mean preoperative oxygen saturations for each infant. We operationalized socioeconomic status with Ontario Marginalization Index dimensions: material deprivation, residential instability, ethnic concentration, and dependency. The Index assesses levels of marginalization based on census tract and has been previously validated for health research in Ontario.11
Brain MRI
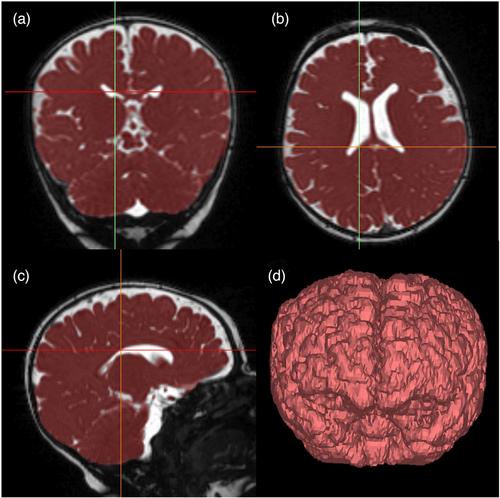
Children with TGA underwent pre- and/or postoperative brain MRI as infants. This became part of routine clinical care at our centre in 2015; infants underwent brain MRI for clinical concerns or if they were participating in a research study before then (eight children in the cohort born before 2015). Brain MRI was performed on a 1.5 T Siemens Avanto (Erlangen, Germany) MRI scanner without sedation. These clinically acquired MRI were reviewed for brain abnormalities by a paediatric neuroradiologist and brain injury severity was scored by a neonatal neurologist (TS). White matter injury was scored as mild (no more than three lesions <2 mm), moderate (more than three lesions or lesion >2 mm and <5% hemispheric involvement), or severe (>5% hemisphere involved).8 Intraventricular haemorrhage severity was graded according to the Papile score.12 The images were reviewed for the presence of stroke, defined as an ischemic lesion involving the cortex or deep grey matter, and hypoxic–ischemic brain injury defined as injury to deep grey nuclei and/or watershed area.13 Moderate–severe brain injury was defined as moderate–severe white matter injury, intraventricular haemorrhage grade 3 or higher, hypoxic–ischemic brain injury, or stroke.8 TBV was determined by segmenting the brain from a three-dimensional steady-state free procession or T1-weighted gradient echo acquisition of the whole head with the use of Mimics software (Materialize, Belgium) as previously described (Figure 1).14
Neurodevelopmental outcomes
As part of routine clinical care, children were assessed at 18 months in the Neonatal Follow-up Program by an experienced occupational therapist or physiotherapist using the Bayley Scales of Infant and Toddler Development, Third Edition, with separate cognitive, motor, and language composite scores (mean 100, SD 15).15
Statistical analysis
Clinical characteristics were compared between infants by timing of TGA diagnosis using Fisher's exact test and a Kruskal–Wallis test for categorical and continuous data respectively. We first examined whether TBV for PMA across the pre- to postoperative periods differed between infants who were diagnosed antenatally compared with those diagnosed postnatally. We used generalized estimating equations to account for repeated measures (pre- and postoperative brain MRI). We used a generalized estimating equation with TBV at pre- and postoperative scans as our primary outcome, the interaction between antenatal diagnosis and PMA at scan as our main independent variable, and PMA at scan, antenatal diagnosis, VSD, and moderate–severe brain injury as covariates. To assess whether associations between neonatal brain volumes and neurodevelopment were modified by antenatal diagnosis, we used generalized estimating equations with primary outcome measures of cognitive, language, and motor scores with the interaction between pre- and postoperative brain volumes by antenatal diagnosis as our main independent variable. TBV, antenatal diagnosis, PMA at scan, VSD, and moderate–severe brain injury were included as covariates. VSD and moderate–severe brain injury were included as potential confounders on the basis of previous work showing associations with both altered brain maturation and poorer neurodevelopmental outcomes.9, 16 We did not adjust for potential factors that could mediate the relationship between antenatal diagnosis and neurodevelopment such as preoperative hypotension and social marginalization. Given there were three separate hypotheses, one each for cognitive, motor, and language outcomes, p-values were considered statistically significant if they were less than 0.017 except when testing for interactions (p < 0.033). Missing observations were considered to be Missing Completely at Random. Statistical analyses were performed using Stata version 15.0 (StataCorp, College Station, TX, USA) and R version 4.0.3 (R Project for Statistical Computing, https://www.r-project.org/).
RESULTS
Clinical characteristics of study participants
The final cohort consisted for 139 children with TGA (Figure S1). Clinical characteristics of infants by timing of TGA diagnosis are presented in Table 1. Infants diagnosed antenatally had a lower Apgar at 5 minutes of age. A greater proportion of infants diagnosed postnatally had preoperative hypotension requiring intervention (postnatal diagnosis 36% vs antenatal diagnosis 14%). We also explored whether there were differences in preoperative oxygen saturations between infants with postnatal and antenatal diagnosis by plotting these measurements, with no difference between groups observed (Figure S2).
| Clinical characteristic | Postnatal diagnosis (n = 62) | Antenatal diagnosis (n = 77) | p |
|---|---|---|---|
| Male sex | 45 (73) | 51 (67) | 0.56 |
| Birth gestational age, weeks | 39.3 (38.7–40.1) | 39.0 (38.0–39.6) | 0.05 |
| Birthweight, g | 3325 (3000–3622) | 3240 (2960–3570) | 0.4 |
| Birth head circumference, cm | 34 (33–35) | 34 (33–35) | 0.61 |
| Apgar at 5 min | 9 (8–9) | 8 (8–9) | 0.01* |
| Mode of delivery: Caesarean sectiona | 12 (19) | 16 (21) | 0.50 |
| Ventricular septal defect | 25 (40) | 31 (41) | 0.86 |
| Prostaglandin | 50 (81) | 70 (91) | 0.08 |
| Balloon atrial septostomy | 51 (82) | 63 (81) | 0.82 |
| Lowest preoperative SpO2 | 50 (40–68) | 51 (35–70) | 0.87 |
| Mean preoperative SpO2b | 84.2 (81.6–86.7) | 85.1 (82.3–87.4) | 0.43 |
| Percentage of preoperative time with SpO2 < 75%c | 8.4 (2.8–15.5) | 7.9 (2.4–13.8) | 0.49 |
| Preoperative hypotension | 22 (35) | 11 (14) | 0.004* |
| Preoperative cardiac arrest | 4 (6) | 1 (1) | 0.17 |
| Day of life at surgery | 9 (7–24) | 8 (6–16) | 0.08 |
| Late surgical repair (>2 weeks) | 20 (32) | 22 (28) | 0.85 |
| Cardiopulmonary bypass | 62 (100) | 75 (97) | 0.5 |
| Cardiopulmonary bypass time, min | 147 (129–182) | 142 (118–167) | 0.30 |
| Cross clamp time, min | 98 (78–116) | 91 (72–112) | 0.34 |
| Circulatory arrest | 3 (5) | 6 (9) | 0.74 |
| Circulatory arrest time, min | 12 (2–16) | 20 (9–25) | 0.43 |
| Postoperative cardiac arrest | 6 (10) | 2 (3) | 0.14 |
| Postoperative NEC | 3 (5) | 6 (8) | 0.73 |
| Postoperative sepsisc | 3 (5) | 5 (10) | 0.72 |
| Postoperative seizuresc | 5 (8) | 2 (4) | 0.27 |
| Postoperative failure to feedc | 17 (27) | 21 (40) | 0.68 |
| Extracorporeal membrane oxygenation | 7 (11) | 7 (9) | 0.78 |
| Social marginalizationd | |||
| Residential instability quintile | 0.89 | ||
| One | 14 (25) | 16 (24) | |
| Two | 8 (14) | 13 (19) | |
| Three | 12 (21) | 12 (19) | |
| Four | 14 (25) | 13 (19) | |
| Five | 9 (16) | 13 (19) | |
| Material deprivation quintile | 0.31 | ||
| One | 12 (21) | 13 (20) | |
| Two | 9 (16) | 16 (24) | |
| Three | 18 (32) | 16 (23) | |
| Four | 9 (16) | 10 (15) | |
| Five | 9 (16) | 12 (18) | |
| Dependency quintile | 0.15 | ||
| One | 20 (3) | 17 (24) | |
| Two | 8 (14) | 21 (30) | |
| Three | 12 (21) | 15 (23) | |
| Four | 9 (16) | 6 (9) | |
| Five | 8 (14) | 8 (13) | |
| Ethnic concentration quintile | 0.84 | ||
| One | 7 (12) | 9 (13) | |
| Two | 13 (23) | 14 (23) | |
| Three | 12 (21) | 17 (24) | |
| Four | 10 (18) | 14 (20) | |
| Five | 15 (26) | 13 (19) | |
| Magnetic resonance imaginge | |||
| PMA at preoperative scan, weeks | 40 (39.4–41.1) | 39.5 (38.6–40.1) | 0.01* |
| Preoperative moderate–severe brain injury | 13 (21) | 11 (14) | 0.15 |
| Preoperative moderate–severe white matter injury | 7 (11) | 7 (9) | 0.56 |
| Preoperative stroke | 10 (16) | 7 (9) | 0.11 |
| Preoperative total brain volume (mm3) | 325 (302–358) | 334 (309–359) | 0.85 |
| PMA at postoperative scan, weeks | 42.1 (41.3–44.1) | 41.9 (40.6–42.9) | 0.05 |
| Postoperative moderate–severe brain injury | 14 (23) | 15 (19) | 0.83 |
| New postoperative white matter injury | 8 (13) | 11 (14) | 1.0 |
| New postoperative stroke | 3 (5) | 5 (5) | 1.0 |
| Postoperative total brain volume (mm3) | 354 (319–395) | 352 (318–387) | 0.55 |
| Died | 3 (5) | 2 (3) | 0.65 |
| Bayley-III scores at 18 monthsf | |||
| Cognitive composite | 100 (95–110) | 103 (95–110) | 0.15 |
| Motor composite | 97 (91–103) | 100 (94–107) | 0.20 |
| Language composite | 94 (79–103) | 97 (89–103) | 0.19 |
- Data presented as n (%) or median (interquartile range).
- a Data available for 94 patients.
- b Data available for 115 patients.
- c Data available for 102 patients.
- d Data available for 124 patients.
- e Magnetic resonance imaging completed: 105 preoperative and 120 postoperative.
- f Data available for 101 children.
- * p < 0.05.
- Abbreviations: Bayley-III, Bayley Scales of Infant and Toddler Development, Third Edition; PMA, postmenstrual age; SpO2, oxygen saturation.
Antenatal diagnosis and TBV
One hundred and twenty-six children underwent pre- and/or postoperative MRI in the neonatal period (preoperative: n = 102; postoperative: n = 112). Pre- and postoperative median TBV differed significantly (preoperative: median 330mm3, interquartile range 304–359; postoperative: median 354mm3, interquartile range 319–390; p < 0.001). Median brain volumes were not significantly different at pre- or postoperative scans by timing of TGA diagnosis (Table 1). We used generalized estimating equations to determine whether pre- and postoperative brain volumes at a given PMA differed between the two groups. The interaction between antenatal diagnosis and PMA was not significantly associated with pre- and postoperative TBV (interaction p = 0.64) adjusting for PMA at scan, antenatal diagnosis, VSD, and moderate–severe brain injury as covariates.
Antenatal diagnosis, TBV, and neurodevelopmental outcomes
One hundred and one children returned for neurodevelopmental assessments at 18 months of age. Clinical characteristics of children who did and did not return for neurodevelopmental follow-up are presented in Table S1. Children who did not return for follow-up assessments were smaller at birth and older at surgical repair, with a greater proportion of children with VSD and late surgical repair.
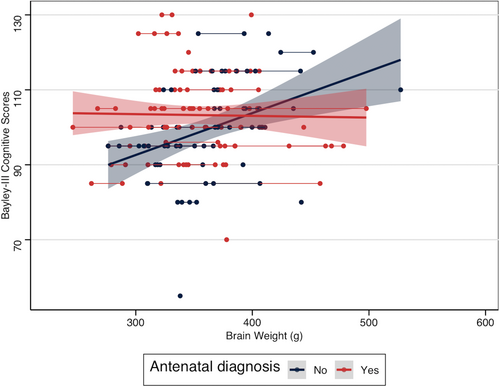
Neonatal TBVs were available for 90 children seen for neurodevelopmental follow-up with cognitive, motor, and language scores available for 90, 84, and 83 children; some children were not cooperative for the full duration of neurodevelopmental assessments. The interaction between pre- and postoperative TBV and antenatal diagnosis was significantly associated with cognitive scores in a simple generalized estimating equation model adjusting for antenatal diagnosis, TBV, and PMA at scan (p = 0.003; Table 2). In infants with postnatally diagnosed TGA, lower TBVs were associated with lower cognitive scores; however, this was not observed in infants with antenatal diagnosis (Figure 2). The antenatal diagnosis by TBV interaction remained significantly associated with cognitive scores even after adding VSD and moderate–severe brain injury to the model (Table 2). The interaction between TBV and antenatal diagnosis was not related to motor or language (p = 0.11) scores. Because the interaction term was not significant, this was removed to examine associations of TBV and antenatal diagnosis with motor and language scores. TBV was associated with motor scores (coefficient = 0.09, 95% confidence interval [0.03–0.14], p = 0.002); there were no associations with antenatal diagnosis, PMA at scan, VSD, and moderate–severe brain injury. Among the variables examined, there were no significant associations with Bayley Scales language scores.
| Variable | Simple model | Complex model | ||
|---|---|---|---|---|
| Coefficient (95% CI) | p | Coefficient (95% CI) | p | |
| Cognitive scores | ||||
| TBV × antenatal diagnosis | — | 0.003* | — | 0.003* |
| TBV | 0.1 (0.04–0.16) | 0.1 (0.03–0.16) | ||
| Antenatal diagnosis | 48.95 (18.79–79.1) | 49.31 (19.25–79.36) | ||
| Postmenstrual age at scan | 0.42 (−0.78 to 1.62) | 0.49 | 0.66 (−0.48 to 1.81) | 0.26 |
| Ventricular septal defect | — | — | −4.6 (−9.73 to 0.53) | 0.08 |
| Moderate–severe brain injury | — | — | −0.98 (−4.78 to 2.82) | 0.61 |
| Motor scores | ||||
| TBV × antenatal diagnosis | — | 0.4 | — | 0.35 |
| TBV | 0.11 (0.04–0.17) | 0.11 (0.04–0.18) | ||
| Antenatal diagnosis | 16.05 (−13.88 to 45.98) | 18.43 (−11.9 to 48.75) | ||
| Postmenstrual age at scan | —0.47 (−1.56 to 0.62) | 0.4 | −0.28 (−1.29 to 0.73) | 0.58 |
| Ventricular septal defect | — | — | −3.53 (−9.23 to 2.17) | 0.23 |
| Moderate–severe brain injury | — | — | −0.39 (−4.06 to 3.29) | 0.84 |
| Language scores | ||||
| TBV × antenatal diagnosis | — | 0.11 | — | 0.07 |
| TBV | 0.11 (0.01–0.2) | 0.11 (0.03–0.2) | ||
| Antenatal diagnosis | 40.49 (−2.8 to 83.77) | 43.83 (1.99–85.68) | ||
| Postmenstrual age at scan | 0.42 (−1.0 to 1.83) | 0.57 | 0.62 (−0.81 to 2.06) | 0.39 |
| Ventricular septal defect | — | — | −4.79 (−12.5 to 2.93) | 0.22 |
| Moderate–severe brain injury | — | — | 3.71 (−0.82 to 8.24) | 0.11 |
- The simple model was adjusted for postmenstrual age at scan whereas the complex model was adjusted for postmenstrual age at scan, ventricular septal defect, and moderate–severe brain injury.
- Abbreviations: Bayley-III, Bayley Scales of Infant and Toddler Development, Third Edition; CI, confidence interval; TBV, total brain volume.
DISCUSSION
In this study, we assessed whether antenatal diagnosis modified associations between pre- and postoperative TBV in infants with TGA with cognitive, but not motor or language, outcomes at 18 months. Although pre- and postoperative TBV did not differ between infants with antenatal and postnatal diagnosis, we found that timing of TGA diagnosis modified the relationship between TBV and neurodevelopmental outcomes. Specifically, in infants with postnatal diagnosis, smaller pre- and postoperative TBVs were associated with lower cognitive scores at 18 months. However, this relationship between smaller TBVs and poorer cognitive outcomes was attenuated in infants with antenatal diagnosis, suggesting that this may be protective. This is consistent with previous findings of improved neonatal brain maturation and neurocognitive outcomes in children with CHD diagnosed antenatally compared with those diagnosed postnatally.6, 8 Further studies are required to elucidate what clinical practices associated with antenatal diagnosis, which is a complex intervention, are protective for brain maturation and cognition in infants with TGA.
Antenatal diagnosis of CHD may lead to changes in clinical management such as delivery at a tertiary care centre, improved perinatal stabilization, and earlier interventions.3, 7, 17, 18 Reduced preoperative morbidity has also been observed in infants with CHD diagnosed prenatally compared with those diagnosed postnatally.7, 19 In infants with TGA, antenatal diagnosis was associated with better preoperative clinical condition including less metabolic acidosis and multiorgan failure compared with infants diagnosed postnatally.20 Similarly, in a recent large retrospective study using the Society of Thoracic Surgeons Congenital Heart Surgery Database, antenatal diagnosis of CHD was associated with decreased preoperative risk factors for cardiac surgery including shock, renal dysfunction, and liver dysfunction in children with CHD who underwent neonatal surgery.4 Consistent with this, we observed a trend towards earlier surgery in the antenatal diagnosis group, which has been associated with reduced preoperative brain injury, more rapid postoperative brain growth, and improved neurodevelopmental outcomes in infants with TGA, possibly through improved oxygenation and hemodynamics.9, 21 Our findings also suggest more favourable preoperative clinical characteristics in infants with antenatal diagnosis, with a smaller proportion of infants having preoperative hypotension than those diagnosed postnatally. We also examined continuous oxygen saturations to assess preoperative hypoxaemia in a subset of patients admitted to the cardiac intensive care unit. We did not find any differences in preoperative oxygen saturations between infants with and without antenatal diagnosis, which may be due to a selection bias in the population of patients for whom we have these data. At our institution, infants with TGA do not routinely receive all their preoperative care in the cardiac intensive care unit. Further studies using continuous physiological data may be helpful in comparing preoperative oxygenation and haemodynamics in infants with antenatal and postnatal diagnoses and how this may potentially contribute to brain maturation and neurodevelopment.
Improved haemodynamics and cerebral oxygenation may be a potential mechanism through which antenatal diagnosis modifies neurodevelopmental outcomes in infants with TGA. Specifically, in animal models of the preterm brain, which has similarities to the immature brain of infants with CHD, exposure to cerebral ischemia has been associated with impaired white matter microstructural maturation and altered dendritic arbor and synapse formation in the cortex, even in the absence of visible brain injury.22 Consistent with this, we previously observed that infants with antenatal diagnosis of CHD, including those with TGA, had faster microstructural white matter and grey matter maturation between pre- and postoperative MRIs compared with infants with postnatal diagnosis.8 Thus, we hypothesize that in infants with postnatal diagnosis, who have more hypotension, there may be additional alterations in brain microstructural development contributing to neurodevelopmental outcomes. This would result in a difference in the relationship between TBV and neurodevelopmental outcomes in infants with postnatal compared with antenatal diagnosis, as infants with postnatal diagnosis may have additional microstructural alterations.
Antenatal diagnosis may be associated with improved neurodevelopmental outcomes in infants with TGA by reducing preoperative brain injury. Infants with CHD are at risk for preoperative brain injury including white matter injury, which has been associated with neurodevelopmental outcomes.16, 23 White matter injury results from hypoxic–ischemic injury to pre-oligodendrocytes in the developing white matter.24 Preoperative hypotension and lower preoperative oxygen saturations have been associated with an increased risk of brain injury in infants with CHD.21, 25, 26 Antenatal diagnosis has also been associated with a reduced risk of preoperative brain injury in infants with CHD, which may have been related to more favourable preoperative clinical characteristics including higher oxygen saturations in the subset of infants with TGA diagnosed antenatally.8 Further, in infants with CHD with aortic arch obstruction, prenatal diagnosis was protective for preoperative white matter injury, but only at the site where infants underwent earlier cardiac surgery, suggesting that protective effects may be due to reduced hypoxemia and improved haemodynamics with early surgery.27 In our cohort, we did not observe a difference in brain injury by timing of TGA diagnosis; there were no differences in timing of surgery between infants with antenatal and postnatal diagnosis.
Although rates of antenatal diagnosis of TGA have improved, there is still a significant proportion of infants with TGA diagnosed postnatally with social determinants of health and geographic factors contributing to rates of antenatal diagnosis.3, 17, 28, 29 In a study of antenatal diagnosis rates of TGA in Ontario, a Canadian province with universal health care, there was significant variability among geographical areas, with the highest prenatal detection rates in Metropolitan Toronto and lowest prenatal detection rates in rural Ontario where there is access to fewer health-care providers.3 Lower socioeconomic status has also been associated with lower prenatal detection rates of TGA.29 Social determinants of health are important predictors of brain maturation and neurodevelopment in children, including those with CHD.30, 31 Socioeconomic status may also modify neurodevelopment after brain injury.32 Thus, given associations of socioeconomic status both with antenatal diagnosis rates and with neurodevelopmental outcomes, it is important to explore relationships between socioeconomic status, antenatal diagnosis, and neurodevelopment in future studies to identify potential strategies to promote optimal outcomes more equitably. We did not observe differences in dimensions of marginalization between infants with antenatal and postnatal diagnosis; however, it would be important to examine this with other measures of socioeconomic status.
We observed that the antenatal diagnosis by TBV interaction was significantly associated with cognitive but not motor outcomes, although motor impairments may be more prevalent at 18 months of age. We hypothesize that this may related to the measure of brain maturation we used, TBV, which may reflect global brain maturation. We may have observed associations with cognitive outcomes at 18 months as cognitive assessments at this age reflect overall cognitive function which may involve many different brain regions. However, motor functions involve more localized areas in the brain. Thus, it would be important to assess whether the same relationships are present with regional brain maturation involving structures important for motor development such as the basal ganglia or corticospinal pathways. Interestingly, a recent study observed associations between fetal TBV and 2-year motor, cognitive, and language outcomes in a cohort including multiple CHD lesion types.33 Our findings may differ because of dissimiliarities in study population (mixed CHD cohort vs TGA only) and timing of brain MRI (fetal vs neonatal). It would be of interest to assess whether similar relationships between antenatal diagnosis, brain maturation, and neurodevelopmental outcomes are present in other CHD lesion types.
Although this is the first study, to our knowledge, to examine associations of antenatal diagnosis with TBV and neurodevelopmental outcomes in infants with TGA, it has some important limitations to consider. In our study, we assessed neurodevelopment at 18 months of age. It would be important to assess whether this interaction between antenatal diagnosis and TBV in infants with TGA is associated with long-term neurodevelopment given impairments may become more apparent over time. There was also a significant proportion of infants who did not return for neurodevelopmental follow-up assessments at our centre, which may affect our findings; thus, it would be important to assess whether similar associations are observed across other cohorts. There were differences in timing of brain MRI, with the antenatal diagnosis group being younger at preoperative, but not postoperative, MRI, which may be related to younger birth gestational age and earlier surgery in this group. The differences in timing of brain MRI may potentially be associated with differences in relationships between TBV and neurodevelopmental outcomes between the two groups; however, PMA at scan was included as a covariate in regression models to account for this potential confounder. Finally, the retrospective nature limits our ability to make any conclusions around causation. We were also unable to collect data retrospectively of maternal health conditions for all the infants in this cohort as our centre is an outborn paediatric hospital that does not provide obstetric/prenatal care. It would be important to consider whether prenatal diagnosis altered management of maternal health conditions, and whether this subsequently led to different outcomes in children with antenatal diagnosis of TGA.
CONCLUSIONS
In this retrospective cohort of children with TGA, we examined whether the interaction between antenatal diagnosis and neonatal pre- and postoperative TBV was associated with cognitive outcomes. In infants with postnatal diagnosis of TGA, smaller TBVs were associated with lower cognitive scores at 18 months; however, this relationship was attenuated with antenatal diagnosis, suggesting that this may be protective. Of note, infants with antenatal diagnosis had less preoperative hypotension and underwent earlier cardiac surgery, which may mediate the relationships between antenatal diagnosis, brain maturation, and neurodevelopment through improved hemodynamic state and cerebral oxygenation. However, further studies are needed to understand the mechanisms through which antenatal diagnosis is associated with brain development and neurodevelopment. Regardless, these findings highlight the importance of antenatal diagnosis in optimizing brain development and neurodevelopment in children with TGA; efforts to improve antenatal diagnosis rates are warranted.
ACKNOWLEDGEMENTS
We thank the children and families who participated in this study. The members of the PCNR Study Group are as follows: Jessie Mei Lim, Amandeep Saini, Ingrid Blydt-Hansen, Steven Ufkes, Davide Marini, Ting Guo, Vanna Kazazian, Robert Greer, Mjaye L Mazwi, Pradeep Krishnan, Linh G Ly, Michael-Alice Moga. TS received fellowship support from CIHR Canada Graduate Scholarship – Master's and Doctoral Awards, the SickKids Research Institute Clinician Scientist Training Program, and the Ontario Ministry of Health - University of Toronto Clinician Investigator Program. VC received support from the SickKids Foundation. This study was supported by the Canadian Institutes of Health Research (MOP-142204).
FUNDING INFORMATION
CIHR (MOP-142204), CIHR Canada Graduate Scholarship, SickKids Research Institute Clinician Scientist Training Program, the Ontario Ministry of Health - University of Toronto Clinician Investigator Program, and the SickKids Foundation.
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest relevant to this article to disclose.
Open Research
DATA AVAILABILITY STATEMENT
Data can be made available upon reasonable request and with appropriate data sharing agreements and ethics board approvals in place.