Antireflux metal stent for biliary obstruction: Any benefits?
Abstract
Endoscopic retrograde cholangiopancreatography with stent placement has been utilized as standard palliative management of distal malignant biliary obstruction (MBO). Compared to plastic stents, metal stents can provide longer-term relief of symptoms. When a large-bore metal stent is placed across the ampulla, patients are predisposed to the risk of cholangitis or stent dysfunction due to reflux of duodenal contents. To mitigate the risk of adverse events associated with the duodenobiliary reflux, efforts have been directed to development of antireflux metal stents (ARMSs). The antireflux property has been introduced through adding of an antireflux valve to the duodenal stent end. Evidence from clinical studies indicates that ARMSs may not only reduce the risk of ascending cholangitis during follow-up but also prolong stent patency time. However, the results of clinical studies testing ARMSs are inconsistent owing to heterogeneous designs of antireflux valves and stent bodies. Metal stents are increasingly indicated for benign biliary strictures and MBO in the setting of neoadjuvant chemotherapy, and therefore, research is warranted to evaluate ARMSs for those indications. Given that endoscopic ultrasound (EUS)-guided transmural biliary drainage has gained popularity, the optimal timing of placing an ARMS in relation to EUS-guided and percutaneous drainage should be investigated. Development and evaluation of ARMSs require an integrative approach utilizing phantom and animal models, measurements of stent mechanical properties, and in vivo functional study after stent placement. In this review article, we summarize updated evidence on ARMSs for MBO and discuss issues that should be addressed in future studies.
INTRODUCTION
Distal malignant biliary obstruction (MBO) is caused by periampullary malignancies, including pancreatic cancer, bile duct cancer, and lymph node metastasis. Endoscopic retrograde cholangiopancreatography (ERCP) with biliary stent placement is commonly carried out to palliate symptoms associated with nonresectable distal MBO (e.g., jaundice, cholangitis).1-3 Randomized controlled trials (RCTs) have demonstrated longer patency times of self-expandable metal stents (SEMSs) compared to plastic stents,4-8 and therefore, SEMSs have served as a first-line treatment strategy for patients with nonresectable distal MBO at a large number of centers. However, during long-term follow-up of patients with a biliary SEMS, we encounter recurrent biliary obstruction (RBO) due to SEMS dysfunction. The issue of RBO has been augmented in the era of intensive and effective anti-tumor treatment as survival time of patients with advanced periampullary malignancy has been prolonged.9-12
The RBO after uncovered SEMS placement for distal MBO is caused predominantly by stent occlusion (Fig. 1a).13-15 To overcome adverse events due to stent occlusion, covered SEMSs have been developed with an expectation of inhibiting tumor invasion and reactive epithelial hyperplasia within the stent.16-20 Furthermore, researchers have attempted to prolong stent patency by developing various functional stents including drug-eluting stents, radioactive stents, biodegradable stents, and silver particle-coated stents.21-23 In addition to the efforts to mitigate the risk of stent occlusion, SEMSs have been recently modified with increased conformability within the bile duct, potentially exerting preventive effect on stent migration.19, 24 The duodenobiliary reflux, which is defined as the reflux of duodenal contents into the biliary tree, is commonly observed in patients with a SEMS placed across the ampulla and may contribute to biliary sludge formation and food impaction, and resultant occlusion of SEMSs.25-27 Under these circumstances, stent migration and the duodenobiliary reflux (biliary sludge and/or food impaction) are now two major causes of RBO after covered SEMS placement (Fig. 1b).13, 15 As a mechanism against the duodenobiliary reflux, antireflux properties have been introduced into biliary SEMSs through adding an antireflux valve to the duodenal end of the SEMS (termed “antireflux metal stents [ARMSs]”). Accumulating evidence implicates the effectiveness of ARMSs on prolongation of time to RBO and reduction of post-SEMS cholangitis. In this review, we summarize current evidence and future perspectives of ARMSs for management of pancreatobiliary diseases.
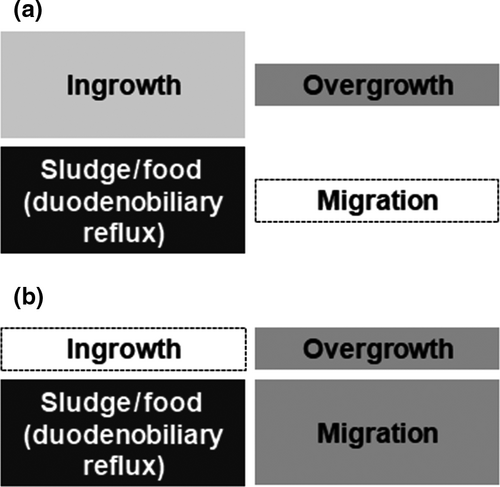
IMPACT OF THE DUODENOBILIARY REFLUX ON BILIARY STENTS
Owing to the duodenal sphincter as a physiological gatekeeper, the biliary system remains sterile in healthy individuals. When a biliary stent is placed across the ampulla, the sphincter function as the mechanical defense mechanism is compromised, potentially provoking the reflux of duodenal contents including duodenal juice and food residue along with contamination of the gut bacteria in the biliary system (Fig. 2).25-27 Microscopic examinations of removed plastic stents suggest that stent occlusion may be provoked based on bacterial biofilms along with dietary fibers and resultant sludge formation.28-31 In an analysis of serial changes of luminal contents in plastic biliary stents, sequential accumulation of bacterial biofilms and biliary sludge was noted.32 Based on these findings, the duodenobiliary reflux has been postulated as a major initiating factor for plastic stent clogging. A clinical study based on barium meal examination after SEMS placement demonstrated the reflux of duodenal contents,26 which has been associated with the formation of biliary stones.33 In a study of patients with a SEMS and a percutaneous catheter in situ, orally-administered radionuclide was detected in the bile obtained through the catheter, suggesting asymptomatic reflux of dietary components.27 In addition to microscopic accumulation of bacterial biofilms, the duodenobiliary reflux of macroscopic food residue may result in occlusion of large-bore stents. In clinical practice, biliary sludge and food impaction as a result of the duodenobiliary reflux have been observed in up to a quarter of patients receiving covered SEMS placement for nonresectable distal MBO (Fig. 2).20, 34-37 Several series suggest that intraductal biliary stent placement without sphincterotomy may reduce the risk of stent dysfunction due to the duodenobiliary reflux and thereby, secure long patency.38, 39 However, in the setting of distal MBO, the distal stent end should often be positioned in the duodenal lumen to fully cover the stricture. Therefore, investigations of antireflux mechanisms for biliary SEMSs may have substantial impact on quality of life among patients with nonresectable distal MBO.
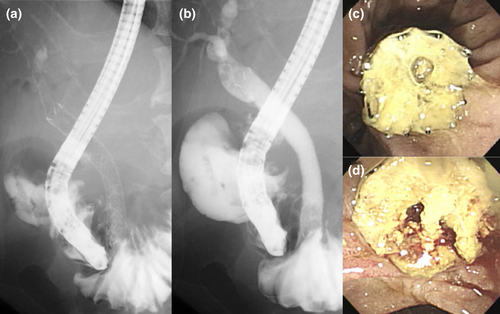
ANTIREFLUX BILIARY STENTS: A DEVELOPING TREATMENT MODALITY
The antireflux mechanism was first examined in esophageal SEMSs to reduce symptoms associated with acid reflux after SEMS placement for the gastroesophageal junction.40-42 Subsequently, antireflux plastic stents harboring a valve against the duodenobiliary reflux at the duodenal end were developed. In a landmark work by Dua et al.,43 an in vitro examination showed that a long windsock-shaped antireflux valve attached to a plastic stent successfully exerted resistance to retrograde pressure while maintaining antegrade bile flow. In a subsequent clinical RCT involving 60 patients with distal MBO, the antireflux plastic stent provided 1.5-fold prolonged patency time compared to a conventional plastic biliary stent (median patency times, 145 days vs. 101 days, respectively).43 However, inconsistent results were reported by subsequent trials investigating the same stent or other types of antireflux plastic stents.44-46
According to the increasing popularity of SEMSs in the setting of nonresectable distal MBO, researchers were motivated to develop ARMSs for this indication. While the designs of antireflux valves in prior studies were heterogeneous,47 ARMSs have been generally associated with lower risks of stent occlusion and non-occlusion cholangitis compared to conventional SEMSs. In 2011, Hu et al.48 reported the first clinical study of an ARMS in patients with nonresectable distal MBO. Following this trial, various types of ARMSs have been developed mainly in Asian countries (Table 1).49-55 For all ARMSs reported, nitinol, an alloy of nickel and titanium, was used for stent walls and if any, anchoring wires for the antireflux valves. Silicone or expanded polytetrafluoroethylene (ePTFE) was used for antireflux valves and if any, covering membranes.
| Author | Year | Antireflux valve | Body | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Shape | Membrane | Support wires | Length, mm | Covered/uncovered | Membrane | Wires | Ends | ||
| Hu et al.48 | 2011 | Hemisphere with a cross-shaped outlet | Silicone | Nitinol | NA |
Partially covered (n = 20) Uncovered (n = 3) |
Silicone | Nitinol | Proximal end flared |
| Lee et al.49 | 2013 | S-shaped | Silicone | Nitinol | 20 | Uncovered | NA | Nitinol | Straight |
| Kim et al.50 | 2013 | Wine glass | Silicone | Nitinol | 10 | Partially covered | Silicone | Nitinol | Both ends flared |
| Hamada et al.51 | 2014 | Funnel | ePTFE | Nitinol | 10 | Fully covered | ePTFE | Nitinol | Straight |
| Hu et al.52 | 2014 | Nipple-shaped with a cross-shaped outlet | Silicone | None | 15 | Partially covered | Silicone | Nitinol | Proximal end flared |
| Hamada et al.53 | 2015 | Long windsock | ePTFE | Nitinol | 22 | Fully covered | Silicone | Nitinol | Both ends flared |
| Lee et al.54 | 2016 | Long windsock | ePTFE | None | 20 | Partially covered | ePTFE | Nitinol | Proximal end flared |
| Hamada et al.55 | 2019 | Funnel | ePTFE | Nitinol | 7 | Fully covered | ePTFE | Nitinol | Straight |
- ePTFE, expanded polytetrafluoroethylene; NA, not available.
ANTIREFLUX METAL STENTS: EVIDENCE FROM CLINICAL STUDIES
Table 2 SUMMARIZES the results of clinical studies that examined ARMSs in patients with nonresectable distal MBO. Overall, the ARMSs have been associated with reduction of stent occlusion and cholangitis occurring on the basis of the duodenobiliary reflux. The antireflux valves reported in the literature include valves that shrink in response to retrograde pressure and closed valves with outlets.
| Author | Study design | No. of patients | Patency (median), months | Recurrent biliary obstruction | Adverse events | ||
|---|---|---|---|---|---|---|---|
| Overall | Causes | Overall | Causes | ||||
| Hu et al.48 | Retrospective, single-arm | 23 | 14 | 6 (26%) |
Migration, 3 (13%) Overgrowth, 2 (8.7%) Ingrowth, 1 (4.3%) |
1 (4.3%) | Bleeding from duodenal tumor, 1 (4.3%) |
| Lee et al.49 | Prospective, single-arm | 32 | 14 | 11 (34%) |
Sludge, 6 (19%) Ingrowth, 4 (13%) Migration, 1 (3.1%) |
0 | – |
| Kim et al.50 | Prospective, single-arm | 5 | 1 | 4 (80%) | Sludge, 4 (80%) | 0 | – |
| Hamada et al.† 51 | Prospective, single-arm | 13 | Not reached | 6 (46%) |
Migration, 4 (31%) Sludge, 1 (7.7%) Unknown, 1 (7.7%) |
0 | – |
| Hu et al.52 | RCT | 56 | 13 | 17 (33%)‡ |
Migration, 5 (9.6%) Ingrowth, 3 (5.8%) Sludge, 2 (3.8%) Overgrowth, 1 (1.9%) Unknown, 6 (12%) |
5 (9.3%) |
Pancreatitis, 2 (3.6%) Cholangitis, 1 (1.8%) Cholecystitis, 1 (1.8%) Bleeding, 1 (1.8%) |
| Hamada et al.† 53 | Prospective, single-arm | 8 | 2 | 4 (50%) |
Sludge, 2 (25%) Migration, 1 (13%) Hemobilia, 1 (13%) |
1 | Cholecystitis, 1 (13%) |
| Lee et al.54 | RCT | 39 | 14 | 7 (18%)§ |
Sludge, 4 (10%) Migration, 3 (7.7%) Valve dysfunction, 2 (5.1%) Ingrowth, 1 (2.6%) Hemobilia, 1 (2.6%) Unknown, 1 (2.6%) |
3 (7.7%) |
Pancreatitis, 2 (5.1%) Cholangitis, 1 (2.6%) |
| Hamada et al.55 | RCT | 45 | 8 | 21 (47%) |
Migration, 14 (31%) Sludge, 6 (13%) Ingrowth, 1 (2.2%) |
9 (20%) |
Cholecystitis, 4 (8.9%) Pancreatitis, 2 (4.4%) Liver abscess, 2 (4.4%) Abscess around the bile duct, 1 (2.2%) |
- RCT, randomized controlled trial.
- † These studies included patients with occlusion of a prior biliary metal stent due to the duodenobiliary reflux, whereas the remaining studies included patients without a previous metal stent.
- ‡ The rates of recurrent biliary obstruction were calculated based on 52 patients who actually underwent stent placement.
- § Stent dysfunction may have occurred for more than one causes.
Following promising results of single-arm pilot studies, Hu et al.52 reported the first RCT comparing an ARMS to a conventional SEMS in patients with nonresectable distal MBO. In this trial involving 112 patients in China, an ARMS harboring a nipple-shaped valve with a cross-shaped outlet successfully reduced the rate of cholangitis after SEMS placement and provided 3-month longer patency time compared to a conventional SEMS (median times, 13 months vs. 10 months, respectively). However, an uncovered SEMS was examined as a comparative group because covered SEMSs were not commercially available during the study period in China, and occlusion due to tumor invasion was a major cause of RBO in the control arm. Therefore, it was not clear how the addition of the antireflux valve contributed to the better clinical outcomes.56 In a RCT reported in 2016, Lee et al.54 first compared an ARMS with a conventional covered SEMS in terms of stent patency. In an analysis of 77 patients with nonresectable distal MBO in South Korea, the ARMS provided 2-fold prolonged patency time compared to the covered SEMS (median times, 14 months vs. 7 months, respectively). Utilizing a dedicated delivery with a supporting bumper, the researchers successfully deployed ARMSs with a quite long windsock-type antireflux valve. Of note, in a proof-of-concept examination utilizing oral barium after SEMS placement, the suppression of the duodenobiliary reflux via the antireflux valve was first demonstrated in the human body.57 In parallel with their efforts, we have sought the effectiveness of an ARMS with a funnel-shaped antireflux valve51, 58 in patients who underwent covered SEMS occlusion due to the duodenobiliary reflux and thus, were considered to be at higher risk of recurrent occlusion after a reintervention.59-61 In addition, our previous pilot study demonstrated the feasibility and safety of the new ARMS as a first-line SEMS for nonresectable distal MBO.58 Based on the promising results of those pilot studies, we conducted an RCT to compare the ARMS with a conventional covered SEMS.55 A major strength of this trial was the use of a conventional covered SEMS harboring the same structure of the stent body. We enrolled 104 patients with nonresectable distal MBO at 11 tertiary care centers in Japan. In our primary hypothesis testing, time to RBO did not differ by the stent type. The median times to RBO were 251 days and 351 days in the ARMS and covered SEMS groups, respectively. The rates of RBO due to the duodenobiliary reflux (biliary sludge or food impaction) were comparable between the groups. Those findings were consistent with the results of a prior retrospective study demonstrating that this type of ARMS was not associated with longer time to RBO compared to a conventional covered SEMS.62 The current ARMS was prone to stent migration during the follow-up period, and it was noteworthy that a substantial number of ARMSs migrated proximally. We therefore speculate that the attachment of the antireflux valve to the duodenal end might inhibit full expansion of this end, potentially resulting in the inward migration. The addition of antimigration properties (e.g., flared end structure, anchoring fins) should be considered before the use of the current ARMS as a first-line treatment option for nonresectable distal MBO can be justified.
In a meta-analysis of those three RCTs, ARMSs were associated with a lower rate of stent occlusion compared to conventional SEMSs, and the rates of clinical success, technical success, and adverse events were comparable between the groups.63 Therefore, ARMSs as a whole are considered an effective alternative to conventional SEMSs for nonresectable distal MBO. However, the designs of antireflux valves were considerably heterogeneous between the studies, and hence, the results of those ARMS studies should be interpreted individually.
The antireflux mechanism has been recently introduced into the lumen-apposing metal stent (LAMS), which is generally utilized for management of acute cholecystitis as well as pseudocyst and walled-off necrosis associated with severe acute pancreatitis.64-70 A clinical study supports the feasibility of an antireflux LAMS with a S-shaped valve for treatment of those complications of pancreatitis.71 Given that endoscopic necrosectomy was additionally carried out after stent removal in this study, modification of the LAMS with a retrievable valve may accelerate the use of this LAMS in this setting.49
IN VITRO MODELS FOR PRECLINICAL EVALUATION OF ANTIREFLUX VALVES
During the developmental process of ARMSs, we should examine designs and materials of the antireflux valve and stent body (Fig. 3). In this setting, experimental data would help to test the function (particularly, resistance to retrograde pressure) and durability of the antireflux valve, potentially providing insights into further modifications. Animal models have been utilized to evaluate not only feasibility of drug-eluting biliary SEMSs72, 73 and biodegradable biliary stents,74, 75 but also antireflux properties of ureteral stents.76-78 However, there has been a lack of data on ARMSs from animal and phantom studies due to the limited access to those experimental systems.
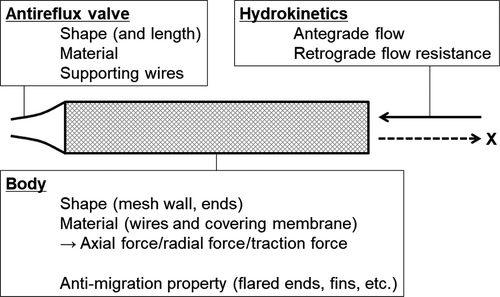
The in vitro model utilized to evaluate antegrade and retrograde flows in ARMSs consisted of a stent holder, flow controllers, and manometers.43, 47, 50 As rates of fluid perfusion were maintained by the flow controller, flow resistance (antegrade and retrograde, separately) was measured via the manometer. This model provided valuable information for determination of the valve design and facilitated clinical use of antireflux biliary stents. Nonetheless, it should be noted that the results of in vitro studies do not necessarily correlate with the performance of the ARMSs in the real world.50 In addition to the hydrokinetics in ARMSs, Kwon et al.47 examined the durability of various types of ARMSs. The researchers customized a bile-flow phantom model and a duodenal pH environmental model, and evaluated long-term (up to 4 months) changes in morphological and functional changes in antireflux valves. In an analysis of our ARMS, the antireflux valve made of ePTFE was prone to degradation owing to the low pH environment (mimicking the duodenal environment), potentially impeding antegrade bile flow.47 This finding may correlate with collapsed valves noted in dysfunctional ARMSs in our clinical study.55
UNMET NEEDS IN ANTIREFLUX METAL STENTS
First, target populations for ARMS placement remain to be elucidated.79 It is of considerable importance to identify individuals who are at high risk of stent dysfunction due to the duodenobiliary reflux and thus may obtain most benefits from ARMSs. Patients with stent dysfunction related to the duodenobiliary reflux tend to undergo a similar biliary event due to the same etiology after subsequent SEMS placement.61 Patients with duodenal tumor involvement are another subgroup for which ARMSs can be an effective management option of biliary decompression.80 For anatomical reasons, patients with distal MBO often develop duodenal tumor involvement at cancer diagnosis or during the follow-up period,81-84 which has been associated with the risk of early dysfunction of SEMSs for distal MBO.80, 85 This association may be explained by reduced peristalsis and narrowed duodenal lumen in this population. The risk can be further increased when duodenal SEMSs are placed for high-level duodenal tumor involvement.85
Second, a further investigation is warranted to clarify the optimal timing of ARMS placement during the life-time clinical course of patients with nonresectable distal MBO. This investigation should be based on refined estimations of survival times of patients.86, 87 Patients with advanced duodenal tumor involvement may be good candidates for not only ARMSs but also endoscopic ultrasound (EUS)-guided transmural biliary drainage, which can be achieved through hepaticogastrostomy or choledocoduodenostomy.88-91 Emerging evidence indicates the feasibility of EUS-guided biliary drainage not only as a salvage procedure after failed ERCP but also as a first-line treatment option for nonresectable distal MBO.92-96 In addition, the preliminary results of clinical studies suggest that EUS-guided drainage may be less susceptible to increased duodenobiliary reflux pressure and thereby provide longer or equal patency times of biliary stents compared to the conventional transpapillary approach.97, 98 Another strength of EUS-guided biliary stent placement is that endoscopic reinterventions may be more feasible because the approach to dysfunctional biliary stents is possible irrespective of the presence of duodenal obstruction. On the other hand, EUS-guided biliary drainage may not be feasible for a fraction of patients, including those with massive ascites, varices, or non-dilated bile duct.83, 99, 100 Percutaneous biliary drainage may maximize the intensity of chemotherapy through securing continuous monitoring of bile discharge and should be considered in cases with refractory cholangitis.101, 102
Third, mechanical properties have not been examined in ARMSs. The mechanical properties that should be evaluated during the developmental process include axial force and radial force.103-105 Axial force has been defined as straightening force of a bent stent103 and has been implicated in the risk of SEMS-related adverse events including pancreatitis and cholecystitis.106, 107 Radial force, defined as expanding force in a radial direction,103 has been associated with the risk of biliary SEMS migration.108 Another consideration should be given to traction force, which is defined as the resistance force required to manipulate a stent delivery while releasing a metal stent.109, 110 In the setting of ARMSs, the attachment of the antireflux valve to the SEMS might increase the traction force because the part of the antireflux valve in the stent delivery is mechanically weaker compared to the adjacent portions and thus, tends to be bent during stent deployment. Indeed, when examining the feasibility of an ARMS with flared ends, we experienced technical difficulties in deploying the stent due to increased traction force, which inhibited clinical use of this stent.53
Fourth, the effectiveness of ARMSs for specific indications other than for nonresectable distal MBO remains unclear. Temporary placement of a covered SEMS is recently utilized for a variety of benign and malignant biliary diseases including carcinomas treated by neoadjuvant chemotherapy,111, 112 benign biliary strictures,113, 114 and bilio-enteric anastomotic strictures;115 therefore, the usefulness of ARMSs can be explored for those indications. In addition, ARMSs may be utilized in the setting of EUS-guided transmural biliary drainage.116
Fifth, further research is needed to compare patients’ quality of life and cost-effectiveness between ARMSs and conventional covered SEMSs.111, 117-119 Quantitative estimation of quality of life is challenging, but may be done based on questionnaires, the number or duration of hospitalizations, the number of reinterventions, etc.93
Finally, there has been no standardized reporting system for evaluation of SEMS outcomes. The standardization would increase the comparability of results of clinical studies.120, 121 The lack of the standardization has decreased the comparability of the reported studies and evidence levels of meta-analyses.122, 123
CONCLUSIONS AND FUTURE PERSPECTIVES
We presented the recent progress of ARMSs for nonresectable distal MBO. Management of distal MBO is crucial to maintain quality of life and secure administration of intensive chemotherapy among patients with aggressive periampullary malignancy. Various types of ARMSs have been developed with promising results of clinical studies, and ARMSs may have the potential of a first-line treatment modality for nonresectable distal MBO as well as that of a salvage procedure for refractory cases. Given the heterogeneity in the designs and clinical outcomes of the ARMSs, these stents should be evaluated individually for the clinical use. It is of considerable importance to identify target populations and optimal timing for ARMS placement in relation to EUS-guided or percutaneous biliary drainage. Indications of covered SEMSs have been expanded to a variety of benign and malignant biliary diseases and hence, the effectiveness of ARMSs should be examined for those specific indications.
Conflicts of Interest
Dr. Nakai declares research funding from Boston Scientific, Century Medical, and Fujifilm. This work was not supported by any of those companies. Dr. Nakai serves as an Associate Editor in Digestive Endoscopy. The other authors declare no conflicts of interest related to this article.
Funding Information
This work was supported in part by a multicenter research grant from the Japanese Foundation for Research and Promotion of Endoscopy. The funder had no role in data collection and analysis, decision to publish, or preparation of the manuscript.