Medical countermeasures during public health emergencies—Does information sharing among health system coalition help?
Abstract
Challenged by constrained healthcare resources, hospitals encounter barriers to accommodating patient demand during public health emergencies. Building on a comprehensive literature review of information and knowledge exchange in healthcare, disaster management, and humanitarian operations management, this study explores the influence of treatment-based medical countermeasures (T-MCM) on ICU bed utilization during the Covid-19 pandemic, investigating whether participation in a regional health registry (HReg) facilitates better management of care capacities. We use a difference-in-differences approach with propensity score weighting to analyze a panel dataset of 735 observations from Michigan hospitals, finding that health systems that used T-MCMs experienced modest reductions of 1.8% of ICU bed utilization. However, the reduction increased by an additional 27.1% among hospitals that participated in the HReg. Hospitals that used T-MCMs without HReg participation experienced a 10.8% increase in ICU bed utilization, suggesting resource overload. These results underscore the importance of adaptive learning and strategic investment in information-sharing infrastructures to optimize healthcare delivery during crises. We discuss theoretical, managerial, and policy implications when managing healthcare capacity during pandemics.
1 INTRODUCTION
Hospitals find accommodating demand due to public health emergencies (PHEs) difficult due to limited healthcare resources and capacities. One infectious disease expert from a Midwest hospital we interviewed during the aftermath of Covid-19 remarked, “Operating under scarcity, we would develop paradigms of priority to treat certain groups of patients before others,” highlighting difficult choices when treating patients in a hospital. The Food and Drug Administration (FDA) authorizes several medical countermeasures to contain PHEs, classified broadly into treatment-based medical countermeasures (T-MCM) and preventative medical countermeasures. T-MCMs reduce incidences, and preventative countermeasures reduce the spread of illnesses during PHEs.1 We assess the former.
When experiencing acute public health crises, hospitals (i.e., health systems) commonly use T-MCMs. The H1N1 outbreak represented a novel context for medical professionals to use oseltamivir and zanamivir to treat infected patients (Rewar et al., 2015). During outbreaks of SARS and MERS, clinicians used novel treatments such as monoclonal antibodies and protease inhibitors to care for patients in the absence of specific antiviral treatments (Nascimento Junior et al., 2020). More recently, Remdesivir was used to treat Covid-19 patients. Humanitarian relief operations also increasingly use novel technologies, such as unmanned aerial vehicles for search and rescue, medical cargo delivery, and emergency mapping in areas without infrastructure (Rabta et al., 2018).
Such contingencies, or their specific uses, are often unapproved, requiring permission to administer T-MCMs under emergency use authorizations when potential benefits of such treatments outweigh potential risks. Inherent uncertainties (e.g., correct timing during care or exact titers of the drug to use according to patient characteristics) add to the complexity of hospitals’ healthcare delivery during PHEs. Adaptive learning regarding treatment administration risks and efficacy is necessary to manage T-MCM treatment, and thus health systems typically invest in various external information structures to acquire treatment knowledge from peer institutions to help physicians learn about T-MCMs as they continue to administer treatments. One such structure forms when an external organization collects patients’ clinical and demographic data in a health registry (HReg) and subsequently shares information among participating hospitals in pursuit of insights into treating patients. Similar such dissemination occurs during humanitarian operations, when cluster leads act as information hubs, filtering and sorting data to allow quicker, more effective communication (Altay & Pal, 2014).
- RQ1. Whether a health system can leverage its participation in a regional HReg to provide better care capacity during a PHE.
- RQ2. Whether health systems that use T-MCMs manage care capacity better during a crisis by learning adaptively from HReg participation.
Panel data of 735 observations were compiled for this study. Because the effectiveness of T-MCM is characterized by treatment type, we consider convalescent plasma therapy (CPT) that Michigan health systems used as an example of T-MCM. We use difference-in-differences (DiD) with propensity score weighting to create a baseline of CPT's efficacy at managing ICU bed utilization due to Covid-19 hospitalizations.2 We observe this outcome because the World Health Organization recommends CPT use in severe and critical Covid-19 patients.3 To operationalize HReg, we utilize clinical data of Covid-19 patients admitted to approximately 40 hospitals in Michigan that participated in Mi-COVID19, a regional HReg coordinated by the Hospital Medicine Safety Consortium (HMS). Results suggest that health systems using T-MCMs experienced a modest decrease of 1.8% of ICU bed utilization. This impact decreased an additional 27.1% when a health system participated in the HReg. Results also suggest that T-MCM hospitals that did not participate in the HReg experienced a 10.8% increase in bed utilization. Health systems that meaningfully integrated data from HReg extracted benefits from the T-MCM, and those that could not worsened, suggesting resource overload.
This study contributes to disaster recovery management literature (Altay & Pal, 2014; Green & Kolesar, 2004; Minje et al., 2022) by examining the effect of information dissemination among (possibly competing) agents during a public health crisis, and how agents improve countermeasures to fight the crisis. We demonstrate the adaptive learning abilities of humanitarian organizations when they adopt new disaster management mechanisms, highlighting information exchanges between peer organizations when improving the efficacies of such mechanisms. This study also emphasizes that organizational leaders can benefit from information dissemination if they use information from the initiative, advocating investment in operational slack that allows organizations to learn from accrued external information.
2 LITERATURE REVIEW AND HYPOTHESES DEVELOPMENT
2.1 Literature review: information exchange
According to organizational information processing theory (OIPT), organizations that operate during disasters and public emergencies rely on various information-sharing structures to maintain performance (Tatikonda & Rosenthal, 2000). Healthcare organizations leverage information-sharing to ensure timely, equitable access to essential services, optimize limited resources, and strengthen resilience against future crises. Their focus extends beyond financial metrics, centering on saving lives, alleviating suffering, and protecting vulnerable communities. Following the 2010 earthquake in Haiti, the United Nations reported that approximately 2000 (often competing) organizations and agencies collaborated on-site, coordinating critical information and resources to benefit the population (Altay & Pal, 2014).
Organizations that boost their resilience through information-sharing concentrate on internal stability, adaptability, and sustained viability, unlike humanitarian and healthcare organizations, and thus the urgency, purpose, and willingness to share information differ among these types of organizations. Because we investigate organizational response and public emergency management in a healthcare context, we conduct a comprehensive literature review of information exchange in disaster management, humanitarian operations, and healthcare management in the top OM journals. We consider all the OM/OR journals in the SCMList, Financial Times 50 Journal List, UTD Journal List, and ABS (4 and 4× only) list. We find 12 journals in our search process.4 We employ a systematic search strategy using the SCOPUS database. We aim to identify peer-reviewed journal articles in business and management that focus on exchanging, transferring, or sharing information or knowledge within contexts such as disaster recovery, disaster management, humanitarian operations, and healthcare.5 We found 375 articles in the process. The search outcome statistics are provided in Table A2. We then reviewed the articles to filter for knowledge or information exchange within or between organizations and identified 40 articles relevant to our study. We provide a detailed review of these articles in Table A3.
Extant research identifies information exchanges between entities as enablers of organizational performance (Adepoju et al., 2023; Fangwa et al., 2024; Jiang et al., 2024). Clear, detailed exchanges between outpatient clinics and patients reduce patient no-shows (Jiang et al., 2024), and feedback loops between policymakers and organizations improve operational efficiency and outcomes (Fangwa et al., 2024). Information exchanges also enhance trust and coordination on service delivery teams (Agha et al., 2022; Drupsteen et al., 2013; Stevens & van Schaik, 2020) and mediate relationships between technology implementation and organizational performance (Dobrzykowski & Tarafdar, 2015, 2017).
Hospitals invest in health information technologies (e.g., EMR, HIT, and health information exchange [HIE]) to improve information flows both within and across healthcare organizations. HIT literature espouses technology in improving information flows between organizational departments (Dobrzykowski & Tarafdar, 2015), resulting in improved adherence to care guidelines and quality (Oh et al., 2018). HIE literature evidences that technology promotes patient interoperability by reducing duplicate procedures (Atasoy et al., 2021; Ayabakan et al., 2017; Li et al., 2022) and improving both operational efficiency (Rahurkar et al., 2015) and patient care quality (Ayer et al., 2019; Janakiraman et al., 2023). Other research investigates poor use of HIE (Vest, 2009) and its negative hospital revenue implications (Frisse & Holmes, 2007; Frisse et al., 2012).
During PHEs, healthcare organizations must reach beyond boundaries to identify alternative care paths and effective treatments. HIE enables them, but many hospitals rely on HRegs to access critical data because disparities exist between how these technologies function. HRegs aggregate public health information from hospitals to develop evidence-based, best patient care. Unlike HIE, which depends on hospital EHRs and requires substantial planning and infrastructure, third parties typically manage HRegs, assembling and disseminating information quickly, though with data security concerns. HIEs often mandate patient consent before sharing identifiable health data, whereas HRegs provide insights into efficacy of clinical procedures of patients without requiring extensive patient-specific details (Gliklich et al., 2014; Chapter 1). HRegs can thus more readily form immediate peer groups among participating hospitals, enabling them to share insights and learn from each other quickly.
Literature that examines how external facilitators enable information dissemination among participating and often competing organizations remains limited. The few studies that do either define facilitators’ characteristics (Akhtar et al., 2012; Quarshie & Leuschner, 2020; Stieglitz et al., 2022) or assess dynamics of information dissemination (Altay & Pal, 2014; Lusiantoro & Pradiptyo, 2022; Yoo et al., 2016). Lusiantoro and Pradiptyo (2022) identify five communications that enable supply chain resilience during public emergencies. Altay and Pal (2014) use an agent-based simulation to show that information quality is essential to resource use and that facilitators’ roles change during the relationship. Mura et al. (2012) demonstrate that practitioners’ knowledge-sharing propensities drive practitioners’ innovative behaviors. No empirical study uses secondary data to examine how third-party-enabled information-sharing impacts organizations’ emergency response performance or helps organizations develop novel mitigations.6 Our context is noteworthy because unlike humanitarian organizations, we suspect that hospitals share little inaccurate information with the registry, though it is unclear whether resource-constrained hospitals participate in the initiation or use of information to improve care during a PHE. This study is thus the first to use secondary data to investigate whether HReg participation creates value regarding how hospitals manage care capacities, and whether hospitals use information to improve untested PHE countermeasures.
2.2 Hypotheses development
2.2.1 Effect of T-MCM on ICU bed utilization
T-MCMs require new processes and involve significant operational changes to existing healthcare (Menor et al., 2002) that inherently change process complexities (either decrease or increase), affecting caregivers’ understanding of the processes and how they execute modifications (Bala & Venkatesh, 2013). Bala (2013) defines three types of process complexity—component, coordinative, and dynamic. Component complexity refers to changes to a process's elements. For example, T-MCMs might simplify care delivery by removing process elements. Tong et al. (2016) found that using a tissue plasminogen activator treatment reduces hospital acute care times, streamlining the process, though such changes also introduce uncertainties, such as unclear evidence of efficacy or a need for new process understanding, particularly during PHEs. Predicting whether T-MCMs reduce or increase component complexity in new operational changes is thus challenging.
Coordinative complexity refers to the interconnectedness of operational work processes, including relationships among activities, information flows, resource allocations, and task sequencing (Wood, 1986). T-MCMs are designed to simplify care processes. By reducing reliance on large teams of care practitioners, they allow hospitals to scale treatment delivery and manage care capacities better during increased demand. However, implementing T-MCMs often requires practitioners to depend heavily on interconnected systems. For example, countermeasures require coordination with external agencies for approvals and resources (Tobian et al., 2022), a process that increases the complexities of delivering care, undermining intended efficiencies.
Dynamic complexity captures the unpredictability of new work processes. T-MCMs have greater dynamic complexities because they include unapproved treatments or uses of treatments. For example, CPT offered an innovative way of treating Covid-19 patients, but it also included challenges such as determining accurate antibody titers and treatment timing (Sahu et al., 2020). Convergence of clinical evidence requires time, and health systems often do not have a priori knowledge of the efficacy and correct use of countermeasures.
Predicting the impact of T-MCMs on process complexities presents a significant challenge. While it is possible to anticipate an increase in dynamic complexity when introducing a new T-MCM administration process, the overall effect on a hospital's component and coordinative complexity remains uncertain. This uncertainty depends on several factors, including (but not limited to) the specific type of countermeasure being implemented, the healthcare system's understanding of the associated processes, and the resource constraints faced during an emergency. Moreover, because it is unclear how each type of complexity contributes to the hospital's overall system complexity, the impact of T-MCM implementation—both in terms of direction and magnitude—on cumulative system complexity remains uncertain. Because extant research has demonstrated an association between process complexities and organizational capacity management (Li et al., 2021; Vickery et al., 2016), whether hospitals can use T-MCMs to effectively manage bed utilization during a PHE remains an open question, as the interplay between these factors is not yet fully understood. We thus test countervailing baseline hypotheses:
H0a.T-MCMs introduced during a public health emergency associate negatively with ICU bed utilization related to Covid-19 patient hospitalizations.
H0b.T-MCMs introduced during a public health emergency associate positively with ICU bed utilization related to Covid-19 patient hospitalizations.
A hospital's goal is lowering ICU bed utilization related to Covid-19 patient hospitalizations because it wants to reduce nosocomial transmission and open acute care capacity to accommodate care needs.
2.3 Effect of participation in HReg on ICU bed utilization
We have been getting reports from ABC hospital that they were using regular masks plus face shields and not seeing any uptick in employee infections. This information helped us streamline our PPE usage…. I was as collaborative as possible with my infectious disease colleagues (content medical experts) across the state and relied greatly on their expertise for the decision to be made…over the course of the pandemic, I became part of the web of knowledge sharing that developed organically.
Disaster management literature (Altay & Pal, 2014; Moshtari, 2016) supports this opinion by evidencing that interorganizational information exchanges allow organizations involved in human relief to access critical resources. Access to privileged resources helps organizations remove redundant effort (Ruesch et al., 2022) and strengthens service capacities (Altay & Pal, 2014). Therefore,
H1.A health system's HReg participation during a public health emergency associates negatively with ICU bed utilization that relates to Covid-19 patient hospitalizations.
2.4 Moderation by HReg
OIPT further suggests that information-sharing is critical to performing uncertain tasks that are associated with new treatment procedures (Tatikonda & Rosenthal, 2000). HRegs provide patient-level, nonexperimental, descriptive, and qualitative information to hospitals. The strength of clinical evidence ranges from meta-analyses of randomized clinical trials at the top to anecdotes and opinions at the bottom. Tucker et al. (2007) argue that nonexperimental and qualitative studies, such as information gathered from HReg, lay between these extremes. These descriptive findings offer healthcare providers strong evidence of the clinical efficacy of the T-MCM, demonstrating its ability to facilitate hospitals to refine treatment protocols, optimize resource allocation, and expedite patient care at the accelerated pace demanded by a PHE (Altay & Pal, 2014).
Thus, HReg expedites participating hospitals’ collective learning process about evolving treatment procedures and effectiveness-enhancing modifications of countermeasures while avoiding those that have proven ineffective for patients, which may ameliorate components and dynamic complexity (Altay & Pal, 2014). For instance, the Swedish Hip Arthroplasty Register enabled clinicians to identify prostheses with higher failure rates, ultimately discontinuing underperforming implants for the current treatment processes (Kärrholm, 2010). Faster information sharing across organizations reduces coordinative complexities by identifying redundancies and mitigating interconnected challenges of these countermeasures (Ruesch et al., 2022). Extant healthcare management literature discusses knowledge-sharing attitudes as enablers of innovation successes (Mura et al., 2012).
Consequently, we believe HReg facilitates faster peer-to-peer learning among hospitals that may help them quickly adjust protocols and identify redundancies in new treatment processes during a PHE. Thus,
H2.The negative association between treatment-based medical countermeasures and ICU bed utilization that relates to Covid-19 patient hospitalizations is strengthened when a health system participates in health registry during a public health emergency.
3 BACKGROUND AND EMPIRICAL STRATEGY
3.1 T-MCM: the case of convalescent plasma therapy
A T-MCM's efficacy and outcome are characterized by the type of treatment that health systems use. The Mayo Clinic facilitated use of CPT through the Expanded Access Program since the FDA approved it on April 3, 2020, and between then and August 23, 2020, several Michigan health systems administered the CPT to enrolled patients with Covid-19 symptoms in ICUs (Joyner et al., 2020).8 We analyze use of CPT by Michigan healthcare systems as an example of T-MCM application by these systems.
Extant studies of the clinical effectiveness of CPT report mixed findings. Many suggest benefits, such as lower 28-day mortality rates, improvements to clinical statuses, and lower risks of death (Ateş et al., 2021; Joyner et al., 2021; Kober et al., 2022). Some that use randomized control trials (RCTs) report positive clinical outcomes of CPT (O'Donnell et al., 2021; Salazar et al., 2021), and yet others that also use RCTs and exploratory analyses report nonsignificant effects of CPT (Gharbharan et al., 2021; Simonovich et al., 2021). One of 24 open-label RCT studies (Bégin et al., 2021) found that a CPT treatment group, comprising general and acute care patients, reports serious adverse effects. A literature review of research that investigates CPT effectiveness appears in Table A1. Extant literature suggests that treatment efficacy depends on factors such as antibody titers and disease stage, requiring ongoing physician learning. We do not examine the clinical effectiveness of CPT, instead assessing it as an example of T-MCM use to understand how it influences, directly and in conjunction with HReg, the operational outcome of ICU bed utilization. We tracked the date when each Michigan health system began using CPT, finding that four began before May 7, 2020, another four after that date, and the remaining seven not using it during treatment of Covid-19. Introduction of CPT across time allows study of how variance in T-MCM use influences intensive care capacity between using and non-using health systems.
3.2 HReg: case of Michigan Covid-19 HReg (Mi-COVID19)
The Agency for Healthcare Research and Quality defines an HReg as an “organized system that uses observational study methods to collect uniform data (clinical and other) to evaluate specified outcomes for a population defined by a particular disease, condition, or exposure, and that serves one or more stated scientific, clinical, or policy purposes” (Gliklich et al., 2014; Chapter 1). Physicians and specialists share (occasionally, directly from electronic health records) patients’ demographics, medical diagnoses, treatments, vital signs, and outcomes when enrolling them in a registry, and law mandates that all registries maintain strict privacy protections. The registry shares amassed data with participating physicians, hospitals, and healthcare organizations to evaluate treatment effectiveness and improve care (American Heart Association, 2022).
To understand the spread of the virus and its influences across communities, HMS coordinated an HReg (Mi-COVID19) to collect demographic data on all patients in Michigan who tested positive or had a discharge code for Covid-19, with health departments, academic institutions, and health organizations contributing to the registry. On April 2, 2020, Mi-COVID19 was available online, beginning with 20 volunteer hospitals, but by the end of the month, more than 40 hospitals representing 10 health systems were contributing, which based our data collection. The purpose of the registry was to identify factors that led to critical Covid-19 occurrences and outcomes; identify patient characteristics, care practices, and treatments that associated with improved outcomes; understand long-term complications for hospitalized patients; identify variability in care; identify processes that associated with better outcomes; and provide health care providers (HCPs) with frameworks for improved care (HMS, 2020). Mi-COVID19 required uploading anonymized patient-level data, with participants granted exclusive rights to access the registry to analyze and identify care processes that fit with patients’ demographics. Participants could also request analyses. The exchange held webinars to share findings and inferences by analyzing shared data, knowledge that was critical to managing care delivery.9
3.3 Data
We analyze health systems as a unit of analysis. Michigan Department of Health and Human Services (MDHHS) mandates that health systems report patient census and Covid-19 treatment units. Health systems report census data biweekly every Monday and Thursday. We began data collection on May 7, 2020, following the CDC's practical guidelines for safeguarding healthcare personnel, patients, and communities against Covid-19. The initial dataset comprised information on ICU bed utilization and admission of Covid-19 patients to ICUs until November 12, 2020. On November 16, 2020, MDHHS ceased reporting system-level data. Across 49 periods, we gathered data twice weekly from all 19 health systems in Michigan. A second dataset was sourced from ESRI's geospatial cloud, comprising hospital bed capacities and county-level demographics for 6090 hospitals. We refined it to isolate information on 106 hospitals that affiliated with the 19 health systems in Michigan. We calculated the aggregate number of ICU beds, average staffed beds in each health system, and mean demographics across the counties in which the hospitals were located. To examine the influence of T-MCM and HIE coalitions on ICU bed utilization for Covid-19 patient hospitalizations, we controlled for several variables. The dataset combined news articles and health system data from January 1, 2020, to November 20, 2020. We integrated this information with preexisting datasets, excluding four single-hospital health systems due to variations in characteristics. Robustness analyses included the complete dataset, and primary analysis included 15 health systems. Appendix B details the entire data collection.
4 Measures
4.1 ICU bed utilization related to Covid-19 patient hospitalization
ICU bed utilization related to Covid-19 patient hospitalizations is not overall ICU bed utilization. Variable ICUUtilCOVIDit was developed by considering the ratio of the total number of Covid-19 patients admitted to ICUs to the total number of ICU beds in the system. ICUUtilCOVIDit has high dispersion, ranging from 0% to 69%, with mean and standard deviation of 10.4% and 9.7%, respectively. A high proportion of ICU beds occupied by Covid-19 patients resulted in a smaller number of ICU beds for other patients, which also increased the probability of infection in ICUs from patients with viremia. From a health system perspective, a lower value for ICUUtilCOVIDit helped provide the intensive care that patients needed while keeping hospital infections low.
4.2 T-MCMs adoption
The T-MCM of the CPT is a binary variable (TMCMit) that captures whether a system used CPT during the Covid-19 pandemic. TMCMit is zero if system i did not use CPT at time t, and 1 if system i began using plasma therapy at time t. Four health systems had TMCMit = 1 across all 49 periods, 4 with TMCMit = 1 for some periods, and 7 systems that did not administer the treatment across all periods.
4.3 Participation in HReg
InfoExchCoalitioni represents the extent of participation in Mi-COVID19, measuring the percentage of hospitals that affiliated with a health system that participated.
4.4 Control variables
For each health system i and time t, we accounted for new Covid-19 cases (AvgDemandit) in a county in which a health system was located. If hospitals in a health system were spread across multiple counties, we considered average Covid-19 cases in those counties. We controlled for time-fixed effects to account for evolving patterns of the dependent variable over time. We used health system-specific variables to match the systems. For example, we used infection prevention and control (IPCit) practice to control for time-varying health system characteristics, and average number of staffed beds in a system (AvgStaffedBedsi) and total number of affiliated hospitals in the system (AffiliatedHospitalsi) to control time-invariant health system characteristics. We also considered average county populations (AvgPopulationi) to control for county-fixed effects. Summary statistics and correlations among the variables appear in Table 1.
| Variable | Mean | SD | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|---|---|
| 1 ICUUtilCOVIDit | 0.10 | 0.10 | – | ||||||
| 2 InfoExchCoalitioni | 0.67 | 0.47 | 0.08* | – | |||||
| 3 ServiceInnovationit | 0.44 | 0.50 | 0.05 | 0.18* | – | ||||
| 4 AvgDemandit | 189.54 | 297.52 | 0.26* | 0.16* | 0.26* | – | |||
| 5 IPCit | 7.377 | 4.78 | 0.07 | 0.42* | 0.29* | 0.04 | – | ||
| 6 AvgStaffedBedsi | 163.09 | 86.52 | 0.26* | 0.42* | 0.47* | 0.56* | 0.24* | – | |
| 7 AffiliatedHospitalsi | 6.80 | 3.41 | 0.14* | 0.29* | 0.34* | 0.02 | 0.49* | 0.33* | – |
| 8 AvgPopulationi | 486,261 | 540,946 | 0.30* | 0.22* | 0.29* | 0.67* | −0.04 | 0.81* | 0.003 |
- * p < 0.05.
4.5 Econometric approach
Our estimation approach addresses two potential issues in the data. First is the possibility of unconditional heteroskedasticity across health systems, which must be modeled explicitly. Second, disparities in the care capacity of health systems in a region, and their competing objectives, can induce correlations in error terms. We use generalized least squares panel regression for estimation because it allows unrestricted modeling of heteroskedasticity and correlation across health systems (Gao & Hitt, 2012; Wooldridge, 2010). We include time controls so that results are robust to specification errors that might derive from time-dependent effects that are common to all health systems. We estimated the model using the xtgls command in STATA 15. We follow Hu and Hoover's (2018) methodology to conduct power analysis, with results suggesting that the sample provides sufficient power to reject null findings at a threshold of 80%. The estimation models consider robust inference clustered around unique health system IDs to address health system-level heteroskedasticity (Wooldridge, 2010).
4.6 Identification strategy: treatment effect of T-MCM on ICU bed utilization
We use DiD with propensity score weighting, which addresses potential endogeneity that associates with a health system's use of a T-MCM (e.g., choosing a T-MCM due to location demographics). We divided the data into treatment and control groups, with the treatment group consisting of the eight health systems that used the T-MCM, and the control group consisting of the seven that reported not using the T-MCM. Four used the CPT throughout the analysis period, and another four started using the therapy some time during. Identification was enabled by interventions created by unique variations in treatment and control groups. To discern intended effects of the T-MCM, we used propensity score weighting to address selection bias in both groups’ observations, ensuring that the groups were comparable before treatment occurred. We used health system-specific variables to match the systems. Table 2 reports summary statistics of the variables used in the treatment and control groups’ analyses. The Table shows that use of the T-MCM might have been endogenous to demand and demographics. For example, the average population in counties in which treatment group health systems were present was 660,507, and that in which the control group systems were present was 348,139. The systems might have used the therapy because larger populations might have increased demand for critical care.
| Variable | Group | ||
|---|---|---|---|
| Treatment | Control | Total | |
| ICUUtilCOVIDit | 0.109 (0.079) | 0.09 (0.11) | 0.104 (0.097) |
| AvgDemandit | 275.98 (375.9) | 121.01 (190.47) | 189.54 (297.52) |
| IPCit | 8.95 (2.95) | 6.12 (5.53) | 7.37 (4.78) |
| AvgStaffedBedsi | 208.83 (93.69) | 126.83 (59.14) | 163.09 (86.52) |
| AffiliatedHospitalsi | 8.09 (3.54) | 5.78 (2.92) | 6.8 (3.41) |
| AvgPopulationi | 660,507.1 (604629.7) | 348,139.8 (438515.5) | 486,261.4 (540,946.4) |
- Note: Standard deviations appear in parentheses.
The propensity score is the probability that an observation unit received treatment conditional on observed covariates. We used propensity scores as sampling weights to reweigh treatment and control observations and thus eliminate bias during average treatment effect estimation and to satisfy overlap restrictions (Bell et al., 2018). Following Imbens and Wooldridge's (2009) recommendations, we use inverse propensity weights. We define , where W = 1 indicates a treated health system, and is the estimated probability of being treated. To compute , we use IPCit, AvgStaffedBedsi, AffiliatedHospitalsi, and AvgPopulationi. We use a binary logit model to estimate the required probability of a system's participation in the treatment group and the natural log of the variables to control for dispersion when required. After obtaining the weights, we estimated DiD models by including them during estimation.
We verified whether the weights balanced the treatment and control groups. Using Guo and Fraser's (2014) suggestion, we compared estimates from weighted and unweighted regressions, in which we considered one covariate (AvgPopulationi) the dependent variable and the treatment indicator (i.e., systems that used the T-MCM) as the independent variable. When we used linear regression with IPCit, AvgStaffedBedsi, and AffiliatedHospitalsi as control variables, the treatment estimate in the unweighted regression was statistically significant (β = −0.104; p < 0.05), suggesting that health systems in denser populations are more likely to use the countermeasure, an endogeneity that must be accounted for. The treatment estimate in the weighted regression was nonsignificant (b = −1.82; p > 0.1), and thus weighted regressions eliminated all significant differences between the two groups, evidencing that the propensity score method balanced the data. We considered another covariate (AvgStaffedBedsi) as the dependent variable and ran both weighted and unweighted regressions, finding similar results that supported the balance. We argue that the parallel trend assumption holds in the pretreatment group, with detailed analyses appearing in Appendix C.
4.7 Identification strategy: effect of HReg on ICU bed utilization
4.7.1 Estimation of direct effect
AvgDemandit represents the propensity score weighted new Covid-19 cases in a county in which health system i is located at t time, and Tt represents the time trend. Publishi measures the number of academic articles health system i published over the observation period. We could not control for the system-level indicator variable because the variables were collinear with InfoExchCoalitioni to explain the variance fully, thereby making publishi redundant. The standard 2SLS estimator requires a linear first-stage regression, and thus we used a linear probability model despite the binary endogenous variable (Bavafa et al., 2018). We use the predicted value from Equations (1) in (4) as a substitute for InfoExchCoalitioni. Results appear in columns 1 and 2 of Table 3. Appendix D evidences the relevance and exclusion restrictions of the instruments.
| Variables |
(1) InfoExchCoalitionit (1st Stage) |
(2) ln(ICUUtilCOVID) (2nd Stage) |
(3) InfoExchCoalitionit (1st Stage) |
(4) InfoExchCoalitionitX it (1st Stage) |
(5) ln(ICUUtilCOVID) (2nd Stage) |
|---|---|---|---|---|---|
| publishit |
0.083*** (0.005) |
– |
0.079*** (0.005) |
0.004*** (0.001) |
– |
| publishit × it | – | – |
0.017 (0.014) |
0.099*** (0.009) |
– |
| it |
−0.382*** (0.044) |
−0.018*** (0.004) |
−0.512*** (0.126) |
−0.394*** (0.089) |
0.108*** (0.014) |
| InfoExchCoalitionit | – |
−0.031* (0.013) |
– | – |
0.053*** (0.017) |
| InfoExchCoalitionit × it | – | – | – | – |
−0.271*** (0.025) |
| AvgDemandit |
0.086*** (0.006) |
0.028*** (0.002) |
0.086*** (0.006) |
−0.005*** (0.001) |
0.019*** (0.002) |
| Time controls | Day–Week | Day–Week | Day–Week | Day–Week | Day–Week |
| Observations | 735 | 735 | 735 | 735 | 735 |
| F-statistic | 17.03 | – | 17.44 | 26.41 | – |
| Wald chi-square | – | 360,226.11 | – | – | 190,944.3 |
| Number of systems | – | 15 | – | 15 |
- Note: Standard errors appear in parentheses.
- *** p < 0.005.
- ** p < 0.01.
- * p < 0.05.
4.8 Estimation of moderation
AvgDemandit represents the propensity score weighted new Covid-19 cases in a county in which health system i is located at t time, and Tt represents the time trend. We used the predicted values from Equation (2) and from Equations (3) in (5) as a substitute for InfoExchCoalitioni and InfoExchCoalitioni × it, respectively. Results appear in Table 3. The F-statistics for the first-stage regressions (Equations 1 and 2) were (F = 17.44, p < 0.001) and (F = 26.41, p < 0.001), respectively. Because they are greater than 10, the relevance of the instrument assumptions was validated (Stock & Yogo, 2005).
5 RESULTS
5.1 Direct effects of T-MCM and information exchange coalition on ICU bed utilization
Health systems began using the T-MCM at different points, and thus captures variation not only across health systems, but within them across time. is the predicted value of InfoExchCoalitionit from Equation (1). The remaining variables were similar to those discussed previously. The coefficient of , , captures the change in relative to baseline ICU bed utilization for a system, and seasonality patterns. If is negative, use of the T-MCM reduced intensive care bed utilization. Coefficient captures the percentage change in for a 1% change in . If and are negative, use of the T-MCM and participation in Mi-COVID19 reduced bed utilization, respectively.
Column 1 in Table 3 reports the first-stage equation of the 2SLS. The number of research articles published by health systems explains their likelihood of participating in an information exchange coalition. Column 2 reports results of the second-stage model represented in Equation (4). The effect of T-MCM use was negative ( = −0.018, p < 0.001), supporting H0a. The effect of participation in the HReg was negative ( = −0.031, p < 0.001), and thus H1 was supported. Because the dependent variable is the natural logarithm of ICU bed utilization, the result is economically meaningful. Using the T-MCM helped a health system reduce ICUUtilCOVID by 1.8%. A 1% increase in the percentage of healthcare participation in an information exchange coalition is associated with a 0.031% decrease to ICUUtilCOVID.
5.2 Moderation by information exchange coalition
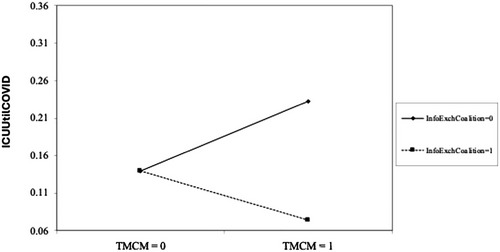
is the predicted value of InfoExchCoalitioni from Equation (2), and is the predicted value of from Equation (3). We controlled for time-fixed effects and the effects of new Covid-19 infections. Columns 3 and 4 in Table 3 report results of the first-stage equations of the 2SLS. We found evidence of the strength of the IV (F > 10; p < 0.001), which satisfies Stock and Yogo's (2005) test of IV relevance. Column 5 reports results for the second-stage equation, suggesting that the coefficient of interaction term it×InfoExchCoalitioni was negative ( = −0.271, p< 0.01), supporting H2. We plot interactions of the variables in Figure 1, finding that hospitals reduced ICUUtilCOVID further by using T-MCM when more affiliated hospitals participated in HReg. The figure shows negative moderation by InfoExchCoalition in the relationship between TMCM and ICUUtilCOVID.
6 ADDITIONAL ANALYSES
6.1 Robustness checks
6.1.1 Additional variables
We tested additional variables that might have confounded relationships during main analyses. We included two time-variant local (environmental) variables—average number of Covid-19 tests (AvgTestingit) and average number of deaths related to Covid-19 (AvgDeathit)—and three health-system-specific variables—a health system's response to the Covid-19 pandemic by becoming familiar with care standards (FacilityResponseit), time-invariant average hospital case-mix index (CMIi), and average number of cases treated during 2020 (AvgCasesi). We used these variables in the propensity score weighting method to remove disparities between the treatment and control groups. We used local variables in the main models represented in Equations (4) (direct effects) and (5) (moderating effects). We used endogeneity-corrected InfoExchCoalition and TMCM×InfoExchCoalition as described above. Results appear in Table E1 of Appendix E (direct effects in column 1 and moderation in column 2). Results are similar to those of the main model.
6.1.2 All health systems in Michigan
During the main analyses, we omitted four single-hospital health systems to assess dynamics of a multi-hospital health system as they used T-MCM. We included all 19 health systems and applied the same econometric strategy as during the main analyses, finding that results were consistent. Results appear in Table E1 of Appendix E.
6.1.3 Alternative operationalization of InfoExchCoalitioni
One assumption during main analyses regarding measuring the extent of InfoExchCoalition by the number of affiliated hospitals that participated in HReg might not hold if the health systems shared information and learned homogeneously across hospitals. We introduced an alternative operationalization of InfoExchCoalitioni to address homogenous information dissemination within health systems, operationalizing it as a binary variable such that InfoExchCoalitioni = 1 when at least one affiliated hospital in health system i participated in information exchanges, and zero otherwise. We thus assume that the entire health system drew information homogenously, even if a few affiliated hospitals participated in Mi-COVID19. Results accorded with main results reported in Table E1 of Appendix E.
6.2 Underlying mechanisms
6.2.1 Adaptive learning by health systems
A negative β0 = b1 + b4 × InfoExchCoalition and β1 = b2 + b5 × InfoExchCoalition for high (mean + 1 × standard deviation) InfoExchCoalition suggests adaptive learning as a health system participated in HReg. Results appear in Table F1 of Appendix F (column 2). When InfoExchCoalition = 1.16 (high information exchange), (β0 = −0.048; p < 0.1) and (β1 = −0.064; p < 0.05).10 Results suggest that as health systems participated in HReg, they adaptively learned about the countermeasure after they used CPT, which improved care capacity management over time. When InfoExchCoalition = −0.125 (low information exchange), (β0 = −0.048; p < 0.05) and (β1 = 0.071; p < 0.05). Results suggest that health systems with low participation in HReg struggled to manage care capacities, potentially missing the information needed to optimize new countermeasures. These results highlight the importance of HReg in enhancing adaptive learning during a PHE.
6.2.2 Meaningful use of HReg
Participating in an HReg might not translate to using acquired information meaningfully. We observed similar disconnects between hospitals using an HIE and physicians using the technology meaningfully (Wani & Malhotra, 2018). We measured extent of HReg use through meaningful use of HIE by affiliated hospitals as a proxy. HealthIT.gov reports data on meaningful use of HIE by hospitals in the country. Hospitals must sign off on stage 2 of meaningful HIT use (dedicated to HIE), and the database records the year of the sign-off. We assessed hospitals with a signoff year until 2019, counting the number of affiliated hospitals by the health systems we assessed, and the percentage of health system hospitals (PercentMUi) that achieved stage 2 status in the meaningful use dataset.
Results ( = −0.027; p < 0.001) suggest that health systems with a greater percentage of hospitals that integrated knowledge from HReg meaningfully lowered ICUUtilCOVID. We plot the marginal effect of InfoExchCoalition interaction for high and low PercentMU (±one standard deviation from the mean) in Figure G1 of Appendix G, finding that health systems with low/high PercentMU experienced higher/lower ICUUtilCOVID with increasing InfoExchCoalition. Patient-level data-sharing required critical resource commitment from the health systems, and such commitment, without necessary value extraction, distracted health systems from managing care capacities during the PHE.
We investigated how moderation of TMCM and InfoExchCoalition changed with high/low PercentMU, introducing PercentMU as a second moderator in the reduced-form model, complicating subsequent interpretation. We thus assessed high and low groups based on observation subsets above and below the mean of PercentMU to observe moderation effects for these groups. We report results of the observation subset above/below the mean of PercentMU (>/≤2.65 [after inverse probability weighting adjustment]) in Table F1 of Appendix F (columns 4 and 5). We report marginal effects in Figures G2A (High PercentMU) and G2B (Low PercentMU) of Appendix G.
Assessing patient populations with greater comorbidities and immunosuppression
The World Health Organization recommended clinical trial application of CPT for acute care Covid-19 patients, but clinical convergence regarding the treatment's efficacy was absent. Various medical committees thus made different recommendations based on relative risk propensities of the treatment. For example, the National Institutes of Health provided a physiological rationale, despite absence of definitive clinical evidence, for CPT use among immunosuppressed patients (since January 2022).11 We assessed the subset of patient populations with greater comorbidity and immunosuppression to observe relationships between constructs in the model. Such patients represented a riskier patient subgroup due to the severity of the SARS-CoV-2 virus, possibly requiring additional resource commitments by the health systems.
Because human immunity (comorbidity) declines (increases) sharply at 65 years,12 we assess a subset of health systems that serve counties with higher populations above 65 years. We enumerated the average population (>65 years) that a health system serves,13 testing the hypotheses using the subsample of health systems with an average county population above the median of the entire sample. We used the same empirical strategy and estimation methodology in the main model. Columns 6 (direct effect) and 7 (moderation) in Table F1 of Appendix F report results. We find that all hypotheses except H1 were supported.
We also assess a subset of health systems that serves counties with higher populations of people suffering from cancer and HIV, primary causes of comorbidity/immunosuppression in ICU Covid-19 patients in the country (Singson et al., 2022). We collected data on cancer incidences and HIV prevalence rates (i.e., number of patients per 100,000 with the disease) in a county in 2020. We enumerated the average cancer and HIV rates in a county that a health system serves, testing hypotheses using the subsample of health systems with average cancer and HIV incidence rates above the median incidence rates of the entire sample. The assumption is that a health system that operated in counties with higher incidences/prevalence disease rates treated greater immunocompromised patients (with HIV/cancer as comorbidity), requiring acute care treatments due to Covid-19. We used the same empirical strategy and estimation methodology in the main model. Columns 8 (direct effect) and 9 (moderation) report results in Table F1 of Appendix F. Results support H0a and H2, and H1 had a significant, reversed sign.
We found no support for H1 in both analyses above, and health systems with higher cancer and HIV rates in the county increased ICUUtilCOVID participation by 7.4%. Among health systems that operated in counties with greater immunocompromised patients, focusing on learning from HReg might have constrained resources for meeting the care needs of acute care patients with comorbidity and Covid-19 complications. Allocation of resources for HReg participation might even have reduced such resources. Such patients have complex care requirements, and healthcare organizations might not have had the extra resources to treat them while learning. More research is needed, but we find that the results accord with Nembhard and Tucker (2011); organizations that operate in complex environments14 perform worse in the short term when they spend resources learning from performance improvement while simultaneously managing daily operations. We thus see no direct marginal advantage of HReg participation, but we found support for the hypothesis that physicians find participation helpful in collecting information to improve novel treatments.
7 DISCUSSION
7.1 Theoretical contribution and implications
Using a quasi-experimental design and a dataset of health systems in Michigan during the Covid-19 pandemic, this study demonstrates the value of participating in an information dissemination coalition facilitated by an external organization (HReg) to manage resource use during a public emergency/disaster. It shows that health systems that participated in HReg during the pandemic managed critical care capacities better. However, additional analyses show that the value depends on several factors. If healthcare systems do not use the information effectively, participation hinders capacity management. During a PHE, healthcare organizations have limited resources, so additional effort made without fully using incoming information diverts attention from care. Contrarily, health systems that use the information effectively enhance their ability to manage care capacities during a pandemic. However, extracting HReg benefits may depend on the type of patients health systems treat. The study finds the systems that treated Covid-19 patients in counties with higher comorbidity did not find HReg participation beneficial. Infected patients with greater chances of comorbidity required resource commitment to treatment, which might not have allowed them to leverage such participation, resulting in greater acute care capacity use.
Our research underscores the importance of dedicating organizational resources to external information exchange initiatives to fully realize their potential benefits. This insight aligns with findings in both the healthcare (Ayer et al., 2019; Janakiraman et al., 2023) and humanitarian operations management literature (Altay & Pal, 2014; Green & Kolesar, 2004; Moshtari & Vanpoucke, 2021; Yoo et al., 2016). For instance, Ayer et al. (2019) demonstrated that the meaningful use of HIEs in emergency departments significantly improves performance, whereas low exchange utilization can hinder operational outcomes. In disaster management and humanitarian efforts, the constantly evolving operational landscape often constrains collaboration and knowledge-sharing initiatives among humanitarian organizations. Our study emphasizes that committing adequate resources is essential to ensuring the sustainability of these relationships. This perspective is further supported by Moshtari and Vanpoucke (2021), who highlight the importance of team-building initiatives, shared training programs, and joint problem-solving efforts to foster and reinforce structural and relational ties among collaborating entities. By committing resources to such activities, organizations can create robust frameworks for collaboration and maximize the benefits of external information exchanges.
By including T-MCM and HReg in an overarching theoretical framework provided by OIPT, we examine the roles played by external information-sharing structures in strengthening the outcome of novel treatment while lacking sufficient clinical evidence. Considering this information-sharing structure helps extend underlying predictions of OIPT. A coalition of peer organizations provides an external information-sharing structure that helps manage uncertainty. Complexities inherent in planning and process management characterize T-MCM that addresses environmental uncertainties. However, having access to such external information-sharing also allows access to a broad range of information that an organization can process to derive insights into handling the situation. Additional analyses evidenced health systems improving T-MCMs adaptively, highlighting how they learn about new processes and treatments. Results emphasize the need to invest in external information-gathering when dealing with complex, uncertain environments, contributing to a new literature domain that investigates how health systems learn about new treatments (Tong et al., 2016; Tucker et al., 2007). Results also apply to contexts beyond healthcare, such as humanitarian relief operations, manufacturing plants, retail organizations, and projects. Managing tasks with uncertain steps or tasks performed in an uncertain environment is challenging, but they can be managed using lateral communication and sharing information (Altay & Pal, 2014; Ramanathan, 2012; Wiengarten & Longoni, 2018).
7.2 Managerial and policy implications
This study offers insights to health system administrators by considering the role HReg participation plays and how it informs practitioners. We show that a 1% increase in the percentage of participation in an information exchange coalition is associated with a 0.031% decrease in ICU bed utilization related to Covid-19 patient hospitalizations. This finding is relevant to the large healthcare systems in our dataset that never participated in the HReg. For example, on November 2, 2020, the health system reported 19.92% ICU bed utilization related to Covid-19 patient hospitalizations. It had 271 ICU beds across 14 hospitals, and it had 54 Covid-19 patients admitted to ICUs that day. If all hospitals within the system had participated in the HReg, the health system would have freed about 2 ICU beds.
Practitioners should know that HReg participation allows a health system to look beyond its boundaries and learn from others. An information exchange coalition provides a structure for acquiring external information, which might enable the development of more robust procedures to offer innovative services. According to one infectious disease expert we interviewed, “The registry [Mi-COVID19] helped us to understand the nuances of the treatment [CPT]. For example, we learned about the right concentration of antibodies in the plasma and the ideal time to administer the treatment to the patients in the treatment process.” Results suggest that the effect of using T-MCM on decreasing ICU bed utilization is stronger when a system participated in the information exchange coalition. A health system that used the CPT at any point and had 1% participation in Mi-COVID19 reduced bed utilization by 0.271%, in comparison to those that had not participated. If one health system with 271 beds had used T-MCM on April 19, 2020, 20% participation in HReg would have freed 2 additional ICU beds. Positive moderation by HReg participation is evident in Figure 1, which shows that if a health system did not participate, it lost the advantage of using T-MCM. To benefit from similar countermeasures, health systems should connect with multiple external entities and learn how they can continue to improve. This study thus motivates health systems to incentivize participation or HCPs in information-sharing coalitions to learn best practices for treating virulent patients better and faster.
7.3 Limitations and future research
Hospitals began using CPT at the onset of the pandemic, without comprehensive understanding of its efficacy. Knowledge of the treatment evolved and was exchanged among peer hospitals, and thus, this study reflects healthcare institutions’ incremental learning. Subsequent medical studies continued to debate CPT effectiveness, but current findings suggest a modest reduction to ICU bed utilization, an outcome attributable to two factors. Concerns arose regarding the treatment's adverse effects leading to higher mortality rates, though consensus did not support this notion, rendering it an unlikely explanation. As hospitals continued to refine treatment protocols based on shared knowledge and best practices, CPT efficacy was expected to be optimized. Future research should use patient-level data to assess how the T-MCM influenced patient care and how hospitals used HReg to improve treatments across patient groups.
This study did not capture fully the extent of hospital participation in HReg. We use meaningful use attestation of HIT (stage 2) as a proxy to assess HReg use, given exigencies of the pandemic, during which medical practitioners were pressured to access timely information for patient care, but actual use of information acquired from HReg remains unknown. Future research should explore nuances of participation when more detailed data become available, offering deeper understanding of the dynamics of HReg engagement during PHEs.
This study uses Michigan health systems as a context, limiting assessment of locational heterogeneity. Future research should assess relationships across multiple states or even countries. Although the effects of T-MCM and HReg on capacity are not limited to healthcare, current data focus only on critical healthcare services. Future research should investigate whether and how these relationships hold across industry contexts.
This study investigates factors that influence ICU bed capacity in health systems. Because new T-MCMs, such as CPT, also come with challenges attributable to lack of evidence of benefits, scarce organizational knowledge, and multiple operational and logistical considerations, this study offers directions that strengthen the effects of T-MCM. Findings advance the theory and practice of managing capacity when organizations experience disruptions characterized by long-term, uncertain demands and lack of capabilities and resources to address them.
ACKNOWLEDGMENTS
Open access funding provided by the Iowa State University Library.
Biographies
Sukrit Pal: Sukrit Pal is an Assistant Professor of Supply Chain Management in the Ivy College of Business at Iowa State University. His research addresses operational and strategic challenges in healthcare and platform services, with an emphasis on process improvement, innovation, labor allocation, and capacity management. Pal has collaborated with scholars and practitioners to explore topics such as ride-hailing platform behavior, healthcare information exchange, vaccine distribution, and hospital risk management. His work has been published in top-tier journals, including the Journal of Operations Management and International Journal of Production Economics, and has earned recognition such as the Best Problem-Driven Analytical Research Paper Award from the Decision Sciences Institute. In addition to serving on the editorial review board of the Journal of Operations Management, Pal is an active reviewer for several leading journals, including Production and Operations Management, Service Science, International Journal of Physical Distribution & Logistics Management, and BMC Psychology. He also contributes to the academic community through doctoral supervision, conference organization, and participation in professional societies such as INFORMS, DSI, and POMS.
Anand Nair: Anand Nair is Professor & Jeff Bornstein Faculty Fellow in the Supply Chain & Information Management academic area at Northeastern University. Nair has worked with organizations in manufacturing, healthcare, and retail sectors on issues such as inventory management, forecasting, pricing, capacity planning, lean systems, manufacturing planning and control, quality management, contracting, product development, process improvement, and technology implementation. His research examines how firms, teams, and individuals learn, adapt, and organize to manage processes, supplies, technology, and innovation, and the associated performance implications. Nair's research articles have been published in leading operations and supply chain management journals. He was selected as the Fulbright-Aalto University Distinguished Chair in 2017–2018. He had served as Department Editor for the Journal of Operations Management and is currently serving as a member of the Senior Editorial Team for the journal. He is serving as a Senior Editor for Production and Operations Management Journal and an Associate Editor for Decision Sciences Journal. He has served as Decision Sciences Institute's Vice-President and Secretary as well as a member of its Board of Directors. He is currently serving on the Executive Committee of the Operations and Supply Chain Management (OSCM) division of Academy of Management.
REFERENCES
- 1 https://www.fema.gov/cbrn-tools/key-planning-factors-bio/kpf-3/2
- 2 Hospitals offer higher care capacities when bed use is low.
- 3 https://www.who.int/news/item/07-12-2021-who-recommends-against-the-use-of-convalescent-plasma-to-treat-covid-19
- 4 Journal of Operations Management, International Journal of Operations and Production Management, Journal of Supply Chain Management, Production and Operations Management, Management Science, Operations Research, European Journal of Operational Research, IEEE Transactions on Evolutionary Computation, Mathematical Programming, Manufacturing and Service Operations Management, Journal of Business Logistics, and Decision Sciences Journal.
- 5 Search string used: (ALL [information OR knowledge] AND ALL [exchange* OR transfer* OR sharin*] AND TITLE-ABS-KEY [“disaster recovery” OR “disaster management” OR humanitarian OR health* OR hospital OR “organization* resilience”]) AND (LIMIT-TO [DOCTYPE, “ar”]) AND (LIMIT-TO [LANGUAGE, “English”]) AND (LIMIT-TO [SUBJAREA, “BUSI”]).
- 6 Starr and Van Wassenhove (2014) acknowledge the need for data-driven research to investigate information exchanges and coordination between organizations.
- 7 We replaced the hospital's name with ABC for confidentiality.
- 8 The Mayo Clinic began enrolling patients with serious or life-threatening Covid-19 complications to access investigational convalescent plasma outside of clinical trials. The FDA subsequently issued emergency use authorization (EUA) on August 23, 2020, permitting physicians to use the CPT without being enrolled in the clinic's EAP. The clinic ceased the EAP after the FDA published the EUA.
- 9 https://www.uofmhealth.org/news/archive/202004/michigan-medicine-teams-blue-cross-blue-shield-michigan-and
- 10 InfoExchCoalition was weighted by inverse probability weighting.
- 11 https://www.covid19treatmentguidelines.nih.gov/therapies/antivirals-including-antibody-products/covid-19-convalescent-plasma/
- 12 https://newsnetwork.mayoclinic.org/discussion/covid-19-keeping-seniors-immunocompromised-people-safe/#:~:text=Please%20courtesy%20%22Jessica%20Lancaster%2C%20Ph,a%20sharper%20decline%20at%2065
- 13 We enumerated the average population across all states with an affiliated health system hospital.
- 14 NICU clinics that treat premature and critically ill children.




