Applying a Modified Version of the Prediction of Alcohol Withdrawal Severity Scale in a Canadian Community Withdrawal Management Setting
Funding: This work was supported by the Ministry of Health, British Columbia, Vancouver Coastal Health Research Institute, F19-02790; Lianping Ti is supported, in part, by the US National Institutes of Health (R01DA052381).
ABSTRACT
Introduction
Severe alcohol withdrawal syndrome (SAWS) can lead to significant morbidity and mortality. The Prediction of Alcohol Withdrawal Severity Scale (PAWSS) has been validated in general acute care environments, but its efficacy in withdrawal management settings remains underexplored. This study aimed to assess the utility of a modified PAWSS and identify appropriate cutoff scores in a community withdrawal management setting in Vancouver, Canada.
Methods
From October 2019 to September 2022, we reviewed charts at Vancouver Detox Centre. Modified PAWSS versions replaced question 9 on the original PAWSS with: (i) breath analysis readings; (ii) alcohol consumption in the previous 24 h; and (iii) clinical assessments. We performed receiver operating characteristic analysis and used Youden's index to determine modified PAWSS' diagnostic accuracy against SAWS presentation, defined by a score of 15 or greater on the Clinical Institute Withdrawal Assessment Alcohol, Revised, seizures or delirium tremens and/or benzodiazepine administration.
Results
Among 228 individuals (165 male, 63 female), 175 (75%) met SAWS criteria during admission. For breath analysis readings, an optimal PAWSS cutoff score had 55% sensitivity (95% confidence interval [CI] 46%–63%) and 74% specificity (95% CI 54%–87%). For alcohol consumption in the last 24 h, a cutoff score of 7 had 44% sensitivity (95% CI 36%–51%) and 85% specificity (95% CI 70%–93%). For clinical assessment, a cutoff score of 6 had 53% sensitivity (95% CI 45%–61%) and 71% specificity (95% CI 58%–85%).
Discussion and Conclusions
Within a community withdrawal setting, the prevalence of SAWS was high, rendering the modified PAWSS less valuable. Although higher cutoff scores improved specificity, poor sensitivity hindered identification of low-risk SAWS individuals.
1 Introduction
Alcohol is the most commonly used intoxicating substance in North America and the prevalence of both high-risk drinking and alcohol use disorders (AUD) is a growing concern [1, 2]. The 2022 National Survey on Drug Use and Health estimated that approximately 8% of noninstitutionalised people living in the US over age 18 had AUD [3]. In 2019, nearly 700,000 (2%) Canadian adults met diagnostic criteria for moderate to severe AUD [4]. The prevalence of AUD and alcohol use represents a high burden of disease with significant healthcare costs [5, 6]. Nearly 20% of deaths among individuals aged 20–49 were attributed to excessive alcohol use and AUD in the US [7]. In 2020, the number of hospitalisations entirely attributed to alcohol in Canada was 279 per 100,000, three times more common than hospitalisations for opioids and cannabis [8, 9]. Despite the individual and systemic harms associated with high-risk drinking and AUD, it often goes unrecognised in the healthcare system [10, 11].
One of the many potential consequences of prolonged alcohol use is the development of severe alcohol withdrawal syndrome (SAWS) when alcohol use is significantly reduced or stopped [12, 13]. The development of SAWS occurs in approximately 20% of patients hospitalised for alcohol management, and treating prophylactically can improve patient outcomes [14]. The most severe clinical manifestations of AWS, including withdrawal seizures and delirium tremens (DT), are associated with higher rates of morbidity and mortality [15, 16]. If left untreated, the prevalence of mortality among patients with SAWS is approximately 3%–5%; however, with early detection and appropriate medical interventions, the expected mortality rate is less than 1% [9, 13, 17]. As a result, specialised and costly inpatient services have been established to assist with withdrawal management.
Although SAWS can be a life-threatening emergency, the vast majority of patients with AUD do not develop SAWS [15]. Prophylaxis or unnecessary prescribing of benzodiazepines, the standard of care for treatment of AWS [18], in patients experiencing uncomplicated withdrawal may lead to unintended outcomes including higher prevalence of benzodiazepine use and higher rates of alcohol use and relapse [19, 20]. The Prediction of Alcohol Withdrawal Severity Scale (PAWSS) is a tool for estimating the risk of developing moderate to severe SAWS and can be used by clinicians to determine appropriate withdrawal management pathways and supports [21]. The PAWSS score is comprised of a 10-item scale that incorporates several known risk factors for SAWS, including previous episodes of alcohol withdrawal, seizures, DTs, alcohol rehabilitation treatment, and concurrent use of other regulated or unregulated substances, among others [14, 21, 22]. A prospective study of hospitalised patients admitted to a general medicine unit in the US showed that when a PAWSS cutoff of 4 was used, the tool was able to identify patients at high risk of SAWS with high sensitivity (93%) and specificity (99%) and good inter-rater reliability (96%) [23].
While recent Canadian guidelines have recommended the use of PAWSS to assess the risk of SAWS and determine appropriate clinical pathways [9], PAWSS has not yet been validated in community care settings, limiting the generalisability of findings to acute care patient populations with low prevalence of AUD and co-occurring substance use. Particularly, the PAWSS cutoff score of 4 may not have the same predictive value in specialised community withdrawal settings that may have a high prevalence of SAWS. Additionally, some community settings do not have access to laboratory services to assess blood alcohol level upon presentation, which is required to complete the PAWSS tool. Therefore, the objective of this study was to determine the optimal cutoff and clinical utility of three modified versions of PAWSS for identifying patients at high risk of developing SAWS in a community withdrawal management setting with a high prevalence of AUD-related admissions.
2 Methods
2.1 Development of a Modified PAWSS
A Modified PAWSS Tool Development Task Group was convened to develop modified versions of PAWSS that would be suitable in community settings. The working group was comprised of 12 healthcare providers and clinical experts from across BC, and a comprehensive literature review, numerous consultations and Delphi methodology were performed [24]. Three modified versions were proposed, replacing Question 9 in the original PAWSS which required a measurement of the individual's blood alcohol level. These were: (i) A breath analysis reading at intake, where individuals received a point for question 9 if they had a breath analysis reading of 200 mg/dL or greater on presentation; (ii) Alcohol consumption in the last 24 h, where individuals received a point for question 9 if they consumed any alcohol in the last 24 h; (iii) Clinical assessment of intoxication, where individuals received a point on question 9 if slurred speech, disinhibition, impaired coordination/gait, labile mood, nausea and vomiting, ataxia, nystagmus, sedation or decreased respiratory effort were present upon admission.
2.2 Study Setting
This study consisted of a chart review of individuals admitted to Vancouver Detox Centre, a community withdrawal management centre, between October 2019 and September 2022. Vancouver Detox Centre is a bed-based withdrawal management site for individuals with complex, co-morbid medical, psychiatric and substance use conditions requiring 24-h monitoring who were referred from hospital, primary care, substance use clinics or self-referral. Individuals accessing this site often experience high rates of homelessness, incarceration, trauma and a history of both inpatient and community withdrawal management. The demand for services is high, resulting in long waitlists for self-referrals and a limited ability to accommodate referrals from hospital and other primary care facilities, highlighting the need for evidence-based screening tools to determine the risk of SAWS before admission.
2.3 Study Sample
Individuals were required to meet all the following inclusion criteria: at least 19 years or older; admitted to Vancouver Detox Centre within the past 24 h or transferred from hospital within the last 48 h and reported alcohol use within the past 30 days; able to communicate in English; and willing and able to provide informed consent. Individuals were excluded if they were admitted by hospital transfer and exceeded the 72-h window for AWS; interventions including pharmacological management and prophylaxis were already implemented; admitted to Vancouver Detox Centre for less than 72 h; had a history of seizure disorder that was unrelated to alcohol withdrawal; presented with severe alcohol withdrawal as defined by a patient Clinical Institute Withdrawal Assessment-Alcohol revised (CIWA-Ar) score of 20 or more on intake or were unable to understand the PAWSS questionnaire. Individuals who met all eligibility criteria were approached by a clinical team member, and informed consent was obtained.
2.4 Study Design
As part of the clinical protocol, individuals were assessed with a physical copy of the modified PAWSS tool (Appendix S1) upon admission. The modified PAWSS was then filed into the individual's paper chart. Next, we conducted a chart review, where demographic and clinical history were extracted from patient electronic medical records and paper-based forms, including nurses' summary notes at the time of admission. Medications prescribed, CIWA-Ar scores, data pertaining to the absence or presence of SAWS, items 1–10 from the three modified PAWSS, and other variables of interest were extracted from paper charts. Ethics approval was obtained from the University of British Columbia Clinical Research Ethics Board.
2.5 Variable Selection
The primary outcome for this study was SAWS, defined consistently with previous studies, as an individual having a CIWA-Ar score of greater than or equal to 15, experiencing seizures or DTs, and/or the presence of withdrawal symptoms severe enough for the primary care team to administer a benzodiazepine protocol with CIWA-Ar during admission [21, 23]. As a sensitivity analysis, we removed all individuals who were administered benzodiazepines but did not meet other criteria for SAWS. The rationale for exclusion of benzodiazepine administration in the sensitivity analyses was that the threshold for prescribing benzodiazepines is relatively low in withdrawal management settings (i.e., CIWA-Ar score of 10) and the benzodiazepine protocol is used to prevent symptoms of severe withdrawal before they emerge [25].
2.6 Demographic and Clinical Variables
A variety of demographic and clinical variables were collected from patient electronic medical records (i.e., clinician assessed) to characterise the study population. The variables were: age (in years); biological sex (male vs. female); recent reported substance use (i.e., substances the patient was using directly before admission to the site, including prescribed and/or unregulated use) including opioids (i.e., heroin, fentanyl and other opioids), nicotine, stimulants (i.e., cocaine, crack cocaine, methamphetamine and other amphetamines), cannabis and benzodiazepines; substance use–related complications in the last year, including seizures, DTs and overdose; depression; anxiety; post-traumatic stress disorder; bipolar disorder and other psychiatric comorbidities.
2.7 Statistical Analysis
As a first step, demographic and clinical data were stratified by the presence of SAWS and summarised using median and interquartile range for continuous variables and frequency and proportions for categorical variables. Categorical variables were analysed using Pearson's χ 2 test and continuous variables with the Mann–Whitney U test to determine factors associated with SAWS. Descriptive statistics were also used to characterise individuals who developed seizures and DTs during their admission to Vancouver Detox Centre.
Next, receiver operating characteristic (ROC) analysis was used to determine the sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) for all possible cutoff scores on the three modified versions of PAWSS. Youden's index, a function of sensitivity and specificity [26], was used to determine the optimal cutoff score for each modified version of PAWSS. The area under the curve was calculated, and 95% confidence intervals (CI) were constructed using the DeLong method. Finally, we calculated the sensitivity and specificity for each individual PAWSS criterion in predicting SAWS. All measures of diagnostic accuracy were calculated using modified PAWSS scores and the outcome of SAWS, as previously defined. All analyses were done with R (version 4.2.0).
3 Results
3.1 Sample Characteristics
Table 1 provides sample demographics, substance use history and comorbid mental health diagnoses stratified by the development of SAWS during admission. Around three-quarters of the sample were male (167; 73%), the median age was 45 years (interquartile range 12.2) and a high proportion reported recent use of substances in addition to alcohol, including tobacco (137; 60%), stimulants (87; 38%), opioids (46; 20%), cannabis (76; 33%) and benzodiazepines (38; 17%). Substance use–related complications were also common, with 59 (26%) and 49 (22%) individuals having a clinical history of alcohol withdrawal seizures and DTs, respectively. Psychiatric comorbidities were prevalent, as indicated by high proportions of depression (126; 55%), anxiety (125; 55%) and other mental health conditions (79; 35%) reported. The majority of individuals (175; 77%) met the criteria for SAWS during admission. 112 (64%) of these individuals were administered benzodiazepines but did not meet any other criteria for SAWS. Significant differences were observed in age (p = 0.02), history of delirium tremens (p = 0.03) and cannabis use (p = 0.01) between individuals who developed severe alcohol withdrawal syndrome during admission and those who did not.
| Characteristic | Total (%) (n = 228) | Severe alcohol withdrawal | p | |
|---|---|---|---|---|
| Yes (%) (n = 175) | No (%) (n = 53) | |||
| Demographics | ||||
| Age (med, IQR) | 45 (19–76) | 43 (19–76) | 51 (23–69) | 0.02 |
| Sex (male) | 167 (73) | 124 (71) | 43 (81) | 0.19 |
| Recent substance use a | ||||
| Opioids | 46 (20) | 31 (17) | 15 (28) | 0.14 |
| Stimulants | 87 (38) | 61 (35) | 26 (49) | 0.09 |
| Cannabis | 76 (33) | 50 (29) | 26 (49) | 0.01 |
| Benzodiazepines | 38 (17) | 34 (19) | 4 (8) | 0.06 |
| Tobacco | 137 (60) | 102 (58) | 35 (66) | 0.40 |
| Substance use–related complications | ||||
| Seizures | 59 (26) | 50 (29) | 9 (17) | 0.13 |
| Delirium tremens | 49 (22) | 44 (25) | 5 (9) | 0.03 |
| Overdose | 15 (7) | 13 (7) | 2 (4) | 0.53 |
| Psychiatric comorbidities | ||||
| Depression | 126 (55) | 103 (59) | 23 (43) | 0.07 |
| Anxiety | 125 (55) | 101 (58) | 24 (45) | 0.15 |
| Post-traumatic stress disorder | 40 (18) | 30 (17) | 10 (19) | 0.93 |
| Bipolar disorder | 16 (7) | 12 (7) | 4 (8) | 0.80 |
| Other | 35 (15) | 24 (14) | 11 (21) | 0.30 |
- Abbreviations: IQR, interquartile range; PAWSS, Prediction of Alcohol Withdrawal Severity Scale.
- a Recent substance use was defined as substance use prior to intake.
3.2 Seizures and DTs During Admission
In total, nine (4%) individuals experienced DTs and one individual experienced seizures during their admission. Among individuals with DTs, the majority had a CIWA-Ar score greater than or equal to 15 (6; 67%) and had a modified PAWSS score greater than 4 (8; 89%) on all three versions of the tool. The person who experienced seizures did not report a history of seizures and had a CIWA-Ar score of 7.
3.3 Modified Versions of the PAWSS
For the breath analysis reading, 176 (68%) individuals had valid readings (Figure 1), of which 164 (93%) had a modified PAWSS score greater than 4 and a mean score of 6 (SD 1.6). Figure 2 compares the tool's sensitivity and specificity at various cutoff points, indicating that according to Youden's index, the optimal cutoff score is 6. This cutoff produces a sensitivity of 55% (95% CI 46%–63%) and a specificity of 74% (95% CI 54%–87%); with a PPV of 90% (95% CI 81%–95%), NPV of 29% (95% CI 81%–95%). Given the importance of avoiding false negative results and the sensitivity of the tool, a cutoff score of 4 would have a higher sensitivity (94%) but a lower specificity (11%). The ROC analysis gave an area under the curve of 0.68 (95% CI 0.59–0.77).
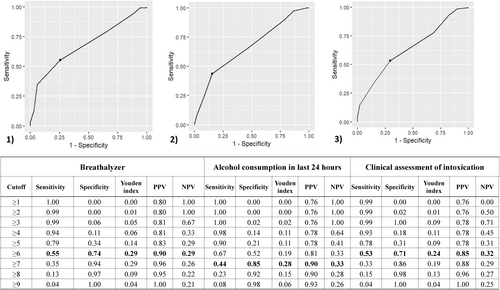
For consumption of alcohol in the last 24 h, 214 (83%) individuals had valid responses, of which 203 (95%) had a modified PAWSS score greater than 4 and a mean score of 7 (SD = 1.6). Figure 2 compares the tool's sensitivity and specificity at various cutoff points, indicating an optimal cutoff score of 7 according to Youden's index. This cutoff produces a sensitivity of 44% (95% CI 36%–51%) and a specificity of 85% (95% CI 70%–93%); with a PPV of 90% (95% CI 81%–96%), NPV of 33% (95% CI 23%–39%). A cutoff score of 4 would have a higher sensitivity (98%) but a lower specificity (13%). The ROC analysis indicates that the area under the curve is 0.66 (95% CI 0.58–0.74).
Finally, for the clinical assessment of intoxication, 214 (83%) individuals had assessment data, of which 194 (91%) had a modified PAWSS score greater than 4 and a mean score of 6 (SD 1.7). Figure 2 compares the tool's sensitivity and specificity at various cutoff points, indicating an optimal cutoff score of 6 according to Youden's index. This cutoff produces a sensitivity of 53% (95% CI 45%–61%) and specificity of 71% (95% CI 58%–85%); with a PPV of 85% (95% CI 80%–93%) and NPV of 24% (95% CI 22%–39%). A cutoff score of 4 would have a higher sensitivity (93%) but a lower specificity (18%). The ROC analysis indicates that the area under the curve is 0.65 (95% CI 0.57–0.73).
3.4 Sensitivity Analysis
As indicated previously, we re-ran the analyses using an alternate definition of SAWS where administration of benzodiazepines was excluded. After removing individuals who were administered benzodiazepines but did not have a CIWA-Ar greater than or equal to 15, seizures, or DTs, 63 (28%) individuals met the criteria for SAWS. The optimal cutoff scores were the same across all modified versions of PAWSS (Appendix S2).
3.5 Individual PAWSS Criteria
Table 2 provides percentages of individual PAWSS criteria and the sensitivity and specificity for each criterion in predicting SAWS. Given the importance of avoiding false negatives and the consequences of missing an individual who develops SAWS, we have highlighted criteria that predict SAWS with high sensitivity (> 80%). Individual PAWSS criteria that met this threshold included: intoxication in the last 30 days (sensitivity = 99%; 95% CI 97%–99%, specificity = 6%; 95% CI 1%–16%), previous treatment for AUD (sensitivity = 81%; 95% CI 97%–99%, specificity = 25%; 95% CI 14%–39%), previous AWS (sensitivity = 97%; 95% CI 93%–99%, specificity = 13%; 95% CI 6%–26%) and history of blackouts (sensitivity = 84%; 95% CI 78%–89%, specificity = 31%; 95% CI 19%–45%). However, it is noteworthy that these highly sensitive criteria have poor specificity.
| Modified PAWSS criteria | Total (%) (n = 228) | Sensitivity (95% CI) | Specificity (95% CI) |
|---|---|---|---|
| Q1: Intoxication in last 30 days | 223 (98) | 0.99 (0.97, 1.00) | 0.06 (0.01, 0.16) |
| Q2: Previous treatment for AUD | 181 (79) | 0.81 (0.75, 0.87) | 0.25 (0.14, 0.39) |
| Q3: Previous AWS | 215 (94) | 0.97 (0.93, 0.99) | 0.13 (0.06, 0.26) |
| Q4: Ever experienced blackouts | 183 (80) | 0.84 (0.78, 0.89) | 0.31 (0.19, 0.45) |
| Q5: Ever experienced seizures | 74 (33) | 0.38 (0.31, 0.45) | 0.85 (0.72, 0.93) |
| Q6: Ever experienced DT | 51 (22) | 0.25 (0.19, 0.32) | 0.87 (0.74, 0.94) |
| Q7: Alcohol with “downers” | 61 (27) | 0.26 (0.19, 0.33) | 0.69 (0.55, 0.81) |
| Q8: Alcohol with any substance | 165 (72) | 0.70 (0.62, 0.76) | 0.17 (0.08, 0.30) |
| Q9a: Breathalyser a | 42 (25) | 0.30 (0.23, 0.39) | 0.97 (0.85, 1.00) |
| Q9b: Alcohol in the last 24 h a | 125 (75) | 0.77 (0.69, 0.84) | 0.35 (0.20, 0.54) |
| Q9c: Clinical assessment a | 34 (21) | 0.23 (0.16, 0.31) | 0.91 (0.76, 0.98) |
| Q10: Autonomic activity | 58 (25) | 0.30 (0.24, 0.38) | 0.90 (0.79, 0.97) |
- Abbreviations: AUD, alcohol use disorder; AWS, alcohol withdrawal syndrome; CI, confidence interval; DT, delirium tremens; PAWSS, Prediction of Alcohol Withdrawal Severity Scale.
- a Incomplete data resulting in a sample size of 166 participants.
4 Discussion
In the present study, we examined several modified versions of PAWSS and their ability to predict SAWS among individuals admitted to an inpatient withdrawal management centre in Vancouver, BC. A key finding from this study is that according to established definitions in the literature [21, 23], an extremely high proportion of individuals met criteria for SAWS during admission. Thus, the pre-test probability of SAWS, according to definitions whereby benzodiazepine administration defines someone as having SAWS, appears so high in inpatient withdrawal management settings as to preclude the value of screening. Indeed, even when higher cutoff scores for all modified versions of PAWSS (compared to the previously validated PAWSS in medically ill hospitalised patients) are utilised, all modified versions of PAWSS had poor sensitivity for predicting SAWS by these definitions. Providing some guidance for future research, we found that there are individual PAWSS items (i.e., intoxication in the last 30 days, previous treatment for alcohol use disorder, previous AWS and having ever experienced blackout) that may be useful in identifying patients that may be at higher risk of SAWS within the context of patients presenting for withdrawal management.
In 2015, a study by Maldonado et al. validated the PAWSS tool and found a cutoff score of 4 to be highly accurate in predicting SAWS among unselected hospitalised patients in the US [23]. In contrast, the modified versions of PAWSS in the setting of an inpatient withdrawal management program did not have high predictive value or diagnostic accuracy in identifying patients at risk of SAWS using the original cutoff score of 4. ROC analysis confirmed that the optimal cutoff score for all three modified versions of PAWSS was at least 6, depending on which modified version was applied. Optimal cutoff scores were determined using Youden's index, which maximises both the sensitivity and specificity of a diagnostic test. However, in this context, it may be more important to have a diagnostic test with higher sensitivity (e.g., a cutoff score of 5) to minimise the number of individuals that are predicted to be at low risk but later develop SAWS.
The poor diagnostic accuracy of the modified PAWSS is likely explained by the differences in setting as well as the prevalence of SAWS. Specifically, the prevalence of SAWS in our study compared to the study undertaken by Maldonado and colleagues was 77% vs. 7%, respectively [23]. It is also noteworthy that the substance use characteristics and healthcare needs of the population that accesses withdrawal management services differ significantly from medically ill patients admitted to a general medical ward [23]. For example, in the hospital setting, only 4% of admissions were for alcohol withdrawal. Additionally, only 1.7% of individuals were diagnosed with alcohol use disorder and 1.5% with a substance use disorder other than alcohol [23]. In inpatient withdrawal management settings such as ours, patients are highly selected and were all accessing the service for alcohol or other substance use withdrawal and reported recent alcohol use; as such, this may preclude the need for a screening tool in this setting. In a population with a high prevalence of a given condition, predictive tools are more effective at confirming diagnoses (ruling in) but less effective at excluding them (ruling out) [27]. Clinicians utilising the modified or original PAWSS scales should consider the study sample characteristics and anticipate that sensitivity may vary across populations with differing prevalence rates of alcohol and other substance use. Future study may evaluate the use of CIWA-Ar protocol without PAWSS screening to determine whether a symptom-driven protocol is sufficient for preventing AWS in this population.
Despite the poor diagnostic accuracy of the modified PAWSS tool in the community inpatient withdrawal setting, we found that individual PAWSS criteria may be more useful for predicting SAWS. Although the majority of PAWSS criteria are static factors which do not provide opportunity for direct intervention, their clinical utility lies in early identification of high-risk individuals. An episode of alcohol intoxication in the last 30 days resulted in the highest sensitivity in predicting AWS in this population. Previous studies have shown that individuals who drink recently and heavily are at greater risk of developing AWS [28], with a significant correlation between the amount of alcohol consumed during the last drinking episode and severity of AWS symptoms [21, 29]. Previous AWS, regardless of severity, also had high sensitivity in predicting SAWS. This is supported by research in both human and animal models showing that alcohol withdrawal symptoms progressively worsen with each episode of withdrawal [14, 21, 30, 31]. Interestingly, we did not find that the history of seizures or DTs on the PAWSS assessments produced a high sensitivity in predicting SAWS, which has been shown in previous work [15, 21]. When we examined clinician-inputted patient electronic medical records of seizures and DTs, we found that DTs were associated with developing SAWS. One possible explanation for this was that there were differences between PAWSS assessments during admission at Vancouver Detox Centre and clinician history in the charts, including electronic medical records. Taken together, these findings suggest that certain PAWSS criteria may better predict SAWS in withdrawal management settings than the tool as a whole, and that we must ensure consistency in how symptoms of severe alcohol withdrawal are defined and documented.
The modified PAWSS may be most useful for clinicians practicing in settings like the emergency department or primary care and among those who are not experienced with AUD. If the modified PAWSS score predicts a high risk of SAWS, this could prompt a consult with an addiction medicine physician who would then assess whether a hospital, community bed-based withdrawal management, home-based withdrawal management or community substance use clinic is required. Effective screening tools at this stage would allow for the identification of individuals for whom costly inpatient services, including benzodiazepine prescribing, should be reserved. A recent study found that the implementation of PAWSS in hospitalised patients resulted in a statistically significant reduction in total median benzodiazepine dose prescribed to individuals with AWS [32]. This is an important finding given the growing body of evidence indicating that benzodiazepine prescribing for alcohol withdrawal can lead to a higher prevalence of benzodiazepine use among people with AUD and may contribute to increases in alcohol use and higher rates of relapse following treatment [19, 33]. A double-blind clinical trial evaluating the effectiveness of gabapentin compared to lorazepam for outpatient treatment of alcohol withdrawal found that individuals receiving gabapentin had lower odds of drinking during and after treatment, experienced less anxiety and less sedation compared to those who received lorazepam [20]. Systematic reviews have also shown that gabapentin and other non-benzodiazepine medications are effective for patients at low risk of developing severe complications from alcohol and reduce the risks and side effects associated with benzodiazepine use [34, 35]. Therefore, the modified PAWSS may be useful in settings where healthcare providers are determining withdrawal management pathways, which may also prevent unnecessary prescribing of benzodiazepines.
A number of limitations should be noted when interpreting findings from the current study. First, this study was subject to selection effect as individuals admitted to Vancouver Detox Centre are primarily individuals at very high risk of developing severe SAWS according to past definitions. This resulted in a very high proportion of participants that met criteria for SAWS. While this suggests that future research on the modified PAWSS in high-prevalence settings is likely of little value, future studies could evaluate the modified PAWSS in lower-prevalence settings to determine the modified PAWSS' ability to predict AWS in various populations with different prevalences of SAWS. Second, participants who were treated for withdrawal symptoms may not have progressed to SAWS, and having use of a benzodiazepine in the definition of severe SAWS likely reflects reverse causation. Although the ideal study would not allow for treatment before the development of SAWS, this would be ethically unacceptable and increase the risk of potential complications including seizures and DTs. Finally, the design of the modified PAWSS allows for interpretation, potentially leading to variability in how the data were collected and resulting analysis.
In conclusion, this analysis found that the overall prevalence of SAWS was extremely high in patients accessing inpatient specialised withdrawal management services. The sensitivity of the modified PAWSS tool for the prediction of SAWS in this population was very poor, regardless of the cut-off score. Despite the modified PAWSS tool providing little value in predicting SAWS in this context, individual criteria on the modified PAWSS may be useful in predicting SAWS. Future studies should explore the value of the tool and individual PAWSS criteria in settings where patients are at lower risk of developing severe SAWS or in settings like the emergency department that triage patients to either inpatient or outpatient withdrawal management.
Author Contributions
Each author certifies that their contribution to this work meets the standards of the International Committee of Medical Journal Editors.
Acknowledgements
The authors thank all study participants for their contributions to the research as well as current and past researchers at Vancouver Detox Centre and the BC Centre on Substance Use that were involved in the study. This research was conducted on the traditional, ancestral and unceded territories of the xʷməθkwəy̓əm (Musqueam), Skwxwú7mesh (Squamish), Stó:lō and Səl̓ílwətaʔ/Selilwitulh (Tsleil-Waututh) Nations.
Disclosure
This study was supported by a Vancouver Coastal Health Research Institute Team Grant (F19-02790) and British Columbia Ministry of Health Narrowing Gaps grant. Lianping Ti is supported, in part, by the US National Institutes of Health (R01DA052381). Nicole Cowan is a Clinical Nurse Specialist who works for Vancouver Coastal Health Specialised Substance Use Services. All other authors report no relevant disclosures.
Conflicts of Interest
Evan Wood is a physician who works for Vancouver Coast Health in the area of withdrawal management. Dr Wood is also a professor of medicine based in the University of British Columbia, a position supported by a Canadian Instituts of Health Research Tier 1 Canada Research Chair, and received salary support from an R01 from the US National Institute in Drug Abuse, paid to the University of British Columbia. Dr Wood's research lab is further supported by Canadian Institutues of Health Research grants to the Canadian Research Initiative in Substance misuse. Dr Wood has also undertaken consulting work in legal matters related to substance use disorders and for a mental health company called Numinus Wellness. where Dr Wood is the former chief medical officer. Dr Wood reports receiving honoraria for non-industry-related academic lectures and conference presentations. Dr Wood has also received payment for expert reports and expert testimony in legal matters pertaining to substance use disorder, including from the Canadian Medical Protective Association and from trade unions representing workers with possible substance use disorder. Dr Wood has received travel support from the Canadian Insitutes of Health Research. All other authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




