Advances in the Study of Necroptosis in Vascular Dementia: Focus on Blood–Brain Barrier and Neuroinflammation
Funding: This study was funded by the Science and Technology Project Founded by the Education Department of Jiangxi Province (GJJ211812 to XRZ, GJJ211813 to LC, GJJ2201928 to LLW), the Provincial Natural Science Foundation of Jiangxi Province (20224BAB206040 to XRZ), Foundation of Students’ Platform for Innovation and Entrepreneurship Training Program :202411843024 to XRZ and S202411843050 to CXS, and the Administration of Traditional Chinese Medicine of Jiangxi Province (2022B1010 to LC).
Yuemin Qiu and Lin Cheng contributed equally to this article.
Lin Cheng is a co-first author.
ABSTRACT
Background
Vascular dementia (VaD) includes a group of brain disorders that are characterized by cerebrovascular pathology.Neuroinflammation, disruption of the blood–brain barrier (BBB) permeability, white matter lesions, and neuronal loss are all significant pathological manifestations of VaD and play a key role in disease progression. Necroptosis, also known asprogrammed necrosis, is a mode of programmed cell death distinct from apoptosis and is closely associated with ischemic injury and neurodegenerative diseases. Recent studies have shown that necroptosis in VaD exacerbates BBB destruction, activates neuroinflammation, promotes neuronal loss, and severely affects VaD prognosis.
Results and Conclusions
In this review, we outline the significant roles of necroptosis and its molecular mechanisms in the pathological process of VaD, with a particular focus on the role of necroptosis in modulating neuroinflammation and exacerbating the disruption of BBB permeability in VaD, and elaborate on the molecular regulatory mechanisms and the centrally involved cells of necroptosis mediated by tumor necrosis factor-α in neuroinflammation in VaD. We also analyze the possibility and specific strategy that targeting necroptosis would help inhibit neuroinflammation and BBB destruction in VaD. With a focus on necroptosis, this study delved into its impact on the pathological changes and prognosis of VaD to provide new treatment ideas.
1 Introduction
Vascular dementia (VaD) results in cognitive impairment and memory loss and is the second-most common type of dementia worldwide after Alzheimer's disease (AD) [1-4]. With the aging of the population, the incidence of VaD is increasing, bringing a heavy economic burden to families and society, and has become a public health problem that seriously threatens human health, but its underlying pathogenesis has not yet been fully elucidated. Given the lack of identifiable therapeutic targets, there is an urgent need to identify effective treatment modalities [5, 6]. Therefore, in-depth exploration of the pathogenesis of VaD to identify therapeutic targets is critical. Numerous clinical studies have found that pro-inflammatory mediators, such as cytokines, chemokines, and interleukins (IL) are significantly elevated in the peripheral blood of patients with VaD. At the same time, reactive microglia and astrocytes around the damaged white matter are significantly activated, indicating that neuroinflammation is a meaningful pathological change of VaD [7, 8]. Additionally, inhibition of glial cell activation and abatement of neuroinflammation are effective in improving cognitive impairment in animal models of VaD [9-11]. Therefore, suppressing neuroinflammation is vital to improving cognitive impairment in VaD. The destruction of the blood–brain barrier (BBB) is a significant cause for aggravating neuroinflammation, leading to neuronal loss [12, 13]. BBB permeability in the brain also increased with increased white matter lesions in patients with VaD [14]. Thus, neuroinflammation and BBB disruption play a key role in the progression of VaD.
Necroptosis is a form of regulated necrotic cell death mediated by receptor-interacting protein kinase 1 (RIPK1) kinase activity, RIPK3, and mixed-lineage kinase domain-like pseudokinase (MLKL) [15-17]. An increasing number of studies have shown that necroptosis is closely related to age-related neurodegenerative diseases and acute neuronal injury [18-22]. Necroptosis mediates endothelial cell (EC) death and neuroinflammation and participates in BBB decomposition after cerebral ischemic/reperfusion injury [23-26]. It has been found that inhibition of RIPK1, RIPK3, and MLKL in necroptosis can rescue neuronal cell loss in xenografted human neurons [21]. Furthermore, the involvement of necroptosis in regulating neuroinflammation and BBB destruction, which mediates cortical neuronal death, has been demonstrated in both cellular and animal models of ischemic brain injury and is closely associated with cognitive deficits in VaD; the possible mechanism is closely related to the RIPK3-mediated inflammatory signaling pathway inducing apoptotic neuronal death in VaD [27-32].
To sum up, the pathogenesis of VaD is very complex, and there is no effective treatment at present. Necroptosis is closely related to VaD, but its exact molecular mechanism remains unclear. This review discusses the molecular mechanism between necroptosis and neuroinflammation and between VaD and the destruction of BBB permeability to guide the prevention and treatment of VaD. Elucidating this mechanism can provide a new perspective for developing the prevention and treatment of VaD.
2 Overview of VaD
Currently, VaD has various clinical diagnostic criteria, which, despite their different characteristics, cover two core elements: (1) to confirm the existence of dementia and (2) to identify vascular disease as the primary pathological cause of dementia [33-38]. Although the underlying mechanism leading to the development of VaD has not been fully elucidated, many studies have clearly shown that the pathogenesis of VaD is closely related to many vascular-related risk factors, including diabetes, hypertension, and hyperlipidemia, which can lead to macrovascular and microvascular lesions, which promote vascular changes in the cerebral cortex and subcortex, including white matter hyperintensity, lacunar infarction, and microhemorrhage, resulting in VaD [39-44]. Ischemia and hypoxia-induced by long-term chronic cerebral hypoperfusion (CCH) can trigger cascade reactions of molecular and cellular injury, over-activate neuroinflammation, lead to pathological changes, such as BBB damage, and promote the occurrence and progression of VaD [45, 46].
Neuroinflammation and BBB destruction are the basic pathological manifestations of VaD and influence each other. Neuroinflammation can increase the permeability of BBB. Conversely, increased BBB permeability can also lead to persistent immune response and chronic neuroinflammatory state, thereby aggravating the vicious circle of the VaD pathological process [47, 48]. Therefore, the focus of this review will be on neuroinflammation and BBB disruption in VaD states (Figure 1).
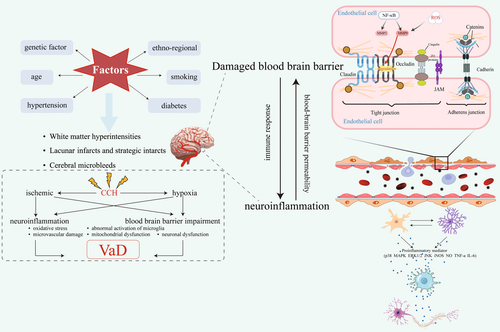
2.1 Neuroinflammation in VaD States
Neuroinflammation is thought to be the main pathogenic factor of VaD [49, 50]. Neuroinflammation in the central nervous system (CNS) is primarily an immune cascade mediated by glial cells due to CNS injury, infection, toxicity, or autoimmunity, eventually leading to neuronal or axonal degeneration or death [51, 52]. Immune cells involved in this process include microglia and astrocytes, but oligodendrocytes are closely related to neuroinflammation. Myelin-associated antigen excitations released by oligodendrocytes can activate peripheral T cells, leading to an autoimmune response. After ischemic stroke, oligodendrocytes derived from oligodendrocyte precursor cells participate in neuroinflammation and contribute to the recovery of nerve function [53]. In addition, neuroinflammation can also deplete oligodendrocytes, the demyelination and remyelination of oligodendrocytes [54]. Neuroinflammation caused by astrocytic and microglial activation was found to be involved in the hippocampal damage process in VaD rats in a study by Sun et al. [55]. Previous studies have demonstrated that activated glial cells induce neuroinflammation via activation of pro-inflammatory mediators, such as mitogen-activated protein kinase (MAPK) p38, extracellular signal-regulated kinase 1/2, c-Jun N-terminal kinase, inducible nitric oxide synthase (iNOS), nitric oxide (NO), tumor necrosis factor-α (TNF-α), and IL-6 [56, 57]. Multiple studies have shown that abnormal activation of microglia in VaD is also an important manifestation of neuroinflammation, and activated microglia can lead to neuronal cell death, tissue matrix degradation, BBB dysfunction, and myelin damage [4, 58-61]. The activation of microglia and reduction of neuroinflammation promote neurogenesis and ameliorate cognitive dysfunction in the VaD mouse model [10]. The above evidence suggests that neuroinflammation is an important pathological manifestation of VaD, and inhibition of neuroinflammation can significantly improve cognitive impairment in VaD.
2.2 BBB Damage in VaD States
The BBB comprises ECs, pericytes, astrocyte endfeet, and basement membranes, which together in their closely related roles with neurons form the neurovascular unit (NVU) [62-65]. Loss of ECs integrity, pericyte degeneration, astrocyte internal membrane swelling, retraction from the vessel wall, loss of tight junctions (TJs) proteins, and cerebral capillary leakage can disrupt the integrity of the BBB, which subsequently leads to CCH [66-68]. Moreover, prolonged CCH also leads to further reductions in ZO-1, occludin, and claudin-5 in TJs, leading to BBB dysfunction and neuroinflammatory responses [69]. The results of animal studies showed that the expression of claudin-5 protein, which maintains the structure and function of the BBB, was significantly reduced in the hippocampus of the VaD mouse model, indicating that the BBB structure was disrupted [70]. In addition, matrix metalloproteinases (MMPs), such as MMP-9 and MMP-2, cleave the extracellular matrix and degrade the TJs of the nuclear basal lamina between ECs, which are essential for the maintenance of the BBB [71]. More convincing evidence shows a significant increase of MMP-2 in the brain and serum of CCH mice and an increase of MMP-9 in the serum of VaD patients [72]. Taken together, these results suggest that increased BBB permeability is an essential pathological manifestation of VaD and that its mechanism of action is related to MMP-2 and MMP-9. However, their mechanisms of action in CCH and VaD have not been fully elucidated.
BBB damage is an early pathological change in cognitive impairment, and BBB damage is considered the leading cause, rather than the result, of aging-related neurodegenerative diseases [73, 74]. Recent studies show that BBB permeability and WM damage are important pathways by which vascular load adversely affects cognitive functioning [75]. The researchers observed loss of capillary pericytes and BBB in the WM of patients with VaD [76]. Furthermore, during disease progression, increased BBB permeability in VaD is accompanied by neuronal loss and WM degeneration [77]. Therefore, BBB destruction is an early change, which has important prognostic value in VaD and provides a critical window of time for treatment.
3 Necroptosis Promotes Neuroinflammation and Aggravates VaD
Necroptosis is mainly initiated by members of the TNF receptor (TNFR) and Toll-like receptor (TLR) families, interferon, intracellular RNA and DNA sensors, and other mediators [78-80]. TNF-α stimulates TNFR1, inducing its activation leading to necroptosis or RIPK1-dependent apoptosis [79, 81, 82]. It is worth mentioning that activation of RIPK1, a pivotal kinase in necroptosis, is one of the main mechanisms of injury in ischemic brain damage [32]. It has been shown that inhibition of RIPK1 activation and TNF-α-induced necroptosis can effectively reduce neuroinflammation and ameliorate brain injury [83, 84]. A variety of inflammatory cytokines, including iNOS, TNF-α, and IL-6, can promote neuronal cell death and BBB destruction [85, 86]. High expression of iNOS and excessive production of NO can also induce neuroinflammation and lead to necroptosis of neurons [87]. In the PND21 mouse model, arsenic-activated microglia via the p38 MAPK signaling pathway led to the overproduction and release of TNF-α. Microglia-derived TNF-α then recruits RIPK1 and RIPK3, activated MLKL, and ultimately induces neuronal necroptosis [88]. In this review, we focus on the effect of necroptosis activated by TNF-α and TNFR1 on neuroinflammation in VaD and its mechanism of action.
3.1 TNF-α Modulates Necroptosis to Exacerbate Neuroinflammation in VaD
TNF-α is one of the most important pro-inflammatory cytokines secreted by various cells, including macrophages and microglia, and regulates necroptosis through the paracrine system [89]. When TNF-α binds to TNFR1 on the membrane surface, it prompts the rapid formation of TNFR1 complex I. This complex I mainly comprises TNFR-associated death domain protein (TRADD), RIPK1, TNFR-associated factor 2 (TRAF2), and cellular inhibitor of apoptosis 1/2 (cIAP1/2) as well as linear ubiquitin chain assembly complex (LUBAC) [52, 90]. Ubiquitinated RIPK1 activates the nuclear factor κB (NF-κB) pathway to initiate cell survival pathways and inflammatory responses. When the NF-κB signaling pathway is inhibited, TRADD in complex I recruits Fas-associated death domain (FADD) and caspase-8 to form complex IIa, and when RIPK1 is deubiquitylated, necroptosis-related complex IIb is formed by RIPK1, FADD, and caspase-8 formation [91]. When caspase-8 is inhibited, microsomes can be formed, comprising mainly of RIPK1 and RIPK3 heterodimerization [92]. There are three main events downstream of TNF-α-mediated necroptosis: phosphorylation of MLKL, assembly of MLKL-containing macromolecular complexes, and disruption of cell membranes [93-95] (Figure 2).
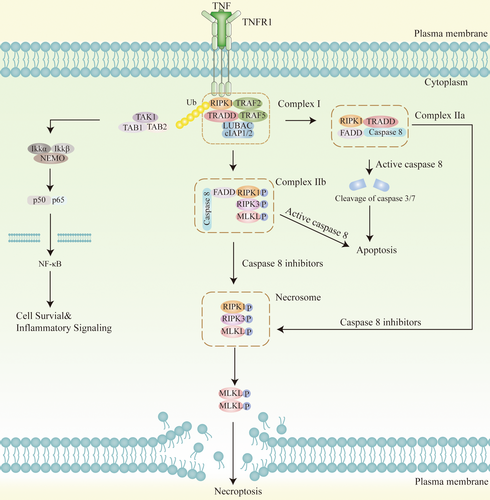
Following phosphorylation and oligomerization of MLKL in RIPK3-phosphorylated microsomes, the N-terminus of MLKL binds directly to phosphatidylinositol phosphates, enabling translocation to the membrane compartment, which leads to membrane rupture and release of cellular contents [96]. In the study by Xu et al. [82] activated neuronal necroptosis is dependent on upstream TNF-α/TNFR1 signaling in both neuronal cell cultures and AD mouse models. It has been demonstrated that ischemic brain injury triggers necroptosis by activating RIPK associated with TNF-α/death receptor, which promotes neuronal cell death [97]. Elevated TNF levels were found in VaD patients' cerebrospinal fluid, suggesting the important role of TNF-α-mediated necroptosis in VaD [98]. By contrast, necroptosis is characterized by plasma membrane rupture, cell swelling, and loss of cell and organelle integrity [99]. Plasma membrane rupture leads to the efflux of cytokines, chemokines, and potassium and the release of damage-associated molecular patterns (DAMPs), triggering activation of the immune system, and neuroinflammation so that necroptosis is highly pro-inflammatory [100-103]. This is supported by a study in which necroptosis and its accompanying inflammatory response could lead to acute injury following ischemic stroke [104]. Necroptosis cells also secrete extracellular vesicles that may be able to modulate the inflammatory response of surrounding cells and are emerging as an important mediator of pro-inflammatory signaling during cell death [105-107]. In summary, TNF-α-mediated necroptosis is closely associated with neuroinflammation in VaD, but its specific molecular mechanism remains to be thoroughly investigated.
3.2 The Effect of Necroptosis of Microglia and Astrocytes on Neuroinflammation
Microglia are immune cells in the brain that maintain the immune defense of the nervous system [108-110]. Microglia are classified into two subtypes: classically activated (M1) cells, the phenotype more likely to be associated with neuroinflammation in neurodegenerative diseases, and alternatively activated (M2) cells, the phenotype promoting inflammatory regression and tissue repair [111-113]. In patients with VaD, a large accumulation of phagocytic activated microglia is seen in the perivascular periphery of the stroke focus [61]. In the CNS, neuroinflammation is characterized by the persistent activation of microglia, the innate immune cells of the CNS, with DAMPs being one of the well-known activators of microglia [114]. In mice with spinal cord injury (SCI), high mobility group box1 (HMGB1) in DAMPs induces pro-inflammatory microglia activation via the receptor for advanced glycation endproducts (RAGE)-NF-κB pathway, and inhibition of HMGB1 or RAGE significantly reduces neuronal loss and demyelination [115].
However, it is noteworthy that the use of the RIPK1 inhibitor Nec-1s did not inhibit microglia-mediated neuroinflammation, but RIPK1 displays scaffold activities in microglial necroptosis. Nonetheless, the exact mechanism of action needs to be validated by further studies [116]. Neuroinflammation and polarization of microglia (M1/M2 phenotype) and activated RIPK3/MLKL-mediated necroptosis promote neuronal loss in an AD mouse model [117]. Moreover, RIPK3/MLKL-mediated neuronal necroptosis regulates the M1/M2 polarization of microglia in the ischemic cortex in primary neuronal culture [118]. It was found that microglia activated by intracerebral hemorrhage (ICH) in ICH rats promote neuronal necroptosis by secreting exosomes and transferring miR-383-3p into neurons [119]. This suggests that necroptosis promotes neuroinflammation and neuronal loss and that targeting necroptosis promotes the conversion of M1 to M2 phenotype, restores microglial phagocytosis, and serves as a therapeutic strategy for VaD.
Astrocyte activation is also known to be closely associated with neuroinflammation. MLKL defects can reduce the loss of tyrosine hydroxylase-positive neurons in Tg-Mlkl mouse models and inhibit the activation of microglia and astrocytes to reduce neuroinflammation [120]. Currently, BBB disruption is the main direction of research in necroptosis of astrocytes. However, the current results indicate that inhibition of necroptosis of astrocytes can inhibit neuroinflammation, and we look forward to further studies on necroptosis of reactive astrocytes in neuroinflammation in the future.
4 Necroptosis Alters BBB Permeability and Exacerbates VaD
Several studies have shown that necroptosis is strongly associated with BBB permeability and that inactivation or genetic inhibition of the RIPK1 kinase reduces BBB permeability or BBB disruption, both in mouse models of ICH and experimental subarachnoid hemorrhage in rats [121-123]. There is increasing evidence that RIPK1 and RIPK3, essential kinases in necroptosis, have a role in increasing BBB permeability in cerebral ischemia or hemorrhage, and that inhibition of necroptosis may have a BBB-maintaining and neuroprotective effect on VaD [25, 83, 122, 124, 125]. In summary, necroptosis is involved in BBB disruption. In the following sections, we will explore in detail the molecular mechanisms of BBB disruption in VaD in terms of ECs, mitochondrial dysfunction, and necroptosis involving astrocytes.
4.1 BBB Destruction by Necroptosis of ECs in VaD
ECs are a core component of the NVU, as they are bound together by TJs to form a selective permeability barrier between the blood and CNS, transport nutrients, and remove waste via specific receptors [126-128]. An early event in the pathogenesis of VaD is dysfunction of the ECs, leading to the loss of BBB integrity [129]. It was shown that EC-epidermal growth factor receptor (EGFR) is a regulator of TNFR1-mediated inflammation and RIP3-dependent necroptosis and that inhibition of the kinase of the EGFR disrupted the formation of complex I and complex IIb and prevented RIP3-dependent necroptosis in ECs [130]. More compelling evidence is that perivascular M1 microglia-induced necrosis of ECs leads to BBB disruption and that anti-TNF-α (infliximab) treatment significantly improves ECs necroptosis, BBB disruption, and improves stroke outcomes [25]. Thus, necroptosis is a potential mechanism leading to ECs dysfunction, which ultimately leads to the destruction of the BBB, resulting in poor prognosis. Thus, preventing necroptosis of ECs in VaD is necessary to protect the BBB as well as improve the prognosis of dementia.
4.2 Mitochondrial Dysfunction and Oxidative Stress Promote Necroptosis and Damage the BBB
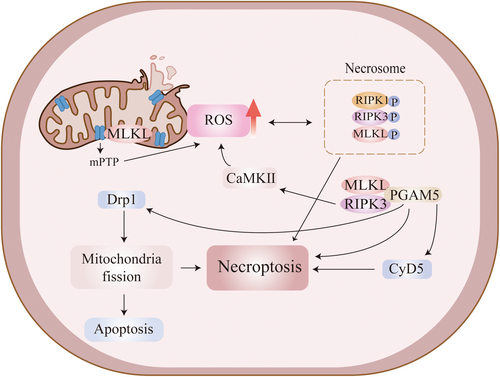
Mitochondrial dysfunction and oxidative stress occur in VaD; moreover, they have been shown to play a key role in the progression of VaD and the development of BBB damage [131, 132]. TNF-α increases RIPK3 expression and promotes the formation of RIPK1/RIPK3 necrosome, leading to the production of large amounts of reactive oxygen species (ROS), causing mitochondrial dysfunction and aggravating organismal damage [133, 134]. ROS induces RIPK1 autophosphorylation on S161, which is essential for RIPK1 activation [135]. ROS activation in microglia triggers neuroinflammation and alters synaptic activity and neuronal transmission [136]. TNF-α-induced necroptosis requires RIPK1/RIPK3-mediated mitochondrial ROS generation at an early stage; RIPK1 is also required to sense ROS during necroptosis execution [137]. It has been demonstrated that ROS scavenger (N-Acetyl-l-cysteine) reduced the expression of p-RIPK3 and p-MLKL proteins and inhibited necroptosis after traumatic brain injury (TBI) in mice [138]. TNF-α-induced necroptosis requires oxidative stress-generated ROS not only upstream but also in the downstream pathway, where phosphoglycerate mutase 5 (PGAM5), a downstream molecule of RIP3/MLKL that regulates necroptosis through activation of cyclophilin D (CypD) and dynamin-related protein 1 (Drp1), plays an important role in oxidative stress-induced necroptosis [139]. It has also been shown that calcium/calmodulin-dependent protein kinase II, a downstream factor of RIPK3, can increase ROS levels and induce mitochondrial dysfunction [140]. In addition, MLKL may also be transferred to the mitochondrial membrane, leading to an over-opening of the mitochondrial permeability transition pore (MPTP), resulting in the overproduction of ROS, which further exacerbates cell death [141] (Figure 3). In conclusion, mitochondrial dysfunction and oxidative stress play an important role in TNF-α-mediated necroptosis, especially for RIPK1/RIPK3 necrosome, and are responsible for the massive production of ROS, which exacerbate BBB damage and thus accelerate disease progression in VaD.
4.3 Effect of Astrocyte Necroptosis in the BBB on VaD
Necroptosis leads to astrocyte death, which acts as a trophic agent for neurons and maintains CNS homeostasis in vivo [142-144]. It has been demonstrated that attenuating vascular stenosis-induced astrogliosis preserves white matter integrity and cognitive function [9]. Zhu et al. [145], in their study on ischemic stroke, showed that RIPK1 enhances the vascular endothelial growth factor D (VEGF-D)/VEGF receptor VEGFR-3 signaling pathways and contributes to astrogliosis and glial scar formation, and RIPK3 and MLKL are also involved in ischemia-induced reactive astrogliosis in a transient middle cerebral artery occlusion (tMCAO) rat model and an oxygen and glucose deprivation and reoxygenation (OGD/Re)-induced astrocytic injury model. On the other hand, the knockdown of RIPK1 prevented OGD-induced astrocyte damage and inhibited the increase in astrocyte lysosome numbers in the ischemic cerebral cortex induced by permanent middle cerebral artery occlusion in a rat model [146]. Y-2 inhibits astrocyte-mediated neuroinflammation and attenuates TNF-α-triggered neuronal necroptosis in cell cultures and AD mice [147]. These results suggest that reducing reactive astrocyte proliferation plays an important role in the disease process of VaD. The RIPK1 inhibitor NEC-1, which can effectively reduce astrocyte proliferation, has the potential to become an important drug to reduce the degree of dementia in VaD. However, its specific mechanism of action needs to be further investigated in depth.
M1 microglia can cause BBB dysfunction and vascular leakage, whereas M2 microglia play a protective role in the BBB [148]. Targeting necroptosis can promote the conversion of M1 to M2 phenotype. Whether it is also possible to target necroptosis in the BBB to play the protective role of M2 microglia on the BBB is something to look forward to in the future.
5 Treatment
5.1 Inhibition of Necroptosis Pathways
RIPK1 inhibitors are potent inhibitors of necroptosis [149]. Nec-1, a blocker of RIPK1, protects the brain from ischemic necroptosis by reducing RIPK1 activation and inhibiting its downstream signaling pathways and is protective against brain injury [150, 151]. Specifically, in the middle cerebral artery occlusion (MCAO) rat model, cerebral ischemia stimulated RIPK1 phosphorylation at Ser166 residue, RIPK3 phosphorylation at Ser232 residue, and MLKL phosphorylation at Ser345 residue, and significantly increased RIPK1, RIPK3, and MLKL levels, whereas Nec-1 attenuated these changes through inhibition of the phosphorylated RIPK1 kinase [152]. Many experiments have demonstrated that inhibition of RIPK1 activation is very effective in preventing ischemic injury [153-155]. Furthermore, in transient OGD (resupply [R]) brain microvascular ECs, administration of Panax notoginseng saponins and NEC-1 could protect against OGD/R-induced necroptosis by inhibiting phosphorylation of receptor-interacting Ser/Thr protein kinase 1 (RIP1), RIP3, and MLKL, suggesting that P. notoginseng saponins and NEC-1 effectively inhibit the occurrence of necroptosis in ischemic stroke by inhibiting the RIP1/RIP3/MLKL signaling pathways [156]. However, it is worth noting that the limited ability of anti-TNF-α antibodies to enter the brain makes TNF-α antibody-based therapies of limited utility in neurodegenerative diseases [157]. Furthermore, in necroptosis cells that have exposed phosphatidylserine in their outer membrane, targeting the pMLKL translocation was more favorable to the reversal of necroptosis events than targeting RIPK1 and RIPK3 activation [105].
The crosstalk between ferroptosis and necroptosis deserves attention. Iron overload is one of the mechanisms of ferroptosis that leads to MPTP opening, which exacerbates RIP1 phosphorylation and leads to necroptosis [158]. Furthermore, heat shock protein 90 (HSP90) induces necroptosis and ferroptosis by promoting RIP1 phosphorylation and inhibiting GPX4 activation [140]. In neuroinflammation, acyl-CoA synthetase long-chain family member 4 (ACSL4) overexpression exacerbates neuronal ferroptosis and pro-inflammatory cytokine release from microglia by catalyzing the esterification of arachidonic acid and adrenergic acid to phosphatidylethanolamine [159]. Cistanche total glycoside capsule has been marketed to treat VaD disease. One of the active ingredients, Cistache deserticola phenylethanoid glycoside, can reduce the formation of lipid peroxides and improve the mechanism of ferroptosis by upregulating the GPX4/SCL7A311 axis and downregulating the ACSL4/LPCAT3/LOX axis, preventing neuroprotective effects and alleviating learning and memory dysfunctions in mice suffering from hypobaric hypoxia [160]. Thus, focusing on the association between ferroptosis and necroptosis may offer a promising therapeutic option for VaD.
5.2 Inhibition of Necroptosis-Mediated Over-Activation of Glial Cells
It has been demonstrated that fetuin-A inhibits oxidative stress and necroptosis in glutamate-treated primary microglia, thereby attenuating the aberrant inflammatory response after trauma [138]. Moreover, necrosulfonamide inhibits astrocyte necroptosis and improves prognosis by preventing the upregulation of protein kinase expression in necroptosis in the tMCAO rat model, blocking translocation of p-MLKL and p-RIPK3 to the nuclear membrane and co-localization at the nuclear membrane [161]. It has been found that melatonin inhibits microglial necroptosis via the A20/RIP3 pathway, thereby reducing cell death, inflammation, and mitochondrial damage [162]. Inhibition of necroptosis of glial cells was demonstrated to be a key target for the treatment of abnormal glial cell death, activation, or neuroinflammation in VaD. Research in this area is still at an early stage and needs to be further strengthened. Currently, more studies in VaD are focused on targeting the TLR4/NF-κB signaling pathway to alleviate neuroinflammation and improve cognitive impairment [163, 164]. Electroacupuncture (EA) has been recognized as a potential treatment for cognitive impairment in recent years, and it has been found that EA can inhibit neuroinflammation by reducing astrocyte necroptosis through down-regulation of the RIP1/MLKL/TLR4 pathway in mice with SCI [165, 166].
5.3 Reduction of Oxidative Stress to Inhibit Necroptosis and Thus Protect Neurons
First, oxidation of vitamin C and the generation of dehydroascorbic acid could regulate neuronal necroptosis under conditions of oxidative stress in neuronal N2a and HN33.11 cell lines and cortical neurons [167]. Second, ketamine as a potent inhibitor of necroptosis may have neuroprotective effects [168]. In the VaD rat model, lycopene prevents memory deficits by inhibiting ROS levels, but whether lycopene exerts its neuroprotective effects by directly eliminating ROS or through downstream pathways activated by ROS remains unknown [169]. Novel glycosylated angiotensin-(1–7) Mas receptor agonist PNA5 reverses cognitive deficits, reduces ROS production, and inhibited inflammatory cytokine production in a preclinical mouse model of vascular cognitive impairment and dementia (VCID) that is induced by chronic heart failure [170]. Novel materials have been developed for the treatment of VaD. Wang et al. designed a novel inhaled nanotherapeutic agent, P/D@Mn/Co3O4, which could effectively inhibit acute and chronic cerebral ischemia symptoms by scavenging ROS and damaged mitochondria from the lesion area in a rat model of stroke and VaD [171]. Neuroinflammation characterized by TNF-α, oxidative stress characterized by ROS and superoxide dismutase were all significantly ameliorated in a rat model of permanent occlusion of the bilateral common carotid arteries (2-vessel occlusion [2VO]) after the use of bisindole analogue (2-(2-(bis(5-chloro-1H-indol-3-yl)methyl)phenoxy) aniline, compound 4ae) in a rat model of 2VO [172]. P53 is a central stress sensor in cells and is closely associated with necroptosis by regulating redox signaling [137]. Hyperbaric oxygen inhibits intracellular p53 levels in repeated cerebral ischemia–reperfusion injury rats, providing new mechanisms and laboratory evidence for the clinical treatment of VaD [173].
In summary, little work has been done to target necroptosis as a therapeutic target for VaD, and inhibition of necroptosis in specific cell types or inhibition of key factors in necroptosis appears very promising. Small molecules that inhibit necroptosis by targeting the kinase activity of RIPK1 have shown promise in several murine models of non-infectious disease and phase-II human clinical trials. This has triggered an investment of more than one billion dollars (USD) into the emerging class of necroptosis-blocking drugs [174].
6 Conclusion
Neuroinflammation and BBB destruction play a crucial role in the disease progression of VaD. In this review, we focus on the molecular mechanisms of necroptosis action in VaD. Targeting necroptosis in VaD significantly inhibits neuroinflammation and BBB destruction and improves the prognosis of VaD, and therapeutic measures targeting necroptosis are expected to be part of the disease modification strategy in VaD. In this review, only the effects of both neuroinflammation and BBB permeability in VaD under necroptosis have been described. However, other aspects of VaD, such as demyelination and axonal degeneration have not been addressed in this study. In future studies, we will further explore the role of other aspects of necroptosis in VaD to provide a multifaceted perspective for the treatment of VaD.
Acknowledgments
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.




