The Gut Microbiota Modulates Neuroinflammation in Alzheimer's Disease: Elucidating Crucial Factors and Mechanistic Underpinnings
Funding: This work was financially supported by Innovative Research Project for Postgraduate Students of Heilongjiang University of Traditional Chinese Medicine (no. 2024yjscx013).
ABSTRACT
Background and Purpose
Alzheimer's disease (AD) is characterized by progressive cognitive decline and neuronal loss, commonly linked to amyloid-β plaques, neurofibrillary tangles, and neuroinflammation. Recent research highlights the gut microbiota as a key player in modulating neuroinflammation, a critical pathological feature of AD. Understanding the role of the gut microbiota in this process is essential for uncovering new therapeutic avenues and gaining deeper insights into AD pathogenesis.
Methods
This review provides a comprehensive analysis of how gut microbiota influences neuroinflammation and glial cell function in AD. A systematic literature search was conducted, covering studies from 2014 to 2024, including reviews, clinical trials, and animal studies. Keywords such as “gut microbiota,” “Alzheimer's disease,” “neuroinflammation,” and “blood–brain barrier” were used.
Results
Dysbiosis, or the imbalance in gut microbiota composition, has been implicated in the modulation of key AD-related mechanisms, including neuroinflammation, blood–brain barrier integrity, and neurotransmitter regulation. These disruptions may accelerate the onset and progression of AD. Additionally, therapeutic strategies targeting gut microbiota, such as probiotics, prebiotics, and fecal microbiota transplantation, show promise in modulating AD pathology.
Conclusions
The gut microbiota is a pivotal factor in AD pathogenesis, influencing neuroinflammation and disease progression. Understanding the role of gut microbiota in AD opens avenues for innovative diagnostic, preventive, and therapeutic strategies.
1 Introduction
Alzheimer's disease (AD) manifests as a relentless neurodegenerative disorder marked by progressive memory impairment and cognitive deterioration, coupled with the accumulation of amyloid-beta (Aβ) plaques and neurofibrillary tangles (NFTs) within the brain [1]. Epidemiological investigations indicate a notable surge in AD-related mortality over the past two decades, cementing its status as the foremost neurodegenerative affliction among the elderly globally [2]. Despite extensive research efforts, the precise pathogenesis of AD remains enigmatic; however, burgeoning evidence implicates neuroinflammation as a central factor in its pathological advancement [3]. Neuroinflammation, predominantly driven by activated neuroglial cells—the innate immune cells of the central nervous system (CNS)—is pivotal in AD [4]. Dysregulation and hyperactivation of neuroglia lead to the excessive secretion of pro-inflammatory cytokines, reactive oxygen species (ROS), and nitric oxide (NO), thereby exacerbating neuroinflammation and neurotoxicity [4-6].
The gut microbiota orchestrates cerebral function and influences the onset and progression of AD. It modulates the equilibrium of the gut–brain axis by interacting with the CNS, including the vagus nerve, immune system, and endocrine system [7]. Alterations in the gut microbiota are associated with elevated levels of inflammatory cytokines, oxidative stress, and neurotoxicity in the brains of both AD patients and animal models [8, 9]. The gut microbiota may impact AD by regulating neuroinflammation, as its metabolites may cross the blood–brain barrier (BBB), eliciting anti-inflammatory and neuroprotective effects on glial cells [10-12]. Furthermore, the gut microbiota influences the infiltration of peripheral immune cells and the exposure of glial cells to systemic inflammatory stimuli, regulates the expression and activity of pattern recognition receptors, and modulates the production and metabolism of neurotransmitters [13-15].
This paper aims to provide a comprehensive analysis of the current role of the gut microbiota in modulating neuroinflammation and glial cell function in AD. Its objective is to enhance our understanding of AD's pathogenesis and to deliberate on the potential advantages and challenges of manipulating the gut microbiota as a novel approach for diagnosing, treating, and preventing AD.
2 Gut Microbiota and AD
2.1 Pathogenesis of AD
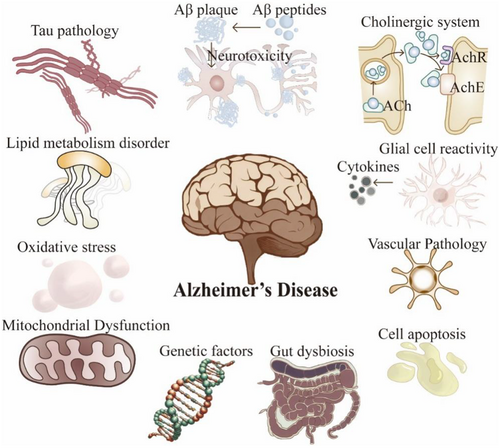
Current investigations propose that the etiology and pathogenesis of AD may be influenced by myriad risk factors (Figure 1): (i) The Aβ cascade hypothesis assumes a central role in AD pathology [16]. (ii) The inflammatory response is pivotal in AD pathogenesis, wherein the resultant inflammatory process can precipitate neuronal damage [17, 18]. (iii) The integrity of the BBB is crucial in AD progression, and its compromised state incites inflammatory and neurodegenerative processes [19, 20]. (iv) Other pathological mechanisms [3, 21]: Apart from the aforementioned factors, various elements such as tau protein modifications, the autophagy–lysosomal pathway, mitochondrial function, cholinergic transmission, oxidative stress, and genetic susceptibility may influence AD progression. Notably, disruptions in the gut microbiota may serve as significant factors in AD pathogenesis, influencing Aβ deposition and neuroinflammation [22]. These interwoven factors collectively contribute to AD progression.
2.2 Gut Microbiota and the Brain
High-throughput sequencing technologies have provided us with a deeper understanding of the diversity and abundance of human gut microbes. The magnitude and composition of these microbiota are shaped by various factors, including gender, age, diet, and geography [23]. Gut microbes not only colonize the body's surfaces and fluids but predominantly inhabit the digestive tract [24]. They form an extensive chemical factory that impacts host health through the synthesis of diverse compounds. The gut microbiome plays a pivotal role in modulating host immunity, digestion, and neural signaling and its association with brain function, potentially offering novel insights into the exploration, comprehension, and treatment of neurodegenerative conditions like AD [25]. The microbial–gut–brain axis represents a complex network of connections between the gut and the brain through the nervous, endocrine, and immune systems via multiple pathways (e.g., metabolite pathways, immune response pathways, neurotransmission pathways, neuroendocrine–hypothalamic axis pathways, and gut and BBB), which may act autonomously or synergistically to influence the onset and progression of AD [22, 26].
2.3 Alterations in the Gut Microbiome in AD
Recent studies have revealed significant discrepancies in the gut microbiota among individuals suffering from AD [27-36] or analogous animal models [37-46], juxtaposed with those of robust health (Table 1). These investigations propose that the alterations noted in the gut microbiome, encompassing reduced microbial diversity, abnormal proliferation or depletion of particular bacterial groups, decreased abundance of probiotics, and variances in microbial metabolites, could represent a pivotal aspect in the pathogenesis of AD [47-51].
| Category | Methodology | Major findings | Refs. |
|---|---|---|---|
| AD patients | 16S rRNA sequencing | Bacterial population in the brain ↑ | [27] |
| 16S rRNA sequencing | Firmicutes and Bifidobacterium ↓, Bacteroidetes↑ | [28] | |
| qPCR | Escherichia/Shigella ↑ and E. rectale ↓ | [29] | |
| Metabolic phenotyping | Metabolite concentrations of tryptophan pathway metabolites in urine and serum ↓ | [30] | |
| PCR for DNA and DNA sequencing | LPS and gram-negative Escherichia coli fragments colocalize with amyloid plaque | [31] | |
| 16S rRNA sequencing | Various changes in microbial populations associated with amyloid positivity and p-tau status | [32] | |
| Behavioral assessment | FMT from healthy donors showed improvements in MMSE score, memory, cognition, mood, and socialization | [35] | |
| MCI patients | 16S rRNA sequencing | The abundance of the genus Ruminococcus, Butyricimonas, and Oxalobacter ↓ | [36] |
| GC–MS and PET | Fecal levels of acetic acid, butyric acid, and caproic acid ↓; Fecal SCFAs in MCI group were negatively associated with Aβ deposition in cognition-related regions | [50] | |
| CSF biomarker quantification | TMAO levels in CSF ↑ | [51] | |
| Adults (4 Age Groups) | qPCR | Bifidobacterium, Faecalibacterium, Bacteroides group, and Clostridium cluster XIVa ↓ with age up to 66–80 years | [33] |
| Animal models | |||
| GF mice | RNA-seq | GF animals display global defects in microglia | [37] |
| APP/PS1 transgenic mice | 16S rRNA sequencing and 1H nuclear magnetic resonance | Proteobacteria and Verrucomicrobia ↑, Bacteroidetes and key metabolic components of SCFAs ↓ | [38] |
| Thy1-C/EBPβ transgenic mice | Immunofluorescent staining and ELISA assays | FMT from AD donors induces Aβ and Tau aggregation, associated with upregulation of the C/EBPβ/AEP pathway, microglia activation, and cognitive impairment | [46] |
| APP/PS1 transgenic mice | 16S rRNA sequencing | Microbiota composition and diversity perturbed | [39] |
| 5xFAD mice | 16S rRNA sequencing and Immunofluorescent staining | Dysbiosis of gut flora (Aβ in gut; trypsin ↓; Firmicutes: Bacteroidetes ratio ↑; Clostridium leptum ↑); C/EBPβ/AEP pathway active in gut with age | [40, 41] |
| 3xTg mice | Immunofluorescent staining | Microbiota accelerates AD pathology with active C/EBPβ/AEP signaling in brain | [41] |
| 3xTg mice | 16S rRNA sequencing and Immunostaining | FMT from SPF mice and AD patients to GF 3xTg mice activates C/EBPβ/AEP signaling, promoting microglial activation and cognitive deficits | [42] |
| P301L mice | 16S rRNA sequencing | Firmicutes: Bacteroidetes ratio↓ | [43] |
| 5xFAD mice | Amyloid quantification and behavioral assessment | 7-day FMT regimen improved the “plaque-busting” and cognitive behavior shown in 5xFAD mice | [44] |
| APP/PS1 transgenic mice and WT mice | ELISA assays and behavioral assessment | FMT from AD donors worsened behavior and increased neuroinflammation; FMT from AD donors to WT increased neuroinflammation | [45] |
| APP/PS1 transgenic mice | ELISA assays and behavioral assessment | FMT from WT donors improved cognitive function, decreased Aβ plaque burden, and decreased levels of soluble Aβ40 and Aβ42 | [38] |
- Note: Arrows: ↑: Increase or higher levels;↓: Decrease or lower levels.
- Abbreviations: Aβ, amyloid-beta; AD, Alzheimer's Disease; AEP, aspartic endopeptidase; C/EBPβ, CCAAT/enhancer-binding protein beta; CSF, cerebrospinal fluid; FMT, fecal microbiota transplantation; GC–MS, gas chromatography–mass spectrometry; GF, germ-free; LPS, lipopolysaccharide; MCI, mild cognitive impairment; MMSE, mini-mental state examination; NMR, nuclear magnetic resonance; PCR, polymerase chain reaction; PET, positron emission tomography; qPCR, quantitative polymerase chain reaction; SPF, specific pathogen-free.
3 Gut Microbiota and Neuroinflammation
Neuroinflammation is the immune response elicited by glial cells within the CNS, typically in reaction to stimuli such as neural injury, infections, toxins, or autoimmunity [52]. In AD, neuroinflammation constitutes the third primary pathological hallmark following Aβ accumulation and NFT formation [53]. Among the principal glial cell types, microglia are distinguished as the predominant innate immune cells of the CNS, serving as the primary responders to Aβ plaques by aggregating in their vicinity [54, 55]. Numerous genome-wide association studies have further identified microglia as the principal cell type expressing AD-related genes [56]. Microglia play a pivotal role in detecting and reacting to environmental changes, eliminating harmful stimuli, and presenting antigens to T lymphocytes, ultimately contributing to neurodegeneration [18]. Astrocytes are integral in maintaining the integrity and metabolic coupling of the BBB, regulating ion and neurotransmitter homeostasis, producing neurotrophic factors, and supporting neuronal activity and synaptic function [6]. Under normal circumstances, microglia and astrocytes perform neuroprotective functions, limiting the extent of neuroinflammation. However, in pathological conditions such as AD, the chronic activation of microglia and astrocytes becomes dysregulated and overactivated, leading to sustained low-grade neuroinflammation and excessive production of pro-inflammatory cytokines, ROS, and NO, which can be detrimental to neurons and synapses [4]. Indeed, neuroinflammation and the activation of glial cells are hallmark features of AD [57].
Neuroinflammation plays a key role in the pathogenic mechanisms of AD, influencing the metabolism of Aβ and tau, the functionality of neurons and synapses, and accelerating the progression and deterioration of AD. It augments the production and deposition of Aβ, a hydrophobic peptide that, if not cleared, aggregates into neurotoxic oligomers and fibrils, leading to neuronal death and synaptic dysfunction [58]. Neuroinflammation activates β-secretase and γ-secretase pathways, enhancing Aβ production while releasing oxidative stress factors, metalloproteinases, and inflammatory cytokines that impair Aβ degradation and clearance [59]. Additionally, neuroinflammation disrupts Aβ transport and elimination, causing its accumulation at the blood–brain barrier. It also catalyzes the aberrant phosphorylation and aggregation of tau [60]. In AD pathology, aberrant tau phosphorylation leads to its detachment from microtubules, culminating in the formation of NFTs and disrupting neuronal metabolism and signal transduction. The activation of various kinases, such as GSK-3β, CDK5, MAPK, and PKA, induced by neuroinflammation, elevates tau phosphorylation levels [54]. Moreover, the secretion of inflammatory cytokines like tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and IL-6 during neuroinflammation exacerbates tau aggregation and dissemination [18]. Neuroinflammation inflicts damage on neurons and synapses, both directly and indirectly, by inducing apoptosis and necrosis through the release of oxidative stress factors, inflammatory cytokines, nitric oxide, and glutamate [4]. Additionally, neuroinflammation disrupts the synthesis, release, and reuptake of neurotransmitters, precipitating synaptic dysfunction and degeneration. The altered expression and signaling pathways of neurotrophic factors further impair neuronal survival and plasticity [61]. Hence, mitigating neuroinflammation emerges as a promising strategy for the prevention and treatment of AD.
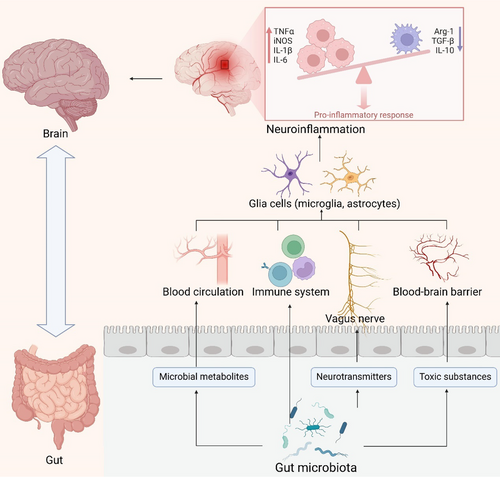
Furthermore, the gut microbiota serves as a significant source of amyloid proteins. Bacterial strains such as Escherichia coli, Bacteroides fragilis, Salmonella typhi, Pseudomonas fluorescens, and Staphylococcus aureus are known to produce amyloid proteins. These strains synthesize proteins like curli, TasA, CsgA, FapC, and phenol-soluble modulators, which facilitate the misfolding of Aβ fibrils and oligomers. This aberration may compromise the host's immune system [62]. Additionally, these bacteria can produce endotoxins such as lipopolysaccharides (LPS), whose levels rise following bacterial infection or alterations in gut microbial metabolic activity due to inflammation, potentially exacerbating neurodegeneration [63]. Notably, this process may impair brain cell function, initiating a vicious cycle between the gut and the brain [64, 65]. Presently, the comprehension of the mechanisms by which the gut microbiota modulates neuroinflammatory processes in AD remains in its nascent stages. Nonetheless, preliminary evidence from extant research indicates that the gut microbiota exerts a substantial modulatory influence on neuroinflammation via four discernible pathways: the metabolite pathways of the gut microbiota, immune response pathways, neural transmission pathways, and the gut–brain barrier (Figure 2). These pathways, either independently or synergistically, impact the onset and progression of AD. Continued research endeavors hold the promise of enhancing the understanding of the intricate and interrelated interactions between the gut microbiota and AD, thereby offering a novel vantage point for the development of innovative therapeutic strategies.
3.1 The Metabolite Pathway
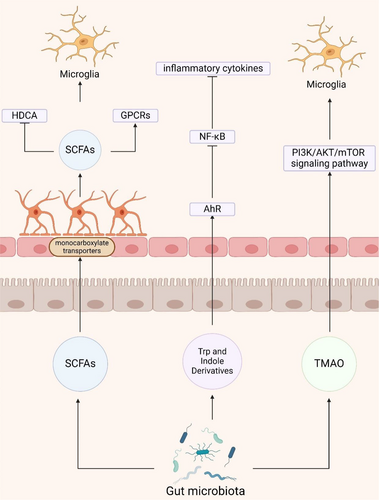
The gut microbiota plays an important role in the function and behavior of the neuroinflammatory system, affecting it through the direct or indirect production of metabolites such as short-chain fatty acids (SCFAs), amino acids, lipopolysaccharides (LPS), and trimethylamine (Figure 3) [66].
3.1.1 Short-Chain Fatty Acids
SCFAs, primarily composed of butyrate, propionate, and acetate, are fermentation by-products of the gut microbiota, predominantly derived from the phylum Bacteroidetes and indigestible carbohydrates such as dietary fibers [67]. These SCFAs permeate the BBB via monocarboxylate transporters on endothelial cells and contribute to maintaining BBB integrity by suppressing pathways associated with non-specific inflammatory responses to microbial infections in vitro [68, 69]. Studies have demonstrated that SCFA mixtures restore the expression levels of claudin-5, occludin, and ZO-1 in the hippocampus, thereby improving BBB permeability [70, 71]. Similarly, using hCMEC/D3 cells as an in vitro model of the human BBB, it was observed that propionate mitigated the LPS-induced disruption of occludin, claudin-5, and ZO-1 localization within cells [69]. Thus, dysbiosis of the gut microbiota may compromise the protective role of SCFAs on the BBB, leading to increased permeability and the infiltration of peripheral inflammatory factors into the brain, ultimately contributing to neuroinflammation [72, 73].
SCFAs exert their physiological effects by acting as endogenous ligands for G-protein-coupled receptors (GPCRs), particularly GPR43 and GPR41, and by modulating gene expression through inhibition of histone deacetylase (HDAC) activity [12]. In vitro studies have demonstrated that acetate displays anti-inflammatory properties in Aβ-induced BV-2 microglia by enhancing GPR41 expression and inhibiting the ERK/JNK/NF-κB signaling pathway [74]. Furthermore, in vitro experiments have indicated that SCFAs can diminish histone deacetylase activity and hinder NF-κB nuclear translocation, thereby directly modulating LPS-induced primary microglial cells [75]. Animal studies have shown that genetic deletion of microglial Hdac1 and Hdac2 significantly ameliorates cognitive deficits in 5xFAD mice by enhancing microglial Aβ phagocytosis [76]. Meanwhile, augmenting butyrate levels through Clostridium butyricum intervention has demonstrated its capacity to suppress microglial activation and reduce pro-inflammatory cytokine levels in APP/PS1 mice [77]. SCFAs directly interact with microglia, attenuating their antigen-capturing capabilities and consequently reducing the production of pro-inflammatory cytokines such as IL-12 and TNF-α [78, 79]. These cytokines play a crucial role in regulating the neuroinflammatory response and Aβ deposition in the brain [75]. Despite numerous studies highlighting the pivotal role of SCFAs in mediating communication between gut microbiota and glial cells, a more exhaustive understanding of the mechanisms underlying SCFA actions in AD, including their effects on other neural cells within the brain, necessitates further meticulous exploration.
3.1.2 Tryptophan and Indole Derivatives
Amino acids, as precursors of bioactive molecules, play a pivotal role, with mounting evidence highlighting the critical involvement of the gut microbiota in the metabolic processing and utilization of essential amino acids, particularly tryptophan (Trp) [80]. The majority of dietary L-tryptophan released in the gut is transferred into circulation via epithelial transport; approximately 10%–20% of L-tryptophan is metabolized by intestinal epithelial cells and the gut microbiota within the intestinal lumen [81]. The gut microbiota can degrade dietary L-tryptophan into bioactive metabolites, namely (i) indoles, (ii) kynurenine (Kyn), (iii) serotonin, and (iv) tryptamine pathways [82-85]. Enterochromaffin cells in the gut are capable of converting dietary L-tryptophan into serotonin, while the gut microbiota can also modulate the synthesis and release of serotonin from enterochromaffin cells [86]. For instance, Reigstad et al. [87] demonstrated that SCFAs produced by the microbiota promote serotonin production in human enterochromaffin cells. Tryptophan hydroxylase 1 (TPH1), the rate-limiting enzyme in serotonin biosynthesis, catalyzes the conversion of L-tryptophan into 5-hydroxytryptophan, which is subsequently decarboxylated to produce 5-hydroxytryptamine (5-HT), or serotonin. Consequently, 5-HT can be further metabolized into melatonin, regulating various features of the gut microbiota, including oxidative stress and inflammation [88].
Gut microorganism-mediated Trp metabolism encompasses pathways such as the aryl hydrocarbon receptor (AhR) ligand pathway, the indole pathway, the Kyn pathway, and the 5-hydroxytryptophan pathway [89]. Metabolites of Trp derived from the gut, such as indole or bacterial tryptophanase, influence astrocytes and microglia via AhR signaling, thereby significantly impacting neuroinflammation in experimental autoimmune encephalomyelitis mice [90, 91]. Additionally, indole metabolites from the gut microbiota activate AhR, inhibit the NF-κB pathway, suppress the formation of NLRP3 inflammasomes, and reduce the production of inflammatory cytokines, thereby improving gastrointestinal function, modulating microglial reactivity, and alleviating neuroinflammation in APP/PS1 mice [92, 93]. Kyn can traverse the BBB to exert its effects within the brain [94]. Furthermore, Kyn treatment has been shown to upregulate the expression of NLRP2 inflammasomes in astrocytes, resulting in the secretion of IL-1β and IL-18 [95]. Although the neuroinflammatory role of gut-mediated Trp metabolites in AD has been explored to some extent, further research is necessary to elucidate the precise mechanisms underlying the actions of different tryptophan metabolites in AD.
3.1.3 Trimethylamine N-Oxide
Trimethylamine (TMA) is synthesized by the gut microbiota during the metabolism of methylamine-containing dietary nutrients [96]. This compound subsequently undergoes hepatic metabolism to form trimethylamine N-oxide (TMAO) via flavin monooxygenase. TMAO crosses the blood–brain barrier, triggering neurodegeneration by activating microglia and astrocytes and enhancing the release of inflammatory mediators [97, 98]. TMAO promotes inflammation and worsens Aβ and tau pathology in D-galactose/AlCl3-induced AD mice through the PI3K/AKT/mTOR signaling pathway [99]. Conversely, administration of the TMA formation inhibitor 3,3-dimethyl-1-butanol diminishes circulating TMAO levels and improves cognitive deficits in APP/PS1 mice by attenuating Aβ pathology and neuroinflammation [100].
3.2 The Immune Pathway
The immune and CNS are intricate networks that regulate various physiological functions in the organism [101]. They exhibit shared characteristics in their function and development, potentially contributing to the pathogenesis of neuropsychiatric disorders. Around 70%–80% of immune cells in the human body reside within the gastrointestinal tract, facilitating direct interactions between gut and immune cells [102]. Microbe-associated molecular patterns produced by pathogenic microbes engage pattern recognition receptors (e.g., TLRs) on host cell surfaces, modulating the production of both pro- and anti-inflammatory cytokines [103]. These cytokines cross the BBB and influence CNS cells, including microglia, thereby shaping the brain's inflammatory environment [104]. The resultant chronic inflammation significantly impacts neurodegeneration [104].
3.2.1 Immune Regulation
Dysregulation of the pro-inflammatory gut microbiota in AD patients may initiate inflammation and promote the formation and aggregation of Aβ proteins [101]. Accumulation of Aβ in the brain triggers intracerebral immune-inflammatory responses via TLRs and CD14, predominantly mediated by microglial cells. This cascade results in the release of various cytokines and the upregulation of antigenic markers, precipitating a neuroinflammatory response [105]. This acute and transient inflammation supports Aβ clearance and neuronal protection [105]. Systemic inflammation resulting from intestinal dysregulation may exacerbate microglial hyperactivation and impair hippocampal plasticity, worsening the onset and progression of AD [106]. Disruption of intestinal barrier (IB) function increases permeability to commensal microbes, microbial-derived products (e.g., metabolites, virulence factors), and other intestinal constituents, leading to aberrant immune-inflammatory responses such as inflammation, allergies, and autoimmune diseases mediated by molecular mimicry and dysregulated T-cell responses [66]. T cells play a crucial role in systemic and mucosal immune responses, initiated by dendritic cells continually sampling the intestinal lumen. This process primarily contributes to the proliferation of regulatory T cells (Treg) [107]. Furthermore, the altered composition of gut microbes and their derived metabolites may stimulate or inhibit the differentiation of initial CD4+ T cells into TH17 cells, which are highly abundant at the mucosal barrier and play a key role in regulating tissue homeostasis. Specific intestinal bacteria induce distinct T-cell subsets; for example, segmented filamentous bacteria drive the differentiation of Th17 cells, while fragile Bacteroides generate Treg expressing the transcription factor Foxp3 [108, 109]. Some commensal microorganisms, such as Bacteroides fragilis, Bifidobacterium infantis, and Firmicutes, as well as certain microbial metabolites, can influence immune responses by affecting different cell types in the immune environment [106]. They induce the differentiation of Treg into effector cells, such as Th1 and Th17 cells, or IL-10 regulatory T cells, promoting either immune activation or tolerance [110]. They induce the differentiation of Treg into effector cells, such as Th1 and Th17 cells, or IL-10 regulatory T cells, promoting either immune activation or tolerance [111-115]. Bacillus-derived poly-γ-glutamic acid specifically signals CD4+ T cells, facilitating selective Treg differentiation [116]. Increased microbiota-induced Th17 differentiation has been linked to behavioral abnormalities from maternal immune activation, potentially migrating from intestines to meninges [107, 117-119]. Intestinal microbe–immune cell interactions regulate T-cell and B-cell dynamics, impacting immune responses in both peripheral and CNS. Gut T and B cell subsets may migrate from gut to meninges, influencing local neuroimmune environments through cytokine production such as IL-17a, IL-10, and IgA antibodies, thereby modulating neuroinflammation [120]. Additionally, microbiota-derived metabolic products like taurocholic acid, histamine, indole, and spermine influence downstream neuropeptides, regulating NLRP6 inflammasomes, IL-10, and IL-18 secretion, which correlate with inflammatory factor levels and Alzheimer's severity [14, 121].
3.2.2 5-Hydroxytryptamine
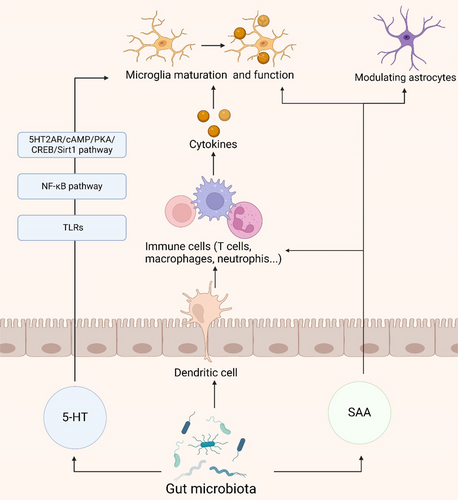
Serotonin, also known as 5-hydroxytryptamine (5-HT), acts as a neurotransmitter with multifaceted roles in the brain and gut, particularly in orchestrating the gut microbiota-brain axis [122]. The gut microbiota influences systemic immune function through modulation of 5-HT production and release from gut enterochromaffin cells [122, 123]. 5-HT impacts the functionality of monocytes and macrophages, governing inflammatory responses and potentially influencing neuroinflammation [124]. Research indicates that 5-HT regulates neuroinflammation by activating the 5HT2AR/cAMP/PKA/CREB/Sirt1 pathway and the NF-κB pathway, controlling the transcription of TLR2 and TLR4 in response to microglial phagocytic stimuli and thereby influencing neuroinflammation [125-127]. Additionally, 5-HT modulates the release of inflammatory cytokines, affecting the activation of immune cells and inflammatory responses, such as TNF-α, IFNγ, IL-1β, IL-17, and IL-6 [128]. Moreover, 5-HT binding to its receptors on microglia triggers the release of cytokine-laden exosomes, providing an alternative mechanism for the modulation of gut-induced neuroinflammation [129]. The synthesis, metabolism, or transport of 5-HT, critical in the inflammatory response, may offer novel avenues for mitigating neuroinflammation in AD.
3.2.3 Serum Amyloid A
Serum amyloid A (SAA), a product of the inflammatory cascade in intestinal epithelial cells, serves as a prominent acute-phase reactant, with the gut microbiota potentially modulating neuroinflammation through the regulation of SAA levels [130, 131]. In the brain tissues of AD patients, SAA localizes with the distribution of senile plaques [132]. A recent study reported that SAA expression in the brains of APP/PS1 mice exacerbates neuroinflammation by hindering astrocyte activation and migration toward Aβ plaques via the p38 MAPK pathway [133]. In vitro, recombinant SAA treatment modulates the functions of astrocytes and microglia, decreasing astrocyte viability while enhancing microglial activity [132]. There are also differences in cytokines and inducible iNOS between the two cell types [132]. PI3K is a common pathway mediating the effects of SAA on astrocytes and microglia, whereas the c-JNK pathway is selectively induced in microglia, and the NF-kB pathway is selectively activated in astrocytes [132]. Furthermore, SAA promotes the differentiation of Th17 cells, increasing the expression of the pro-inflammatory cytokine IL-17, which induces the production of cytokines such as IL-1β, IL-6, TNF-α, and IL-22 [131, 134]. SAA orchestrates neuroinflammation, regulates cholesterol metabolism, and activates glial cells, impacting AD [135].
In this immune pathway, the intestinal microbiota has a role beyond immune system modulation. Its regulation of 5-HT, mediation of inflammatory responses through SAA and interactions with neurons provide insights into the complex pathogenesis of neuroinflammation (Figure 4). These findings not only enhance our understanding of neuroimmune interactions but also suggest new avenues for potential therapeutic strategies to mitigate neuroinflammatory processes.
3.3 The Vagus Nerve Pathway
The vagus nerve (VN), a pivotal component of the autonomic nervous system, comprises 80% afferent fibers and 20% efferent fibers. It intricately traverses the gastrointestinal tract, functioning as a critical neural pathway [136]. By facilitating bidirectional information transmission with visceral organs via motor and sensory fibers, the VN regulates organ function and maintains internal organismal homeostasis [137]. This nerve serves as a pivotal link between the intestinal microbiota and the brain, playing a crucial role in the neural-immune and gut–brain axes (Figure 5) [137].
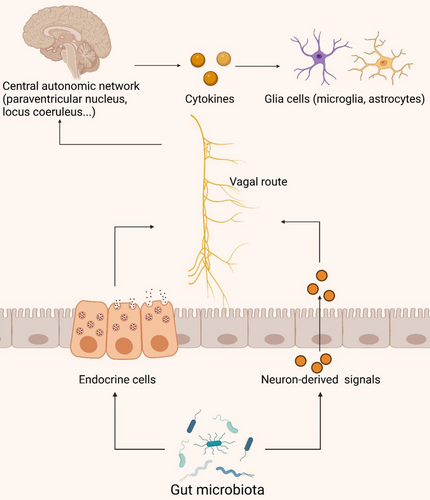
Intestinal endocrine cells interact directly with VN afferent fibers, transmitting information to the central autonomic network for analysis and integration (including the paraventricular nucleus, locus coeruleus, hypothalamus, and limbic system encompassing the thalamus, amygdala, and hippocampus) [138]. Research indicates that chronic VN stimulation in rats with AD can enhance their memory, likely through modulation of glutamate receptors [139]. VN stimulation activates the locus coeruleus, triggering catecholamine release in the hippocampus and neocortex, enhancing synaptic plasticity, and reducing levels of inflammatory signaling factors (such as TNF-α, IL-1β, and IL-6) [140-142]. These pro-inflammatory molecules may access the CNS via the bloodstream or VN afferent fibers, triggering neuroinflammatory responses, activating microglial cells and astrocytes, and leading to neuronal damage and cognitive impairment [143-145]. Furthermore, VN efferent fibers can synthesize and release acetylcholine (ACh), influencing cholinergic neurons. Activation of ACh released by VN efferent fibers inhibits TNF-α secretion, demonstrating an anti-inflammatory effect through binding to α-7 nicotinic ACh receptors on macrophages [146]. While the VN's role in the gastrointestinal domain is clear, the exact operational pathways and complex processes are still under investigation. Concurrently, increasing research underscores the VN's significant role in elucidating the interplay between gut microbiota and neuroinflammation.
3.4 The Gut–Brain Barrier
The IB consists of the epithelial layer that lines the intestinal tract, along with associated elements such as the mucous layer, tight junctions, and immune cells, orchestrating the selective passage of intestinal contents to safeguard against pathogens and toxins [147]. The BBB is constituted by specialized brain endothelial cells within microvessels, meticulously regulating the exchange of molecules and nutrients between the bloodstream and brain tissue [148]. Dysbiosis of the intestinal microbiota, characterized by reduced diversity, inflammation, and toxicity, compromises IB integrity, potentially triggering or exacerbating inflammation at the IB and permitting the unchecked translocation of pathogenic microbiota across the BBB (Figure 6) [149]. Persistent systemic inflammation can perturb BBB structure, increasing permeability and precipitating neuroinflammation, neurodegeneration, and age-related cerebral changes [150, 151].
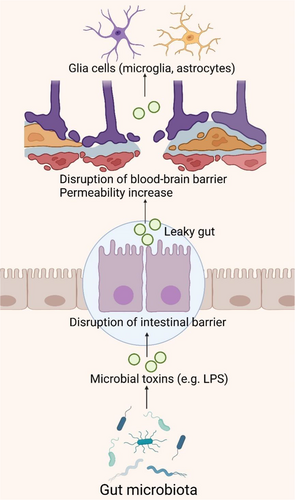
Specific Gram-negative bacteria in the gut can produce substantial quantities of amyloids, lipopolysaccharides (LPS), or endotoxins, breaching the IB and BBB to provoke robust pro-inflammatory and innate immune responses within the CNS, potentially modulating signaling pathways and pro-inflammatory cytokine production associated with AD [152-154]. LPS, a prevalent endotoxin, acts as an immunostimulant by being transported to the surface of myeloid cells via LBP, where they bind to membrane-bound CD14, forming a complex that subsequently activates the TLR4-MD2 complex. The activation of TLR4 initiates a signaling cascade involving MyD88, IRAK, and TRAF6, ultimately leading to the activation of NF-κB and MAPK through the NIK and TAK1 pathways. This cascade results in the release of inflammatory cytokines such as IL-1β, IL-6, and TNF-α, thereby instigating an inflammatory response [155]. Research has identified the accumulation of bacterial-derived LPS within the neuronal parenchyma and around the periphery of neuronal nuclei in the hippocampus and superior temporal gyrus of AD patients [156, 157]. This occurrence is attributed to the synergistic effects of bacterial LPS and amyloid-like proteins, which may exacerbate intestinal permeability, leading to elevated cytokine levels such as IL-17A and IL-22, known to correlate with AD [158, 159]. Moreover, LPS stimulation of the enteric nervous system induces the production of pro-inflammatory cytokines TNF-α, IL-1β, and IL-6, selectively activating TLR4 on astrocytes and microglia, triggering NF-κB pathway activation, escalating cytokine production, inducing neuroinflammation, and fostering Aβ deposition, crucial in inflammatory signaling associated with AD [160-162]. This triggers NF-κB pathway activation, increasing cytokine production, inducing neuroinflammation, and Aβ deposition, playing a key role in the inflammatory signaling cascade in AD patients [163-165]. Additionally, during aging, vascular impairments, or degenerative diseases, harmful metabolites originating from the gut microbiota may permeate into the systemic circulation and cerebrovascular system, accumulating at both systemic and cerebral levels [166]. This accumulation can elevate ROS and activate the NF-κB signaling pathway, thereby upregulating pro-inflammatory miRNA-34a. Consequently, this downregulates TREM2 expression, impairing microglial phagocytic function and resulting in Aβ accumulation [158, 159].
4 Prospective Therapeutic Strategies for AD
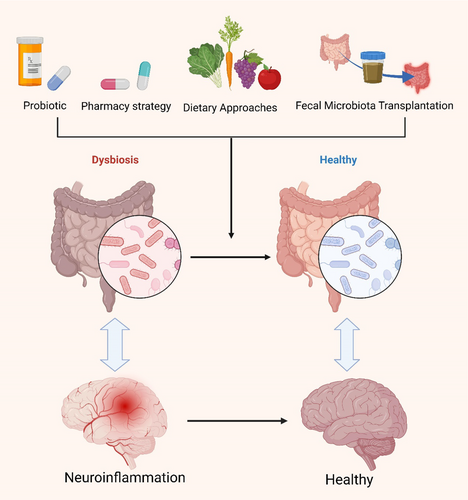
The intricate mechanisms by which the gut microbiota influences AD pathogenesis offer promising avenues for future therapeutic interventions. Compared to conventional brain-targeted treatments, strategies focusing on the gut microbiota possess unique advantages, not only circumventing the challenge of the BBB but also allowing for more precise modulation of host–microbe interactions, thereby achieving faster therapeutic outcomes. These approaches are also regarded as safer, given their lower side effect profile, particularly through interventions such as dietary modifications, supplementation with probiotics and prebiotics, fecal microbiota transplantation, and other microbiota modulators, which can effectively reshape the composition of the gut microbiome, yielding beneficial effects on neurological disorders and alleviating pathological conditions (Figure 7; Table 2) [10, 35, 38, 138, 167-178]. Moreover, an increasing body of research suggests that certain traditional Chinese herbal monomers, extracts, and compound formulations may exert potential preventive and therapeutic effects in AD by modulating the composition, diversity, and abundance of the gut microbiota. This further underscores the importance of the gut–brain axis in neurodegenerative diseases and highlights that gut microbiota-targeted therapies represent not only an emerging field in AD treatment but also a novel perspective for enhancing overall health.
| Strategy | Model | Therapeutic method | Therapeutic outcome | Refs. |
|---|---|---|---|---|
| FMT | APP/PS1 transgenic mice | Transplantation of fecal microbiota from wild-type mice to transgenic mice expressing APP, PSEN1, and MAPT | Improvement in pathological Aβ plaques, neurofibrillary tangles, neuroglial reactivity, and cognitive impairment | [170] |
| 82-year-old male AD patient | FMT from the patient's cognitively sharp wife | Rapid improvement in AD symptoms (significant increase in MMSE score) | [35] | |
| 90-year-old female AD patient | FMT from a 27-year-old male | Significant improvement in MMSE, MoCA, and CDR scores | [171] | |
| Dietary approaches | Sprague–Dawley rats | Dietary fiber | Interference with the formation of soluble neurotoxic Aβ aggregates | [174] |
| APOE4 transgenic mice | Dietary inulin | Elevation of gastrointestinal microbiota metabolites (SCFAs, tryptophan-derived metabolites, bile acids); reduction in hippocampal inflammation gene expression | [173] | |
| Probiotic | Elderly individuals (≥ 65 years old) | Probiotic supplementation(BGN4 and BORI) | Heightened serum levels of BDNF | [175] |
| 3xTg-AD mice | Lactobacillus lactis carrying plasmid encoding human p62 protein | Improved memory, regulated ubiquitin-proteasome system and autophagy; reduced amyloid burden, oxidative stress, and neuroinflammation | [176] | |
| AppNL-G-F mice | Lactic acid-producing bacteria | Reduced gut inflammation and permeability; minimal impact on brain plaque deposition or cognitive improvement | [177] | |
| Mice injected intracerebroventricularly with Aβ25-35 | Bifidobacterium breve strain A1 | Decreased expression of inflammation and immune response genes in the hippocampus | [141] | |
| 3xTg-AD Mice | Probiotic formulation (SLAB51) | Reduced Aβ aggregation and partially restored compromised neuronal proteins | [140] | |
| C. elegans | Lactobacillus rhamnosus HA-114 | Demonstrated neuroprotective properties | [178] | |
| Pharmacy strategy | 5XFAD transgenic mice | GV-971 (sodium oligo-mannurarate) | Attenuated intestinal dysbiosis and regulated neuroinflammation | [169] |
| APP/PS1 transgenic mice | Long-term administration of broad-spectrum antibiotics | Reduced amyloid plaque deposition, increased soluble Aβ levels; mitigated neuroinflammatory responses by reducing plaque-associated gliosis and altering microglial morphology | [172] |
- Abbreviations: AD, Alzheimer's disease; APP, amyloid precursor protein; Aβ, amyloid-β; BDNF, brain-derived neurotrophic factor; CDR, clinical dementia rating; FMT, fecal microbiota transplantation; MAPT, microtubule-associated protein Tau; MMSE, mini-mental state examination; MoCA, Montreal cognitive assessment; PSEN1, Presenilin-1; SCFAs, short-chain fatty acids.
5 Conclusions and Discussion
AD is witnessing an alarming global surge in prevalence, with the foremost challenge to treatment lying in the incomplete elucidation of its pathogenesis. Current FDA-approved therapies offer only marginal benefits, emphasizing the critical need to explore innovative therapeutic strategies and targets. Inflammatory signals are central to AD pathogenesis, mediated by the bidirectional communication of the gut–brain axis. This study delves into the intricate ways in which gut microbiota modulates AD progression through diverse mechanisms such as metabolite production, immune regulation, preservation of intestinal and BBB integrity, and neurotransmitter synthesis.
The vital role of a healthy gut microbiota in maintaining immune homeostasis includes: (i) fermenting dietary fibers to produce SCFAs, which regulate immune responses by binding to receptors on both intestinal and peripheral immune cells, thereby promoting the secretion of anti-inflammatory cytokines and inhibiting pro-inflammatory factors. This immunomodulatory effect reduces systemic inflammation, which in turn alleviates neuroinflammation; (ii) It helps preserve the integrity of the intestinal epithelial barrier, preventing harmful substances such as pathogens and toxins from entering the bloodstream; (iii) It fosters the generation of Tregs, reducing the release of pro-inflammatory cytokines; and (iv) Through the BBB, it modulates microglial activation, keeping them in an anti-inflammatory and tissue repair mode, thereby diminishing neuroinflammation. Conversely, dysbiosis promotes chronic, low-grade inflammation, exacerbating neuroinflammatory pathways linked to attention deficit disorder [37, 157, 169, 170]. These mechanisms underscore the pivotal role of the gut microbiota in regulating both local and systemic immune responses, thus influencing the trajectory of neuroinflammation and attention deficit disorder. A well-balanced gut microbiota supports anti-inflammatory processes and preserves the integrity of the gut barrier, which is crucial in preventing systemic inflammation from impacting the brain [155, 179, 180]. These mechanisms highlight the critical role of gut microbiota in modulating both local and systemic immune responses, consequently shaping neuroinflammation and the trajectory of AD progression. Dysbiosis has been linked to the promotion of neuroinflammation, while a balanced microbiome appears to offer neuronal protection and mitigate the advancement of AD. Although this paper predominantly focuses on bacteria, it is imperative to recognize that the gut microbiota encompasses viruses, phages, fungi, and other microorganisms, whose roles in AD pathogenesis merit further investigation. Mechanistic insights into how gut microbiota metabolites impact human health, alongside translational research and multifactorial studies—including age, diet, ethnicity, environment, and physical activity—remain crucial areas for future exploration [181].
In conclusion, this study underscores the fundamental influence of gut microbiota on AD pathogenesis, advocating for a deeper investigation into its diversity and impact as a driver for more efficacious therapeutic interventions. In particular, further clarification of the interplay between gut microbiota and immune homeostasis is necessary, as chronic inflammation stemming from dysbiosis plays a central role in exacerbating neuroinflammation and neuronal damage in AD. Future studies should aim to establish causal links between gut microbiota and AD, paving the way for novel microbiome-targeted treatments that may alter the disease's course.
6 Current Challenges and Future Perspectives
The significance of the gut microbiota to human health was recognized by scientists over a century ago, but research was hindered by limited methodologies, particularly the inability to culture anaerobic bacteria within the gut microbiome. The advent of modern technologies, such as anaerobic culturing, DNA fingerprinting, next-generation sequencing, and real-time quantitative PCR, has significantly advanced the study of the gut microbiota [182, 183]. At this stage, defining a healthy microbiota is exceedingly challenging. The abundance and diversity of gut microbiota exhibit considerable individual variability (e.g., gender, race, genetic background, environmental factors, dietary habits, etc.), necessitating further research employing metagenomic analysis and integrating multiple omics approaches such as proteomics, genomics, and metabolomics, rather than solely relying on 16S rRNA gene sequencing to elucidate the regularity of gut microbiota structure and strain levels in AD patients. For high-risk populations, such as individuals with a family history of AD and the elderly, more research on the effects of microbiota-based interventions for AD, potential interactions with other therapies, appropriate sample sizes, and longer follow-up studies should be considered. Currently, methods for modulating the gut microbiota mainly include probiotics, prebiotics, fecal microbiota transplantation, antibiotics, etc. However, the efficacy and safety of these methods require further clinical trials and long-term observations for validation. Additionally, when administering drugs, it is crucial to consider the impact of the medication on other microbiota interventions. It is worth noting that although therapeutic approaches targeting the gut microbiota have certain advantages, strategies for gut microbiota modulation in AD are still in the research stage. Despite some preliminary research suggesting the potential benefits of gut microbiota modulation for AD, the clinical translation of microbiome-based therapies remains challenging, thus requiring continuous research efforts to unravel the complexity of the microbiota–gut–brain axis and fully exploit its potential. With the increasing maturity of technology and methodological innovations, further exploration of the causal relationship between the gut microbiota and AD, elucidation of the molecular mechanisms of microbiota–gut–brain axis neuroinflammation regulation, discovery of more gut microbiota-related AD biomarkers, and development of more effective and personalized gut microbiota modulation therapies are warranted. The plasticity of the human gut microbiome provides an exciting opportunity for the development of personalized microbiota-based therapies for AD.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The authors have nothing to report.




