In vivo Administration of TLR9 Agonist Reduces the Severity of Experimental Autoimmune Encephalomyelitis. The Role of Plasmacytoid Dendritic Cells and B Lymphocytes
Although it is known that infections can increase risk of exacerbation of relapsing-remitting multiple sclerosis (MS), some microbial products trigger a regulatory function and provide a link between microbial recognition and suppression of autoimmune diseases 1. The family of Toll-like receptors (TLRs) has been shown to play the crucial role in developing and directing the response of DCs to microbial invasion 2. TLR-9 is of particular interest, because it is expressed constitutively on pDCs and B lymphocytes, and its ligands, the unmethylated cytosine–phosphate–guanosine oligodeoxynucleotides (ODN-CpG), act as very effective activators of these cells 3. pDCs and B cells may present important functions in the pathogenic and regulatory mechanisms in MS 4, 5. Recent studies have demonstrated that ODN-CpG may also reduce the severity of some autoimmune diseases 6. The beneficial effect of the ODN-CpG may be due to the activation of the tolerogenic properties of the pDC and B lymphocytes. In the present study, we aimed to investigate the role of pDCs and B lymphocytes in the beneficial effect of ODN-CpG treatment in MS experimental model, experimental autoimmune encephalomyelitis (EAE). As far we know this is the first work with in vivo administration of ODN-CpG in EAE.
Experimental autoimmune encephalomyelitis was actively induced in the C57Bl/6 mice by immunization with MOG35-55 peptide (Appendix S1). To test whether administration of the CpG oligonucleotide can modulate the evolution of EAE, we treated mice with five consecutive subcutaneous doses (−5 to 0 days) before immunization with the MOG peptide. The CpG-treated (5 μg/dose) mice had significantly less severe EAE than the control group (Figure 1A). The reduction of the disease was accompanied by significant reduction of specific proliferative response of encephalitogenic T lymphocytes in the lymph node (Figure 1B) and a decrease of encephalitogenic cells infiltrated in the CNS (data not shown). Very interestingly, in vitro depletion of pDCs abrogates the inhibition of the specific proliferative response, suggesting the tolerogenic effect of these cells (Figure 1B). Our results are in agreement with previous study that clearly demonstrated that TLR9 −/− C57Bl/6 mice exhibited more severe EAE symptoms than wild-type mice, suggesting the regulatory effect of the stimulation of TLR9 7. As both pDCs and B lymphocytes present the TLR9, we investigate the activation of tolerogenic functions in these two cells population. Flow cytometry analysis demonstrated that the percentage of both pDCs and B lymphocytes did not alter with the treatment in the spleen or lymph nodes (Figure 1C and D). However, qPCR demonstrated that the expression of IDO was significantly increased in pDCs sorted from lymph nodes of ODN-CpG-treated animals, while the B lymphocytes express more IL-10 in the same group (Figure 1E). Interestingly, the adoptive transfer of both pDCs (Figure 2B) and B lymphocytes (Figure 2C) significantly reduces the severity of EAE confirming that suppressive functions were activated in the two cells populations. The enhancement of IL-10 and IDO expression, in B lymphocytes and pDCs, respectively, may trigger the conversion/expansion of regulatory T cells 8. Indeed, our results show a slightly increase of CD4+Foxp3+ T cells in the spleen and a significant increase of these cells in the lymph nodes of ODN-CpG-treated animals (Figure 1F and G). The increase of Foxp3 was confirmed by the expression of the Foxp3 mRNA (Figure 1H). The major mechanism of Foxp3+ regulatory T cells is the expression and release of IL-10 and TGFβ. These cytokines can modulate the autoaggressive response during EAE evolution and other autoimmune pathologies 8, 9. The Figure 1H demonstrated that the treatment with ODN-CpG significantly increases the expression of IL-10 and TGFβ, which may explain the reduction of the disease with the transfer of these cells.
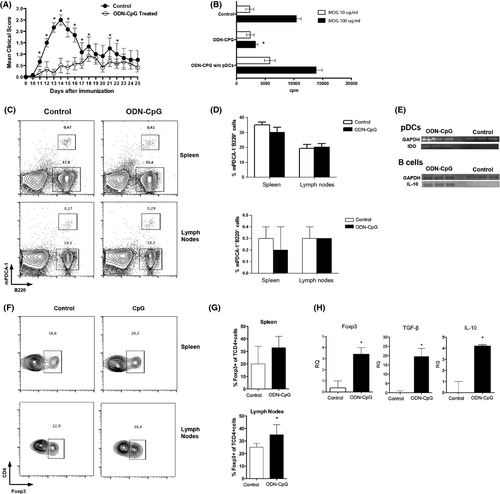
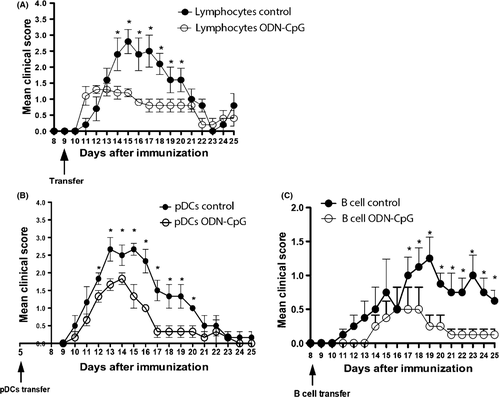
To confirm the activation of regulatory T cells by the treatment with ODN-CpG, T lymphocytes from treated mice and control group treated, only with ODN, were transferred (9d.a.i.) to mice immunized with MOG35–55 before the onset of the disease. The Figure 2A showed that the mice that received T lymphocytes from the CpG-treated ones developed a less severe EAE than those that received T lymphocytes from mice untreated with CpG.
In the EAE model, after successful T cell priming, autoaggressive lymphocytes subsequently migrate into the CNS causing tissue damage 10. The normal immune responses depend on appropriate levels of immunosuppression. In pathological situation such as in autoimmune disease, the immunosuppression is essential to return the inflammatory response to normal levels. Here, we demonstrated that pDCS and B lymphocytes acquire a tolerogenic profile upon the administration of an agonist of TLR 9, ODN-CpG, which significantly reduces the severity of EAE. The enhancement of immunomodulatory molecules, such as IDO, IL-10, and TGFβ, contributes to build a tolerogenic environment in the peripheral immune organs. This tolerogenic environment favors the conversion/expansion of regulatory T cells. Foxp3+ regulatory T cells also release IL-10 and TGFβ, which will contribute to maintain the tolerogenic environment.
Taken together, we demonstrated that the activation of a tolerogenic profile of pDCs and B lymphocytes via TLR9 by ODN-CpG resulted in increase of immunosuppressive mechanisms, which lead to a significant reduction of EAE.
Acknowledgment
This work was supported by grants from FAPESP (#2011/18728-5; 2012/04565-0).
Conflict of Interest
The authors declare no conflict of interest.




