Neuroprotective Effects of the MAO-B Inhibitor, PF9601N, in an In Vivo Model of Excitotoxicity
Summary
Background
PF9601N [N-(2-propynyl)-2-(5-benzyloxy-indolyl) methylamine] is an inhibitor of monoamine oxidase B (MAO-B), which has shown to possess neuroprotective properties in several in vitro and in vivo models of Parkinson's disease (PD). As there is evidence that excitotoxicity may be implicated in the pathophysiology of several neurodegenerative diseases, the aim of the present work was to investigate the effects of PF9601N in an acute in vivo model of excitotoxicity induced by the local administration of kainic acid during striatal microdialysis in adult rats.
Methods
The basal and evoked release of neurotransmitters was monitored by HPLC analysis of microdialysate samples and tissue damage was evaluated histologically “ex vivo.”
Results
PF9601N (40 mg/kg, single i.p. administration) reduced the kainate-evoked release of glutamate and aspartate and increased taurine release, but it had no effect on the release of dopamine, DOPAC, and HVA. PF9601N pretreatment also resulted in a significant reduction in the kainate-induced astrocytosis, microgliosis, and apoptosis.
Conclusions
The results suggest PF9601N to be a good candidate for the treatment of neurodegenerative diseases mediated by excitotoxicity.
Introduction
Factors that may contribute to cell death in neurodegenerative disorders include genetic predisposition, environment, oxidative stress, inflammation, and energy failure. There is also some evidence that excitotoxicity, induced by glutamate, leading to calcium overload in affected neurons, may be an etiological factor in a variety of neurodegenerative disorders such as Alzheimer's, Parkinson's, and Huntington's diseases, multiple sclerosis, and amyotrophic lateral sclerosis (see 1-6).
Although most studies on the role of excitotoxicity in neurodegenerative disorders have concentrated on the NMDA receptors 7, 8, non-NMDA receptors also appear to be involved 1, 5, 9, 10. It has also been suggested that kainate receptors might be a target for the development of new pharmacotherapeutic approaches in Parkinson's disease (PD) 11, 12. Kainate is a cyclic analogue of glutamate that is commonly used to study the mechanisms by which excitotoxicity induces cell death 13. It provokes neuronal death by both apoptotic and necrotic processes 14, 15. It also increases astrogliosis and activates microglia 16-19. Kainate-induced neurodegeneration in rodents has been considered a useful model to elucidate the pathogenesis of excitotoxicity in neurodegenerative diseases 20.
l-Deprenyl (selegiline) is a MAO-B inhibitory propargylamine derivative, which was introduced as an adjunct to levodopa therapy in PD and subsequently shown to possess neuroprotective properties in several PD models and to slow the rate of the disease progression (see 21, 22). Cruces et al. 23 described a series of propargylamine derivatives. Among these, the acetylenic tryptamine derivative PF9601N [N-(2-propynyl)-2-(5-benzyloxy-indolyl) methylamine] (Scheme 1) was shown to be a more potent and selective MAO-B inhibitor than l-deprenyl 24.
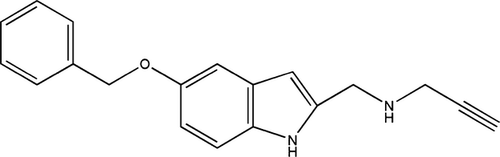
PF9601N (see Scheme 1) showed neuroprotective properties in vivo, against the toxicity induced by MPTP 25 and the intrastriatal injection of 6-hydroxydopamine 26. In vitro studies showed PF9601N to prevent the cell death induced by mitochondrial complex I inhibition and to maintain the mitochondrial membrane potential 27. It was also protective against endoplasmic reticulum stress 28, 29 and able to prevent the dopamine-induced damage to SH-SY5Y cells 30 as well as to reduce reactive oxygen species 31. The antiapoptotic effects of PF9601N appear to involve prevention of transcription factor p53 stabilization and its subsequent transcriptional activity 28.
In this study, we investigated the possible beneficial effect of PF9601N in an acute in vivo model of excitotoxicity induced by the intrastriatal perfusion of kainate during striatal microdialysis in adult rats. Changes in extracellular amino acids and monoamines were studied by HPLC analysis of the microdialysates. After microdialysis, cerebral tissue was evaluated immunohistochemically in terms of the ability of PF9601N to reduce the kainate-induced astrogliosis and microglial reactivity and to exert an antiapoptotic effect.
Materials and methods
Animal Housing
All experiments involving laboratory animals were performed according to the Italian Guidelines for Animal Care (D.L. 116/92), which were in accordance with the European Communities Council Directives (86/609/ECC), with all efforts to minimize animal suffering and the number of animals necessary to collect reliable scientific data. Formal approval to conduct the experiments was obtained from the animal subjects review board of the University of Florence. No alternatives to in vivo techniques are available for this type of experiment.
Male Wistar rats weighing 200–220 g (Harlan, Milan, Italy) were housed in transparent cages under controlled conditions of temperature (23 ± 1°C) and humidity, with free access to food and water and with a 12-h light/dark cycle.
In vivo Experiments
Surgery and Microdialysis Procedure
As previously described 32, rats were anesthetized with chloral hydrate (400 mg/kg, i.p.) and placed in a stereotaxic apparatus. A concentric microdialysis guide cannula (CMA/Microdialysis AB, Stockholm, Sweden) was implanted vertically in the right neostriatum and fixed to the skull with self-curing acrylic (Kerr Italia, Salerno, Italy) and the skin was sutured. Stereotaxic coordinates for the neostriatum, relative to bregma, were AP 0.7, L 3.2, DV −5.5 mm 33.
The microdialysis experiments were performed on freely moving rats 24 h later, starting at 9.00 AM. The artificial cerebrospinal fluid (aCSF) comprised the following: 140 mM NaCl, 3 mM KCl, 1.2 mM CaCl2, 1.0 mM MgCl2, 1.2 mM Na2HPO4, 0.27 mM NaH2PO4, and 7.2 mM glucose (pH 7.4). The dialysis probe (4 mm probe tip; CMA/Microdialysis AB) was inserted into the guide cannula and perfused with aCSF (rate 3 μL/min) via polyethylene tubing (i.d. 0.38 mm) connected to a 1-mL syringe mounted on a micro-infusion pump (CMA/100; CMA/Microdialysis AB). After a 90-min stabilization period, the dialysate samples were collected every 20 min. Three samples, shown in Figures 1 and 2 as −60 min, −40 min, and −20 min, were collected to measure the basal extracellular concentrations of neurotransmitters under resting conditions, before 50 mM KCl was locally applied during collection of the subsequent fraction indicated as +20 min, to represent the first 20-min fraction collected of the potassium-stimulated period. After collecting three more fractions, the rats were anesthetized (chloral hydrate 400 mg/kg i.p.), during collection of the 80-min fraction, just before an excitotoxic concentration (1 mM) of kainate was applied to the neostriatum for 20 min (during collection of the 100-min fraction) through the dialysis probe. The control PF9601N alone treated group also received chloral hydrate anesthesia during collection of the 80-min fraction. Further dialysate samples were then collected every 20 min, up to 180 min. PF9601N pretreatment consisted in a single i.p. administration of 40 mg/kg dissolved in the vehicle dimethylformamide, 3 h before the intrastriatal kainate perfusion; the untreated group received an i.p. administration of the vehicle, 3 h before the application of kainate. The dialysate samples were either analyzed immediately or frozen before analysis. In some experiments, dialysates were split, using 10 μL for amino acid and 50 μL for amines and metabolites determinations.
Rats were killed by decapitation 48 h after kainate stimulation. Then, the striatum was isolated, fixed in 4% formaldehyde in phosphate-buffered saline (PBS) for 72 h, and embedded in paraffin. Finally, 5-μm-thick sections were cut using a microtome (Polaron, Watford, UK) and examined by light microscopy (Nikon Eclipse E800; Nikon Instrument SpA, Florence, Italy) to verify the correct placement of the probe. Only data obtained from rats with correctly implanted probes were included in the results.
For histological studies, sections were first deparaffinized, gradually hydrated, and finally washed twice in 0.1 M PBS (pH 7.4) containing 0.3% Triton X-100 (PBS-0.3Tx).
Amino Acid Determinations
The concentration of the excitatory amino acids, glutamate and aspartate, and the inhibitory amino acids, taurine and GABA, was measured by HPLC with fluorimetric detection, as previously described 34 with minor modifications. Briefly, one 10 μL aliquot of each microdialysate sample was treated with mercaptoethanol and o-phthalaldehyde (OPA) to derivatize the amino acids. The OPA derivatives were then separated on a 5 μm reverse-phase Nucleosil C18 column (EC 250 × 4.6 mm; Macherey-Nagel, Duren, Germany) at room temperature, using a mobile phase consisting of methanol and potassium acetate (0.1 M, pH adjusted to 5.48 with glacial acetic acid) at a flow rate of 1.0 mL/min in a three linear steps gradient (from 25% to 90% methanol). HPLC analysis was carried out using a Shimadzu (Shimadzu Italia S.r.l., Milan, Italy) HPLC system, comprising LC-10AVP pumps, SIL-10ADVP refrigerated autoinjector and RF-551 fluorescence detector (λex = 340 nm and λem = 455 nm, for the amino acid OPA derivatives). The manufacturer's software (Class-VP™ 7.2.1 SP1 Client/Server Chromatography Data System, Shimadzu Italia S.R.L., Milan, Italy) was used for controlling the system and for chromatographic peak recording and integration.
Determination of Dopamine and Metabolites
In some experiments, the concentrations of dopamine and its metabolites, HVA and DOPAC, in microdialysate samples were analyzed using HPLC with coulometric detection 35. Aliquots (50 μL) of the collected microdialysate fractions were injected into the HPLC apparatus, equipped with a Macherey-Nagel, 125/3 nucleosil 100-5 C18 AB column and a μBondapak 10 μm 125A C18 Pre-column (Waters, Milan, Italy). The mobile phase, comprising 75 mM sodium dihydrogen phosphate monohydrate, 3 mM 1-octanesulfonic acid sodium salt, 1.2 mM EDTA, 8% acetonitrile, adjusted to pH 3.4 with phosphoric acid, was run isocratically, at a flow rate of 0.8 mL/min. The coulometric detector consisted of 3 ESA cells (Model 6210; Alfatech SpA, Genoa, Italy) (12 electrodes set at the following potentials: E1 −250 mV, E2 −200 mV, E3 −200 mV, E4 −80 mV, E5 −80 mV, E6 0 mV, E7 100 mV, E8 200 mV, E9 300 mV, E10 350 mV, E11 400 mV, E12 400 mV) and an ESA 5600A CoulArray Detector (Alfatech SpA). Chromatograms were processed using the CoulArrayWin MFC Application software. The detection limit was 0.20 nM for dopamine and 0.50 nM for its metabolites.
Immunohistochemistry
For astrocyte staining, after rehydration, tissue sections were treated with 0.5 M HCl for 30 min and incubated in a blocking solution, comprising 0.2 M glycine, 10% fetal bovine serum, 0.2% gelatin, and 0.3% Triton X-100 in PBS (PBS-0.3Tx) for 1 h. Subsequently, sections were incubated overnight at 4°C with anti-GFAP antibody (1:1000) in a solution containing 0.2 M glycine, 5% FBS, 0.2% gelatin, and PBS-0.3Tx. The sections were washed twice with PBS-0.3Tx containing 0.48 g/L levamisole, to block endogenous alkaline phosphatase activity, and then incubated for 1 h with the secondary anti-rabbit antibody coupled to biotin (1:400) in 0.1 M PBS also containing 0.48 g/L levamisole. After rinsing, the antigen–antibody complex was visualized using the Vectastain ABC-AP kit (1:200). Alkaline phosphatase was developed with 5-bromo-4-chloro-3-indolyl phosphate (BCIP) and nitro-blue tetrazolium chloride (NBTC) substrate solution until a specific blue color was developed.
For the histochemical staining of microglia, sections were incubated in 2% H2O2 and 70% methanol in 0.1 M PBS for 10 min to block endogenous peroxidase activity. After two further washes in PBS containing 0.1% Triton X-100, sections were incubated in 6 μg/mL of biotinylated Lycopersicon esculentum lectin in the previous solution for 2 h at 37°C. Sections were washed again and incubated with HRP–streptavidin–peroxidase (1:200) in 0.1 M PBS for 1 h at room temperature. Finally, sections were washed and developed using 0.05% 3,3′-diaminobenzidine (DAB) and 0.01% H2O2 in 0.1M PBS. Sections were dehydrated in increasing concentrations of ethanol and cleared with xylene, and coverslips were mounted with DPX.
All sections were visualized in a NIKON Eclipse TE 2000-E fluorescence microscope, and images were captured with a Hamamatsu C-4742-80-12AG digital camera. All slides were coded, so that quantitative analysis could be performed by an observer blinded to the treatment group. The number of positively stained cells, microglia or astroglia, was counted in six randomly chosen fields, using the Metamorph® software (Molecular Devices, Sunnyvale, CA, USA). Counts were expressed as the number of positive cells per mm2.
TUNEL Assay
This was used to evaluate the degree of apoptotic damage, analyzing the extent of DNA fragmentation. Terminal deoxynucleotidyl transferase biotin-dUTP nick end labeling immunohistochemistry, using the Klenow fragEL™ DNA Fragmentation Detection Kit (Inalco, Milano, Italia), was applied to identify apoptotic nuclei according to the manufacturer's instructions, as previously described 32. Briefly, all steps were carried out at room temperature unless otherwise stated. Sections were first deparaffinized in xylene and rehydrated through a graded series of ethanol in water. Sections were then digested with proteinase K (1:100) for 20 min, washed in two changes in PBS, incubated for 5 min in 3% H2O2 in methanol (100%) to block endogenous peroxidase activity, and again washed twice with PBS. Section then underwent treatment with Klenow equilibration buffer (1:10) for 30 min, followed by incorporation of a mixture of biotin-labeled and unlabeled deoxynucleotides with the enzyme TdT (terminal deoxynucleotidyl transferase) for 90 min in a humidified chamber at 37°C. After two further washes with PBS, the sections were covered with 100 μL of stop solution for 5 min to stop the reaction and then washed in three changes of PBS. Biotinylated nucleotides were detected using a streptavidin-horseradish peroxidase (HRP) conjugate (1X) (30 min); after two further washes with PBS the chromogen, 3,3′-diaminobenzidine (DAB: 1 mL H2O, one tablet of DAB and one tablet H2O2/urea) was added and left from 5 to 15 min until the brain sections had turned pale brown. Counterstaining with methyl green (10 min) was performed for the morphological evaluation and characterization of nonapoptotic normal cells.
Slides were dehydrated in increasing ethanol concentrations, cleared in xylene, and coverslipped with DPX mountant (VWR, Lutterworth, UK).
Slides containing tissue sections were coded, so that all quantitative analyses could be performed by an observer blinded to the treatment group. Apoptotic and nonapoptotic nuclei were identified by a brown and a light blue color, respectively, using a Nikon Eclipse E800 microscope (Nikon Instruments S.p.A, Firenze, Italy) equipped with a JVC SLR camera. Thus, two counts were made for each image, the number of TUNEL-positive and the number of methyl green-stained cells, in six fields randomly chosen. The number of TUNEL-positive nuclei was then expressed as a percentage of the total number counted (apoptotic + nonapoptotic). Quantitative image analysis was performed using the public domain software Scion Image (Scion Corporation, Frederick, MD, USA).
Statistical Analysis
Statistical analysis of amino acid and amine concentrations in microdialysate fractions was performed on the original concentration values (nM), whereas, for illustrative purposes only, concentrations were expressed as percentages of their respective basal values. The area under the concentration time curve (AUC), normalized to the time unit corresponding to one 20-min fraction, was used for statistical analysis. Mean basal values were obtained from the −20-min fraction, collected immediately before K+ stimulation, or from the 80-min fraction, collected before kainate stimulation. Mean values for the stimulated K+- and kainate-induced output were obtained from the stimulated AUC values. Confidence intervals (95% CI) of means and the one-sample test were used for statistical significance of the evoked output. When appropriate, data were analyzed by ANOVA, followed by the Bonferroni's test for post hoc multiple comparisons, setting the probability level for statistical significance at P < 0.05 and using the program Prism 5.0 for Mac OS X (GraphPad Software Inc., La Jolla, CA, USA). N represents the number of animals used in each analysis.
Materials
The following commercial products were used: 17 amino acid stock solution (Pierce, Rockford, IL, USA). GABA, 2-aminoethanesulfonic acid (taurine), kainate, o-phthaldialdehyde, 2-mercaptoethanol, paraffin, NBTC, BCIP dipotassium salt, eosin, DAB, Hoechst 33258, N,N-dimethylformamide, hematoxylin, eosin, and all other chemicals not listed (Sigma-Aldrich, Milan, Italy); glacial acetic acid, chloral hydrate, and formaldehyde (Merck, Darmstadt, Germany); methanol (BDH, UK); xylene, ethanol, hydrogen chloride, hydrogen peroxide, gelatin from bovine skin (Panreac, Barcelona, Spain), Triton ×100 (Probus, Cornwall, UK), glycine, Tris-HCl (USB), (-)–tetramisole hydrochloride (levamisole), magnesium chloride solution (Fluka, Milan, Italy), glutamine, fetal bovine serum (Gibco, Grand Islands, NY, USA), Vectastain ABC-AP (alkaline phosphatase) kit, Vectastain ABC-peroxidase kit, HRP–Streptavidin, anti-rabbit tyrosine hydroxylase (TH), antibody anti-NeuN, goat anti-rabbit biotinylated IgG, horse anti-mouse biotinylated IgG (Vector Laboratories, Burlingame, CA, USA); anti-rabbit glial fibrillary acidic protein (GFAP) (Dako, Glostrup, Denmark), mouse anti-ssDNA (Apostain) (Bender Med Systems, Atlanta, GA, USA), Alexa Fluor 488 goat anti-mouse IgG (Molecular Probes, Eugene, OR, USA). All other salts were from Merck, and [N-(2-propynyl)-2-(5-benzyloxy-indolyl) methylamine] (PF9601N) was synthesised by the procedure of Cruces et al. 23.
Results
In vivo Experiments
Extracellular Levels of Amino Acids in the Striatum
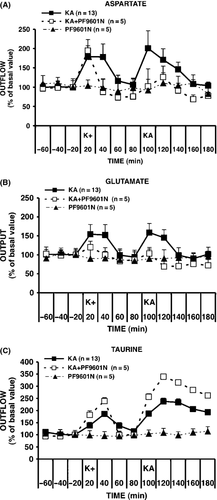
In the absence of any stimulation or treatment, basal levels of aspartate, glutamate taurine, and GABA were found to be stable over the entire period of sample collection (up to 180 min). Three groups of rats were used to study the effects of PF9601N on the basal and K+- and kainate-evoked release. Basal, K+-, and kainate-evoked outputs of the amino acids, aspartate, glutamate, taurine, and GABA, were monitored in one group in the absence and in one in the presence of PF9601N pretreatment, while in a third group of rats, PF9601N pretreatment was administered in the absence of K+ and kainate stimulation to determine any possible effects on basal levels and their stability over time. The time courses of the extracellular concentrations of aspartate, glutamate, and taurine are shown in Figure 1A–C, respectively. The basal extracellular concentrations observed in the three groups, K+/kainate (N = 13), K+/kainate + PF9601N (N = 5), and PF9601N alone (N = 5), respectively, were (mean ± SEM, nM): 218 ± 36, 292 ± 70, and 243 ± 53 for aspartate, 858 ± 142, 835 ± 88, and 857 ± 66 for glutamate, 1459 ± 94, 1052 ± 126, and 1027 ± 180 for taurine, and 41 ± 5, 48 ± 10, and 45 ± 7 for GABA. None of these amino acids showed a statistically significant difference in the basal levels of the three treatment groups. Intrastriatal administration of K+ (50 mM) or kainate (1 mM) induced an increase in the output of aspartate, glutamate, and taurine. The peak increase induced by K+ was 179, 154, and 187% of basal values, for aspartate, glutamate, and taurine, respectively; that induced by kainate was 201%, 168%, and 207% of basal value, for aspartate, glutamate, and taurine, respectively. Mean evoked AUC values (nM, 20 min) ± SEM, together with their statistical parameters, are shown in Table 1. Pretreatment with PF9601N did not affect the K+-evoked release of aspartate or GABA but induced a significant reduction in the kainate-evoked release of aspartate and of both, the K+- and kainate-evoked release of glutamate. In contrast, PF9601N induced an increase in the kainate-evoked release of taurine, from 207 to 342% of basal values. This increase was shown to be statistically significant by ANOVA followed by the post hoc Bonferroni's multiple comparison test (F2,20=4.366, P < 0.0001; kainate vs. kainate plus PF6091N, P < 0.05). Pretreatment with PF6901N alone with anesthesia did not affect the basal output levels of the 4 amino acids, which were stable for the whole collection period. However, as previously reported 32, no kainate-induced GABA release could be observed when kainate stimulation was performed under anesthesia.
| Amino acidAUC (nM, 20 min) | K+ | KA | ||||
|---|---|---|---|---|---|---|
| K+ Vehicle(n = 13) | K+ PF9601N(n = 5) | None PF9601N(n = 5) | KA Vehicle(n = 13) | KA PF9601N(n = 5) | None PF9601N(n = 5) | |
| Aspartate | ||||||
| Evoked (mean ± SEM) | 120 ± 34 | 129 ± 39 | −2 ± 17 | 138 ± 43 | 82 ± 42 | 7 ± 11 |
| 95% CI of the mean | (45–195) | (20–239) | (−50 to 45) | (45–231) | (−34 to 199) | (−23 to 37) |
| One-sample test | P = 0.0045 | P = 0.0304 | NS | P = 0.0070 | NS | NS |
| Glutamate | ||||||
| Evoked (mean ± SEM) | 303 ± 96 | 38 ± 72 | −23 ± 46 | 354 ± 81 | 33 ± 34 | 58 ± 53 |
| 95% CI of the mean | (94–512) | (−162 to 238) | (−151 to 105) | (176–531) | (−59 to 129) | (−89 to 205) |
| One-sample test | P = 0.0083 | NS | NS | P = 0.0010 | NS | NS |
| Taurine | ||||||
| Evoked (mean ± SEM) | 694 ± 111 | 784 ± 117 | −34 ± 14 | 1295 ± 138 | 1852 ± 118 | 55 ± 24 |
| 95% CI of the mean | (452–935) | (459–1108) | (−74 to 6) | (994–1597) | (1525–2180) | (−12 to 122) |
| One-sample test | P < 0.0001 | P < 0.0026 | NS | P < 0.0001 | P < 0.0001 | NS |
| GABA | ||||||
| Evoked (mean ± SEM) | 68 ± 15 | 54 ± 15 | 0.8 ± 5 | −2 ± 2 | 8 ± 3 | 13 ± 5 |
| 95% CI of the mean | (35–101) | (11–96) | (−12 to 14) | (−7 to 4) | (−0.1 to 14) | (−3 to 22) |
| One-sample test | P < 0.0010 | P = 0.0243 | NS | NS | NS | NS |
- K+, 50 mM KCl locally for 20 min; KA, 1 mM kainate locally for 20 min; PF9601N, 40 mg/kg i.p. [N-(2-propynyl)-2-(5-benzyloxy-indolyl) methylamine] 3 h before KA; Vehicle, N,N-dimethylformamide i.p. 3 h before KA; Evoked, mean net output obtained from the area under the K+ or KA-evoked concentration time curve (AUC), normalized to one time interval of 20 min and subtracted of basal output; Concentration values were fmol/μL of perfusate (nM); 95% CL (Confidence Limits) not including zero and the probability level of the one-sample test were taken as an indication of the statistical significance of the mean evoked output of each treatment group; Means ± SEM were derived from number of observations/animals, n = 4–6; NS, not significant.
Extracellular Levels of Dopamine and Its Metabolites
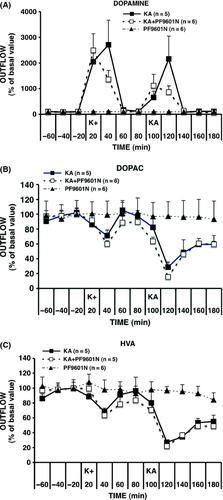
The basal levels of dopamine and its metabolites, HVA and DOPAC, in the absence of any stimulation or treatment, were found to be stable over the entire experimental sample collection period (180 min). Time courses of the extracellular concentrations of dopamine, HVA and DOPAC are shown in Figure 2A–C, respectively. The basal concentrations observed in the three groups, K+/kainate, K+/kainate + PF9601N, and PF9601N alone, which were not significantly different, were (mean ± SEM, n = 4–6) in nM: 1.76 ± 0.33, 1.88 ± 0.10, and 1.58 ± 0.30 for dopamine, 662 ± 36, 800 ± 67, and 807 ± 150 for DOPAC, 320 ± 25, 453 ± 45, and 399 ± 46 for HVA. As shown in Table 2, the intrastriatal administration of 50 mM K+ or 1 mM kainate induced a statistically significant increase in dopamine output together with a decrease in that of the metabolites, HVA and DOPAC. K+- and kainate-evoked changes were not affected by PF9601N pretreatment. PF6901N alone with anesthesia did not affect the basal output levels of dopamine and metabolites, which were stable for the whole collection period.
| Amine AUC (nM, 20 min) | K+ | KA | ||||
|---|---|---|---|---|---|---|
| K+/KA Vehicle | K+/KA PF9601N | None PF9601N | K+/KA Vehicle | K+/KA PF9601N | None PF9601N | |
| Dopamine | ||||||
| Evoked (mean ± SEM) | 26.90 ± 6.77 | 23.03 ± 6.19 | −0.007 ± 0.088 | 15.15 ± 4.38 | 14.41 ± 4.31 | −0.001 ± 0.029 |
| 95% CI of the mean | 8.09–45.71 | 7.11–38.94 | −0.24 to 0.25 | 2.99–27.31 | 0.708–28.12 | −0.083 to 0.080 |
| One-sample test | P = 0.0165 | P = 0.0137 | NS | P = 0.0258 | P = 0.0442 | NS |
| DOPAC | ||||||
| Evoked mean ± SEM | −98.46 ± 21.38 | −144.20 ± 29.40 | 1.33 ± 23.67 | −286.90 ± 37.72 | −335.2 ± 43.15 | 2.47 ± 26.81 |
| 95% CI of the mean | −157.8 to −39.09 | −219.8 to −68.60 | −64.37 to 64.04 | −391.6 to 68.59 | −446.2 to −224.3 | −71.98 to 76.92 |
| One-sample test | P = 0.0100 | P = 0.0045 | NS | P = 0.0016 | P = 0.0006 | NS |
| HVA | ||||||
| Evoked mean ± SEM | −50.62 ± 8.33 | −72.70 ± 16.63 | 6.27 ± 8.96 | −124.00 ± 35.70 | −147.1 ± 27.11 | −8.34 ± 13.03 |
| 95% CI of the mean | −77.13 to −24.10 | −115.4 to −29.96 | −18.61 to 31.16 | −223.31 to −24.84 | −223.3 to −71.81 | −44.51 to 27.82 |
| One-sample test | P = 0.0090 | P = 0.0072 | NS | P = 0.0255 | P = 0.0056 | NS |
- n, Number of animals; NS, not significant.
- K+, 50 mM KCl locally for 20 min; KA, 1 mM kainate locally for 20 min; PF9601N, 40 mg/kg i.p. [N-(2-propynyl)-2-(5-benzyloxy-indolyl) methylamine] 3 h before KA; vehicle, N,N-dimethylformamide i.p. 3 h before KA; evoked, mean net output obtained from the area under the K+- or KA-evoked concentration time curve (AUC), normalized to one time interval of 20 min and subtracted of basal output; Concentration values were fmol/μL of perfusate (nM); 95% CL not including zero and the probability level of the one-sample test were taken as an indication of the statistical significance of the mean evoked output of each treatment group.
Ex vivo Experiments
Striatal Immunohistochemistry of Glial Population
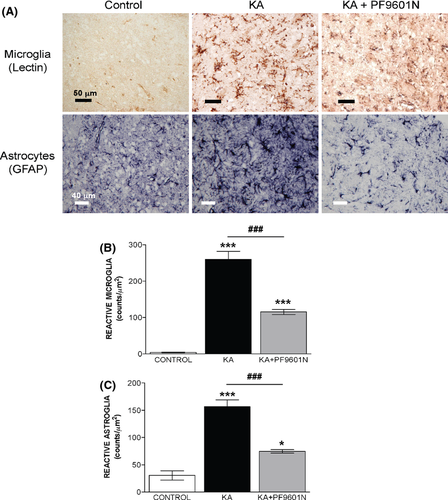
Representative microphotographs of striatal microglia (Lycopersicon esculentum lectin histochemistry) and astrocyte (GFAP immunostaining) response states are shown in Figure 3A. The numbers of activated microglia and astrocytes (mean ± SEM, number of cells/mm2, n = 4) observed in the three groups, PF9601N alone, KA, and KA + PF9601N, are shown in Figure 3B,C. The increase in microglial and astrocytic reactivity observed in the striatum after local kainate application in rats pretreated with PF6091N or vehicle was evident, from the number of lectin- and GFAP-positive cells as well as their staining intensities and morphological features. The staining/morphology score was assessed using the scale of Colburn et al. 36. According to that scale, rats treated with PF9601N alone were scored as “baseline,” resting conditions, being unperturbed and well spaced, consistent with the low counts/mm2 of lectin+ (microglia)- and GFAP (astrocytes)-positive cells, 40.0 ± 0.9 and 30.7 ± 8.5, respectively. The local application of KA induced significantly higher counts/mm2 of both lectin+- (260.0 ± 21.8, 650% of control) and GFAP-positive cells (156.7 ± 12.7, 510% of control), together with an “intense” staining/morphological score (+++). However, the staining/morphological response induced by kainate in rats pretreated with PF9601N was evaluated as “mild” (+), and the lectin+ and GFAP-positive cell counts were much lower, at 115.5 ± 6.8 and 74.7 ± 3.3 cells/mm2, 289% and 202% of control, respectively (Figure 3B,C).
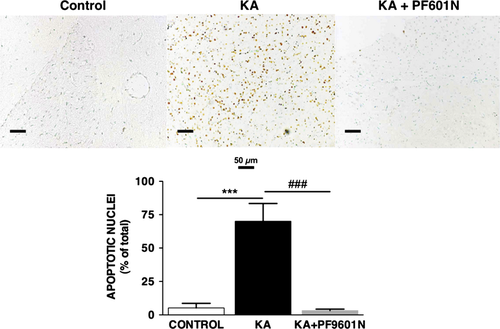
Striatal Immunohistochemistry of Apoptotic Nuclei
Representative microphotographs of apoptotic nuclei in the striatum, determined by the TUNEL assay, are shown in Figure 4. Intrastriatal administration of kainate induced a significant increase in the presence of apoptotic nuclei, 70 ± 13% (n = 6), as compared to 5.2 ± 3.4% (n = 5) in the control group. Pretreatment with PF9601N reduced the number of apoptotic nuclei to control values (3.2 ± 1.1; n = 6) and those that received PF9601N alone (Figure 4 lower panel).
Discussion
The propargylamine derivatives l-deprenyl (selegiline) and rasagiline have been reported to delay the progression of the PD 22, 37-39 and also to improve the motor complications in patients treated with l-DOPA 40, 41. They are currently licensed in Europe and North America for the symptomatic improvement of early PD, to reduce off-time in patients with more advanced PD and motor fluctuations related to levodopa therapy, but not to cover disease modifications [see ref. 42]. As discussed by Schapira 42, because of the limitations of the currently available animal models of PD, further work will be necessary to show whether the experimental evidence that such compounds exert neuroprotective properties applies to the disease in humans [see 42]. The propargylamine derivative PF9601N has previously been shown to have neuroprotective properties in several in vitro and in vivo models of neurodegenerative diseases and also to prolong the effects of exogenously administered levodopa in different experimental models of PD 26, 43, 44.
The present studies indicate that the protective effects of PF9601N extend beyond PD. It also prevents the excitotoxic damage induced by kainate, in a process that involves decreasing the evoked release of the excitatory amino acids and increasing the output of the inhibitory and neuroprotective amino acid taurine. The significant decrease in the kainate-evoked release of the excitatory amino acids, aspartate and glutamate, resulting from PF9601N pretreatment suggests a protective effect against excitotoxicity. However, the effects of PF9601N may not be confined to the neuron, because both aspartate and glutamate are also present in some glial populations 45 and the present work has shown that the glial activation induced by kainate is prevented by PF9601N pretreatment. It has been previously reported that kainate can stimulate glial cells, and this may contribute to the neuronal damage induced by the toxin 17.
The kainate-evoked release of taurine may be a protective response that balances excessive stimulation and the corresponding osmotic disequilibria (see 46). Much of this taurine release in the striatum appears to be from a non-neuronal source, possibly glial 47. The physiological functions of taurine include osmoregulation and modulation of calcium transport. It has been claimed to act as a neuromodulator, neurotransmitter, and neuroprotective molecule against glutamatergic toxicity 48. This would be consistent with the increase in taurine release observed after PF9601N pretreatment having a protective function. The reduction in glutamate and aspartate release indicates that the effects of PF9601N are not at the level of kainate receptor activation, although future work should involve direct studies on its possible interactions with other glutamate-receptor subtypes.
Although GABA is released from striatal terminals under basal conditions 34 and by lower concentrations of kainate (100 μM) 49, the failure to detect it in the present experiments can be attributed to anesthesia that was necessitated by the convulsive effects of the high concentration of kainate required for excitotoxicity 32. Anesthesia with chloral hydrate is also known to reduce, but not to abolish, the release of aspartate evoked by 100 μM kainate, with no significant effect on glutamate or taurine release 49. In the case of dopamine, chloral hydrate anesthesia has been shown to be without effect on the recovery of dopamine 50 but to enhance the K+-stimulated release of dopamine in the striatum 51. In contrast to the results of Hamilton et al. 52 who used the same anesthetic procedure with Sprague-Dawley rats (300−350 g) and reported chloral hydrate to reduce basal dopamine levels in the striatum, we could find no significant difference from controls.
The death of nigral dopaminergic neurons caused by kainate has been previously reported, but this appears to be a secondary phenomenon that does not arise for many days after the initial insult and represents a consequence of the primary toxic events, see 53-55. The mechanisms underlying this indirect dopaminergic toxicity are not fully understood, and it has been suggested that it may result from the loss of striatal interneurones 55, although the high levels of released dopamine and the gliosis observed in the present work could also contribute. In the acute situation, studied in the present work, the protective effects of PF9601N do not appear to involve this system, because pretreatment did not, itself, have any significant effects on the basal levels of dopamine or its metabolites and neither did it significantly affect the kainate-evoked dopamine release. The large release of dopamine evoked by KA was of similar magnitude to that observed in microdialysis after the administration of other glutamate-receptor agonists 56, 57, which was interpreted as being due to a facilitation of DA release. Such a mechanism has also been proposed by others 58-61, whereas Rodriguez et al. 62 suggested inhibition of DA uptake to be involved. The decreased levels of DOPAC observed in the present work, but not investigated in those previous microdialysis studies, would suggest inhibition of dopamine uptake to be involved, because dopamine oxidation to DOPAC does not occur in the extracellular medium. Further work will be necessary to investigate the extent to which this dopamine release contributes, perhaps through the generation of reactive oxygen species, to the kainate toxicity toward other nerve cells.
PF9601N is a highly selective MAO-B inhibitor and the lack of significant effects on dopamine metabolites would be consistent with the dominant role of MAO-A in basal dopamine metabolism in the rat and mouse striatum 63, 64. The extent, if any, to which MAO-B inhibition may contribute to the protective effects would require further investigation, but the neuroprotective effects of propargylamine derivatives in several other systems have been shown to be independent of MAO inhibition (see 22).
Pretreatment with PF9601N reduces the astroglial reactivity and microglial activation resulting from kainate excitotoxic damage. Microglial cells have been reported to be the first population to react against CNS lesions. Their reactions, which appear quite homogeneous and independent from the type of lesion, include changes in their morphology, proliferation and an increase in surface molecules 65. It is difficult to distinguish p53 induction between microglia and macrophages, because they share intracellular and membrane markers, such as lectin domains and CR3 66. However, these results suggest a possible immunomodulatory effect of PF9601N, because it is able to prevent the microglial reactivity induced by kainate.
Thus, the protective effects of PF9601N, which have been previously observed in several models of PD, could also be of value in the therapy of neurodegenerative disorders that involve excitotoxicity. Further studies on the protective effects of chronic treatment with PF9601N would be merited.
Acknowledgments
This work was performed in the framework of the European Actions COST D34 and COST CM1103. We are grateful for financial support from the Ministerio de Ciencia e Innovación MICIN (Madrid, Spain, Grant Ref: SAF2006-08764-C02-01 and SAF2009-07271). ECRF (Firenze, Italy), Università degli Studi di Firenze (Italy), Science Foundation Ireland and ERAB (Brussels, Belgium).
Conflict of Interest
The authors declare no conflict of interest.




