Positive Allosteric Modulation of GABAB Receptors Ameliorates Sensorimotor Gating in Rodent Models
Summary
Background
Converging evidence points to the involvement of γ-amino-butyric acid B receptors (GABABRs) in the regulation of information processing. We previously showed that GABABR agonists exhibit antipsychotic-like properties in rodent models of sensorimotor gating deficits, as measured by the prepulse inhibition (PPI) of the acoustic startle reflex. The therapeutic potential of these agents, however, is limited by their neuromuscular side effects; thus, in this study, we analyzed whether rac-BHFF, a potent GABABR-positive allosteric modulator (PAM), could counter spontaneous and pharmacologically induced PPI deficits across various rodent models.
Methods
We tested the antipsychotic effects of rac-BHFF on the PPI deficits caused by the N-methyl-D-aspartate glutamate receptor antagonist dizocilpine, in Sprague-Dawley rats and C57BL/6 mice. Furthermore, we verified whether rac-BHFF ameliorated the spontaneous PPI impairments in DBA/2J mice.
Results
rac-BHFF dose-dependently countered the PPI deficits across all three models, in a fashion akin to the GABABR agonist baclofen and the atypical antipsychotic clozapine; in contrast with these compounds, however, rac-BHFF did not affect startle magnitude.
Conclusions
The present data further support the implication of GABABRs in the modulation of sensorimotor gating and point to their PAMs as a novel promising tool for antipsychotic treatment, with fewer side effects than GABABR agonists.
Introduction
Ample evidence shows that γ-amino-butyric acid (GABA), the main inhibitory neurotransmitter in the CNS, is implicated in schizophrenia pathogenesis 1-3. In particular, several clinical investigations have documented deficits in the expression of the metabotropic GABAB receptors (GABABRs) in the cortex and hippocampus of schizophrenia patients 4-6. The mechanisms supporting the involvement of GABABRs in psychotic disorders remain elusive; however, recent findings suggest that the dysfunction of this receptors in the cortex leads to alterations of glutamate signaling and excitatory/inhibitory imbalances 7, 8, which contribute to the aberrant information processing and cognitive deficits in schizophrenia 9, 10.
In keeping with this background, our group and others showed that the prototypical GABABR agonist baclofen (BAC) countered the disruption of prepulse inhibition (PPI) of the acoustic startle reflex produced by the blockade of N-methyl-D-aspartate glutamate receptors (NMDARs) in rats 11, 12 and mice 13. This endophenotype is widely regarded as a heuristic index of sensorimotor gating. This cognitive function governs the detection of perceptual salience by enabling pre-attentional information filtering 14, 15; notably, PPI deficits are featured in schizophrenia and related neuropsychiatric disorders 15, 16.
In subsequent studies, we found that BAC rescued the marked deficits in sensorimotor gating present in DBA/2J mice 17, a strain that features antipsychotic-sensitive PPI deficits 18.
These findings collectively highlight GABABR as a highly promising target for antipsychotic treatment. Indeed, Daskalakis and George 19 hypothesized that GABABR activation may be the mechanism underlying the unique ability of the antipsychotic agent clozapine (CLO) in reducing the severity of negative symptoms in schizophrenia. While BAC monotherapy does not elicit significant antipsychotic effects 20, preliminary studies have documented its therapeutic effectiveness as an adjunctive treatment 21, 22. The therapeutic employment of BAC in schizophrenia is limited by several factors, including its poor ability to cross the blood–brain barrier, its short latency of action 23, as well as its potentially severe side effects, including muscle flaccidity, sedation, loss of reflexes, and, at higher dosages, bradycardia, respiratory depression, hypothermia and coma 24. A new class of GABABR-positive allosteric modulators (PAMs) has been recently developed 25 to harness the therapeutic potential of GABABR activation without eliciting the side effects of BAC. The mechanism of these compounds is based on the enhancement of the effects of GABA on GABABRs 26, 27. One of the most potent drugs in this family, rac-BHFF [(R,S)-5,7-di-tert-butyl-3-hydroxy-3-trifluoromethyl-3H-benzofuran-2-one] 25, has been shown to produce behavioral effects without altering motor coordination and nervous reflexes, and with a better safety index than GABABR agonists 25, 28-31. Here, we show that rac-BHFF elicited antipsychotic-like effects on the pharmacologically induced and spontaneous gating deficits in rodent models, as assessed through the paradigm of the prepulse inhibition (PPI) of the acoustic startle reflex; although these effects were comparable with those of BAC and CLO, rac-BHFF did not induce the same alterations of startle reflex associated with these drugs.
Materials and methods
Animals
We used behaviorally naïve male Sprague-Dawley rats weighing between 250 and 300 g and male DBA/2J and C57BL/6J between 18 and 24 g. Animals were housed 4/cage in a room maintained at a temperature of 22°C and kept under an artificial 12/12-h light/dark cycle. Animals were given ad libitum access to food and water and handled for 5 min daily to minimize experimental stress. All experimental procedures were approved by the ethical committee of the University of Cagliari and carried out in strict accordance with the guidelines for experimental animals care (European Economic Community [86/609; DL 27/01/92, number 110]).
Drugs
rac-BHFF (Hoffmann-La Roche, Basel, Switzerland) was suspended in a mixture containing Cremophor EL, 1,2-propanediol and distilled water (4:1:30 ratio) and administered intragastrically (per os, PO) at an injection volume of 10 mL/kg. The NMDAR antagonist dizocilpine (DIZ; Sigma-Aldrich, Milan, Italy) was dissolved in 0.9% saline and administered subcutaneously (SC). BAC (Tocris Cookson, Bristol, UK) was dissolved in saline and administered intraperitoneally (IP). CLO (Sigma-Aldrich) was dissolved in a single drop of 1 N HCl and diluted with saline; the pH was adjusted to 7 with NaHCO3. Parenteral injections were administered in injection volumes of 1 mL/kg for rats and 10 mL/kg for mice.
Experimental Procedures
Acoustic startle reflex and PPI were measured in 4 sound-attenuated chambers (Med Associates, St Albans, VT, USA) with fan ventilation. Each chamber consisted of a Plexiglas cylinder (9 cm diameter for rats and 3.2 cm diameter for mice) mounted on a piezoelectric accelerometric platform and connected to an analogue–digital converter. Background noise and acoustic bursts were conveyed by two separate speakers, properly spaced from the cylinder so as to produce a variation of sound within 1 dB across it. Both speakers and startle cylinders were connected to a main PC computer, which detected and analyzed all chamber variables by means of specific software. Before every testing session, acoustic stimuli and mechanical responses were calibrated via specific devices supplied by Med Associates.
The experimental procedure was based on the protocols described in Frau et al. 32. Briefly, 3 days before the experiment, animals went through a brief baseline startle session. Animals were exposed to 70 dB background white noise for a 5 min acclimation, followed by presentation to a randomized sequence of twelve 40 ms acoustic pulses of 115 dB, interposed with three trials in which an 82 dB prepulse preceded the 115 dB pulse by 100 ms. Subsequently, treatment groups were established so that average startle responses and % PPI {calculated as: 100 − ([mean startle amplitude for prepulse + pulse trials/mean startle amplitude for pulse-alone trials] × 100)} were equivalent across groups. On the testing day, each animal was placed in the cylinder for a 5-min acclimation to 70 dB background white noise, which continued for the remainder of the entire session. The session consisted of three consecutive blocks of trials. Unlike the first and the third block, during which animals were presented with only five pulse-alone trials of 115 dB, the second block displayed a pseudorandom sequence of 50 trials, including 12 pulse-alone trials, 30 trials of pulse preceded by 74, 78 or 86 dB prepulses (ten for each level of prepulse loudness) and eight no stimulus trials, where only background noise was delivered. Intertrial intervals (ITI) were selected randomly between 10 and 15 seconds. The duration of pulses and prepulses was 40 and 20 ms, respectively. Prepulse–pulse delay amounted to 100 ms.
Data Analysis
Normality and homoscedasticity of data were verified by Kolmogorov–Smirnov and Bartlett's tests. Data were compared across groups by one-way or two-way ANOVAs, as appropriate. As no interaction between prepulse levels and treatment were found in the statistical analysis, %PPI values were collapsed across prepulse intensity to represent average %PPI. Post hoc analyses were performed using Tukey's test with Spjøtvoll–Stoline correction. Significance threshold was set at 0.05.
Results
Effects of rac-BHFF on the PPI Deficits Induced by DIZ in Sprague-Dawley Rats
In the first experiment, we investigated whether rac-BHFF pretreatment could prevent the PPI disruption induced by the NMDAR antagonist DIZ in Sprague-Dawley rats. Animals were pretreated with either rac-BHFF (25–50 mg/kg, PO) or vehicle (control) and 60 min later were given an injection of either DIZ (0.1 mg/kg, SC) or saline (control). Animals were subjected to PPI testing 5 min after the last treatment.
No significant differences in startle amplitude were found between pretreatment and treatment groups (Figure 1A) [interaction: F2,47 = 0.10; NS]. Conversely, we found a significant pretreatment × treatment interaction (Figure 1B) [interaction: F2,47 = 4.62, P < 0.05] between groups. Post hoc analyses revealed that the highest dose of rac-BHFF significantly reversed DIZ-induced PPI deficits (P < 0.05; Tukey's test).
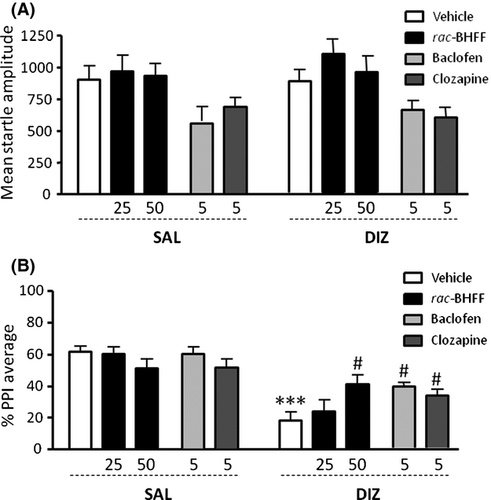
In contrast, BAC (5 mg/kg, IP) produced a significant reduction in startle amplitude [main effect: F1,37 = 9.44; P < 0.01] (Figure 1A); furthermore, it significantly prevented the PPI impairments caused by DIZ [interaction: F1,37 = 4.89; P < 0.05; P < 0.05 for vehicle-DIZ vs. BAC-DIZ comparisons] (Figure 1B). As expected, CLO (5 mg/kg, IP) elicited similar effects, with a general reduction in startle magnitude [main effect: F1,37 = 7.17; P < 0.05] (Figure 1A) and a significant reversal of DIZ-mediated PPI deficits [interaction: F1,37 = 5.27; P < 0.05; P < 0.05 for vehicle-DIZ vs. CLO-DIZ comparisons] (Figure 1B).
Effects of rac-BHFF on the PPI Deficits Induced by DIZ in C57BL/6J mice
Next, we studied whether rac-BHFF (6.25–12.5 mg/kg, PO) exhibited antipsychotic-like effects in C57BL/6J mice treated with DIZ (0.3 mg/kg, IP). In parallel with our results on rats, no differences were found between groups for startle amplitude (Figure 2A) [Interaction: F2,59 = 1.99, NS]; however, we detected a marked pretreatment × treatment interaction (Figure 2B) [F2,52 = 5.32, P < 0.01]. Specifically, the PPI-disruptive effects of DIZ (P < 0.01) were prevented by rac-BHFF treatment at a dose of 12.5 mg/kg (P < 0.001; Tukey's test). As previously shown 17, BAC (5 mg/kg, IP) did not affect startle amplitude in C57BL/6J mice (Figure 2A); notably, the PPI analysis indicated main effects for both pretreatment (BAC) [F1,41 = 7.00; P < 0.05] and treatment (DIZ) [F1,41 = 8.13; P < 0.01], but no significant interactions [F1,41 = 2.32; NS] (Figure 2B). Conversely, CLO (5 mg/kg, IP) reduced startle magnitude [main effect: F1,41 = 7.84; P < 0.01] (Figure 2A) and significantly countered the PPI disruption induced by DIZ [interaction: F1,41 = 4.81; P < 0.05; P < 0.05 for vehicle-DIZ vs. CLO-DIZ comparisons] (Figure 2B).
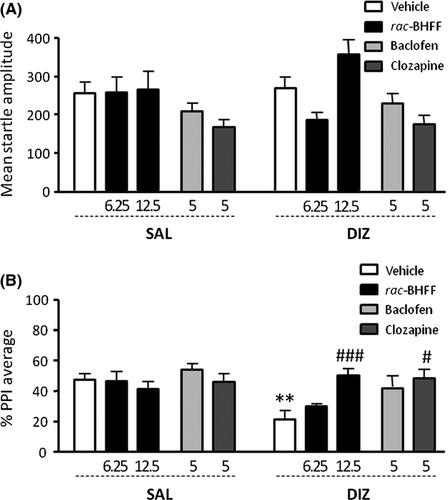
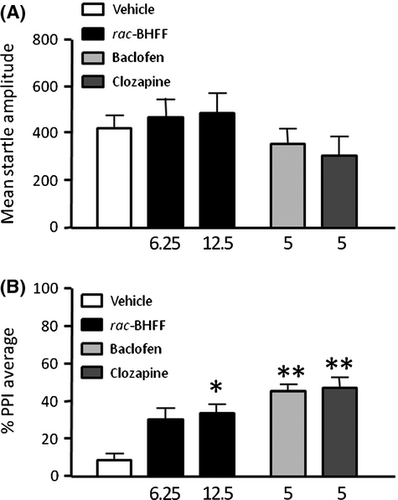
Effects of rac-BHFF on the Spontaneous PPI Deficit Displayed by DBA/2J Strain of Mice
In the last experiment, we evaluated the intrinsic effects of rac-BHFF on the spontaneous low PPI baseline displayed by DBA/2J mice. Animals were treated with VEH or rac-BHFF (6.25–12.5 mg/kg, PO) and subjected to PPI sessions 60 min later. The GABABR PAM did not elicit any changes in startle amplitude at any dose (Figure 3A) [F2,35 = 0.99, NS]. In contrast, low PPI in DBA mice was countered by rac-BHFF [F2,35 = 4.63 P < 0.05)], specifically at the dose of 12.5 mg/kg (P < 0.05; Tukey's test) (Figure 3B). BAC and CLO failed to affect startle amplitude, but significantly countered DIZ-induced PPI deficits [BAC: F1,20 = 10.28; P < 0.01; CLO: F1,20 = 12.36; P < 0.01] (Figure 3B).
Discussion
The results of this study showed that rac-BHFF, a potent GABABR PAM, dose-dependently reversed DIZ-induced PPI deficits in both SD rats and C57BL/6J mice and rescued the PPI impairments displayed by DBA/2J mice. Overall, these effects were akin to those elicited by the GABABR agonist BAC and the atypical antipsychotic CLO and substantially confirm previous findings by our group and others 11-13, 18 on the therapeutic potential of GABABR activators on sensorimotor gating deficits induced by NMDAR blockade. Notably, in contrast to BAC and CLO, rac-BHFF did not significantly reduce the magnitude of startle reflex, irrespective of the animal model and dose. It is also worth mentioning that the same doses of rac-BHFF that elicited antipsychotic-like effects in our models also failed to affect locomotor responses or other spontaneous behavioral manifestations in the home cage [R. Frau and V. Bini, unpublished observations]. Taken together, our findings complement previous preclinical data on the beneficial effects of GABABR PAMs 33, 34 and highlight this class of compound as a novel putative avenue for antipsychotic therapy with fewer side effects than GABABR antagonists.
One of the key problems in the potential employment of BAC as an add-on treatment lies in the exacerbation of locomotor impairments and sedative effects caused by dopamine D2 receptor antagonism; indeed, in preliminary studies, we observed that the association of BAC and antipsychotic drugs, such as CLO and haloperidol, was not suitable for behavioral studies in rodents, in view of the serious impairments in neuromuscular coordination produced by such combinations. From this perspective, GABABR PAMs may afford a safer and tolerable alternative as antipsychotic adjunctive therapies for schizophrenia or related disorders. Future clinical and preclinical studies are warranted to evaluate this interesting perspective and validate the potential usefulness of rac-BHFF and similar agents in the treatment of psychoses.
The PPI of the acoustic response refers to the reduction in the response amplitude to a sudden and intense startling stimulus [pulse], when it is immediately preceded by a weaker nonstartling prestimulus 35. This phenomenon is widely regarded as a dependable index of sensorimotor gating integrity and is typically impaired in schizophrenia 17. In rodents, DIZ and other NMDAR antagonists produce marked PPI deficits, which are sensitive to CLO and other atypical, but not typical, antipsychotics 36-38. The effects of NMDAR antagonists on sensorimotor gating are in line with the well-known psychotomimetic effects of these drugs 39 and other alterations of informational processing 40.
Although the mechanisms by which DIZ and other NMDAR blockers impair PPI remain unclear, several studies point to a key role of the prefrontal cortex (PFC) and hippocampal regions in these phenomena 12, 41. These areas are characterized by a large density of pre- and postsynaptic GABABRs, which finely regulate basal glutamatergic and dopaminergic functions 42. Accordingly, disturbances in GABABR expression or function may affect informational salience by altering the inhibitory/excitatory balance of several neurotransmitter systems in corticolimbic regions.
It has been reported that MK-801 and PCP stimulate cortical glutamate release in PFC and hippocampus 43. In this scenario, rac-BHFF could counteract the disinhibition of neuronal activity produced by exaggerated NMDAR stimulation in these areas or, alternatively, modulate distinct forebrain pathways under the control of non-NMDA glutamatergic receptors, such as AMPA and kainate 44. Alternatively, rac-BHFF may counter DIZ-mediated PPI deficits by acting on the pallidotegmental nucleus (PTn). This predominant GABAergic area exhibits high levels of GABABRs and acts as an interface between the brainstem and forebrain regions implicated in PPI regulation regions 45. Accordingly, DIZ has been recently shown to induce PPI deficits through alterations of the giant neurons of this region; notably, GABABR activation reversed these impairments by stabilizing the hyperactivation of these nuclei 13.
rac-BHFF also significantly ameliorated the PPI deficits on DBA/2J mice, a strain characterized by spontaneous gating impairments sensitive to antipsychotics 19, as well as other phenotypes reminiscent of schizophrenia symptoms, such as poor exploration as well as high aggression and social avoidance 46-49.
The antipsychotic-like actions of rac-BHFF in this murine strain may be related to their reduced expression of GABABRs and NMDARs across the cortex and hippocampus, in comparison with C57BL/6J mice 18, 50.
Several limitations in this study should be acknowledged. First, our analysis was limited to the behavioral analysis of startle reflex and PPI, but did not include the testing of other paradigms with great relevance to the negative and cognitive symptoms of schizophrenia-spectrum disorders, such as object recognition and social interaction test 51, 52. Second, we did not evaluate the effects of rac-BHFF in animal models with high translational validity with respect to schizophrenia, such as DISC1- and neuregulin1-deficient mice, or rodents subjected to chronic administration of DIZ or other NMDAR antagonists, which may have better simulated the neurobiological impairments associated with schizophrenia 53-58. Third, our research did not encompass testing of GABAB negative allosteric modulators or antagonists, which may be essential for a full definition of the role of these receptors in schizophrenia-related endophenotypes.
Although further investigations are needed to address these limitations, our findings extend and support for the role of GABABRs in the pathophysiology of psychiatric disorders associated with sensorimotor gating disturbances and point to PAMs of these targets as interesting therapeutic tools to treat cognitive deficits and negative symptoms in schizophrenia.
Acknowledgments
The present study was supported by National Institute of Health (NIH) Grant R21HD070611 (to M.B.) and a Research Grant from the Tourette Syndrome Association (to MB). This study was also partially supported by subawards (to M.B.) from the NIH grants P20 GM103638 and UL1 TR000001 (formerly UL1RR033179), awarded to the University of Kansas and University of Kansas Medical Center. The authors are indebted to the EU COST Action CM1103 “Structure-based drug design for diagnosis and treatment of neurological diseases: dissecting and modulating complex function in the monoaminergic systems of the brain”. The authors are grateful to Sean Godar for his insightful comments on the manuscript, and Romina Pes for her valuable support in the execution of the experiments.
Conflict of Interests
The authors declare no conflict of interest. Pari Malherbe and Andrew W. Thomas are employees of Hoffmann-La Roche.




