Advances in host-based screening for compounds with intracellular anti-mycobacterial activity
Funding information: Department of Atomic Energy, Government of India, Grant/Award Number: RTI 4006; National Centre for Biological Sciences
Abstract
Intracellular pathogens interact with host systems in intimate ways to sustain a pathogenic lifestyle. Consequently, these interactions can potentially be targets of host-directed interventions against infectious diseases. In case of tuberculosis (TB), caused by the bacterium Mycobacterium tuberculosis (Mtb), while effective anti-tubercular compounds are available, the long treatment duration and emerging drug resistance necessitate identification of new class of molecules with anti-TB activity, as well as new treatment strategies. A significant part of the effort in finding new anti-TB drugs is focused on bacterial targets in bacterial systems. However, the host environment plays a major role in pathogenesis mechanisms and must be considered actively in these efforts. On the one hand, the bacterial origin targets must be relevant and accessible in the host, while on the other hand, new host origin targets required for the bacterial survival can be targeted. Such targets are good candidates for host-directed therapeutics, a strategy gaining traction as an adjunct in TB treatment. In this review, we will summarise the screening platforms used to identify compounds with anti-tubercular activities inside different host environments and outline recent technical advances in these platforms. Finally, while the examples given are specific to mycobacteria, the methods and principles outlined are broadly applicable to most intracellular infections.
Take Away
- Host based systems are disease relevant platforms for screening anti Mtb compounds.
- New target, chemical spaces open up for drug discovery in these platforms.
- New advances enable sensitive detection, identification of subtle phenotypes.
- Overview of host based chemical screens for identification of anti-Mtb compounds.
1 INTRODUCTION
The discovery and development of potent tuberculosis (TB) drugs in the mid-20th century and successful treatment of TB patients with these drugs are often hailed as one of the greatest achievements of modern medicine. However, since then, no new TB drugs were approved for over half a century (Chhabria & Jani, 2010) until the recent success with Bedaquiline, Delaminid and Proteomanid (Bahuguna & Rawat, 2020; Pai et al., 2016). This lag has contributed to the re-emergence of the disease, resulting in a scenario today where, well over a century after its discovery as the causative agent of TB, the bacterium Mycobacterium tuberculosis (Mtb) remains the ‘most successful’ pathogen and the leading cause of death globally due to a single infectious agent (World Health Organization, 2018). A large part of this success is due to the ability of the bacterium to invade the host system and form distinct niches that are hard to eradicate. TB therapy therefore requires long treatment duration of 6–9 months for drug-sensitive strains and up to two years for drug-resistant strains. While the long treatment duration clears the infection in most cases, it also results in prolonged exposure of tolerant sub-population of Mtb to the drugs. This, combined with poor patient compliance for the gruelling regime and the absence of new drugs, provides a fertile ground for emergence of drug resistance. Indeed, multi- and extensively drug-resistant TB (MDR, XDR-TB), requiring even longer treatment regimens, has emerged as a major global health threat, reaching pandemic proportions in several regions (World Health Organization, 2018). Thus, the pressing need of the hour is development of new drugs and drug combinations and exploration of new target spaces, with the twin and concomitant objectives of effective and complete elimination of Mtb from the system and significant shortening of the treatment duration. In this review, we will focus on the advances in recent drug discovery and screening efforts on mycobacteria inside different host systems.
2 HISTORICAL PERSPECTIVE ON ANTI-TB DRUG DISCOVERY
In 1882, Robert Koch discovered the cause of tuberculosis and named the bacilli as M. tuberculosis (Mtb) (Cambau & Drancourt, 2014; Sakula, 1982). In 1894, Hinshaw and Feldman tested Streptomycin, discovered earlier by Schatz, Bugie, and Waksman, in guinea pigs infected with virulent M. tuberculosis and observed striking effect of the drug (Feldman, Hinshaw, & Mann, 1945). Meanwhile, para-aminosalicyclic acid (PAS) was developed by Jörgen Lehmann, which later combined with streptomycin showed longlasting effects against tuberculosis (Mitchison, 2005). However, both drugs had side effects and patients relapsed, indicating drug resistance (Capel & Mitchell, 1955; Hinshaw, Feldman, & Pfuetze, 1946). The next important breakthrough in anti-tubercular chemotherapy was the recognition of benefits of isoniazid (1951) in both experimental and human tuberculosis (Murray, Schraufnagel, & Hopewell, 2015). Based on the discovery that the vitamin nicotinamide has an anti-tuberculosis activity, chemists started to synthesise new molecules based on nicotinamide. Attempts to enhance the activity of nicotinamides and thiosemicarbazones lead to the synthesis of β- and γ-pyridylaldehyde thiosemicarbazones, which showed activity against tuberculosis. In 1952, isonicotinic acid hydrazide (INH) was discovered as a result of these studies. The compound was extraordinarily active compared to any other compound discovered till then (Fox, 1952) (Vilch & Jacobs, 2007). However, resistance to isoniazid was soon observed. Similarly, resistance emerged to another molecule with potent anti-TB effect, pyrazinamide (Muschenheim, 1955), thus demonstrating a need for combination therapies with other existing drugs. In 1961, the synthesis of dextrorotatory form of 2,2′-(ethylenediimino)-di-1-butanol [ethambutol] was reported (Thomas, Baughn, Wilkinson, & Shepherd, 1961). It showed higher activity compared to streptomycin during infection with Mtb H37Rv in mice and was also active against isoniazid- and streptomycin-resistant strains (Thomas et al., 1961; Wilkinson, Shepherd, Thomas, & Baughn, 1961). Investigation into the antibiotic properties of the soil bacterium Nocardia mediterranei led to the discovery of rifamycins in 1957. Rifampin was first used against tuberculosis in 1966 and subsequently used in combination therapy with isoniazid and ethambutol, which enabled the shortened therapy of 9 months and improved cure rates (Lancet, 1976). Other drugs such as cycloserine, ethionamide, kanamycin and capreomycin discovered during this time have played important role in treating drug-resistant TB (Murray et al., 2015). The current chemotherapy of tuberculosis is a combination of isoniazid (INH), rifampin, ethambutol and pyrazinamide taken for six to nine months, that has remained largely unchanged for the past many decades.
The most recently approved anti-TB drug Bedaquline, a novel diarylquinoline, was a hit in a phenotypic screen by Johnson & Johnson (J&J) in 2004, from over 70,000 chemicals that were tested for growth inhibition of Mycobacterium smegmatis (Gestel et al., 2005). Subsequent studies identified conclusively that it acts by inhibiting the ATP synthase of Mtb, a new drug target (Andries et al., 2005; Koul et al., 2007). The TB drug development programme by Otsuka Pharmaceutical Co., Ltd, in place since 1990s screened for agents with enhanced efficacy, reduced side effects and minimal drug–drug interactions against Mtb (Liu et al., 2018). The screen picked up a class of compounds that inhibit mycolic acid synthesis (Matsumoto et al., 2007). Further refinement led to the selection and discovery of a nitroimidazole class compound (Matsumoto et al., 2006; Sasaki et al., 2006), delamanid, which is approved for use in adult pulmonary multi-drug-resistant (MDR)-TB (Tsubouchi, Sasaki, Ishikawa, & Matsumoto, 2016). As seen above, current standard care anti-TB drugs have been discovered largely by the effort of focused testing of synthetic and natural compounds in different phenotypic Mtb infection assays and testing in animal models.
Conventional approaches to anti-Mtb drug discovery, and anti-bacterial drug discovery in general, have focused on identifying compounds that kill the bacteria directly, usually targeting pathways that are distinct in Mtb with no or minimal overlaps in the human host cells. Consequently, currently available anti-TB drugs such as Rifampicin, Isoniazid and Ethambutol act on Mtb pathways that have no mammalian equivalents. Rifampicin acts by specifically inhibiting bacterial RNA polymerase (Levin & Hatfull, 1993). Isoniazid is generally thought to inhibit mycobacterial cell wall biosynthesis (Davidson & Takayama, 1979; Takayama, Wang, & David, 1972) but is shown to have other mechanisms including generation of reactive oxygen species due to the action by mycobacterial enzyme KatG and depletion of nucleic acid pools (Timmins & Deretic, 2006). Although the exact molecular mechanism of ethambutol is unclear, it is thought to act by inhibiting the synthesis of a key cell wall component arabinogalactan (Lee, Mikusova, Brennan, & Besra, 1995; Takayama & Kilburn, 1989). For reasons of minimising emergence of drug resistant strains, it is preferred if the target spaces for newer drugs are distinct from these. Indeed, Bedaquiline and Delaminid, two new TB drugs, have new target pathways, namely, ATP synthesis and mycolic acid synthesis, respectively (Andries et al., 2005; Matsumoto et al., 2006).
3 CURRENT APPROACHES IN ANTI-TB DRUG DISCOVERY
New approaches for the discovery of anti-Mtb drugs are based on either phenotypic screening or target-based strategies. While these two approaches are complementary, the modalities, methodologies and challenges are different. Phenotypic screens can directly identify compounds with the desired phenotype, that is, compounds with anti-Mtb activity. This has the advantages of selecting for compounds that are bioavailable, crossing barriers such as cell wall and could be pro-drugs (i.e., converted within host cells into an active form). Moreover, since the approach is usually not biased to a specific pre-defined target, it has the potential to uncover new targets spaces. However, a significant drawback is that the mechanism of action of compounds is not known (Moffat, Vincent, Lee, Eder, & Prunotto, 2017). Extensive downstream experimentations are usually needed to identify the molecular targets. Knowledge of mechanism of action is important in anti-bacterial drug discovery since any emerging resistant bacterial strain is likely to be resistant to drugs having similar mechanisms of action. Thus, drugs with distinct target spaces are preferred for combination therapies. Target-based screening is a powerful approach to identify such molecules. However, hits with strong activity on targets in vitro do not always show activity against the whole bacteria and infection assays (Grzelak et al., 2019; Kumar, Chettiar, & Parish, 2017; Pethe et al., 2010). This could be due to several reasons, including the significant barriers, compounds being effluxed out, or being metabolised into inactive forms. Significant amount of medicinal chemistry and multiple iterative rounds of testing are usually required in such cases to ensure hits from target-based approaches are effective in cells. Even when they are active on cells, the possibility of additional targets inside the cells cannot be ruled out. The current pipeline for anti-TB drugs in different phases of pre-clinical and clinical trials includes compounds with diverse sets of targets emerging from both target based and phenotypic approaches (Libardo, Boshoff, & Barry III, 2018; Villemagne et al., 2012; World Health Organization, 2020)
Initial whole Mtb screens were based on screening drug libraries against Mtb grown in rich media with glycerol as carbon source. Such screens identified compounds that target growth inhibition or killing of the bacteria growing in rich media conditions. However, they typically yield similar chemical classes as hits (Bandodkar, Shandil, Bhat, & Balganesh, 2020; Kumar et al., 2017), possibly due to the same target spaces being explored. These assays do not fully capture the in vivo characteristics of Mtb such as the extreme slow growth and distinct environments and niches. Hence, attempts have been made to simulate different ‘in vivo’ conditions in liquid culture media, such as reduced pH, hypoxia, starvation and growth in specific lipid conditions. However, the precise nature of such in vivo niches and how faithfully they can be captured in in vitro growth conditions remain open questions. Nevertheless, screens against whole Mtb in different conditions have direct relevance since a significant fraction of Mtb inside the necrotic granuloma core are extracellular, often exhibiting exuberant growth in the foamy macrophage and necrotic cell debris (Cambier, Falkow, & Ramakrishnan, 2014). The different screening conditions and integration of datasets from such screens are discussed extensively in other reviews (Parish, 2020; Yuan & Sampson, 2018). We will not elaborate on this here since our focus is on intracellular screening platforms.
4 RATIONALE FOR DRUG SCREENING WITHIN INFECTED HOST CELLS FOR NEW GENERATION ANTI-TB COMPOUNDS
The host cell environment offers not only significant challenges but also new possibilities for discovery of new anti-TB compounds. A hit compound must reach the target in its active form inside an Mtb infected cell. However, to be effective, the compound must not be toxic to the host cells in the concentrations used, must be permeable and not be metabolised or effluxed out. On the other hand, given the biology of host–pathogen interactions and the dependence of the pathogen on host cellular processes, new target spaces open up within the infected cell system.
The new target spaces inside the infected cell can be of both bacterial and host origins. New bacterial targets inside infected cells could include Mtb processes that are active only within the hosts such as cholesterol (VanderVen et al., 2015) or triacylglycerol metabolism (Martinot et al., 2016). Such targets typically do not feature in conventional whole bacteria-based screens and are excellent examples of anti-virulence targets, that is targets that are not required or essential for bacterial growth outside of the infection context. Exploitation of such host active bacterial targets could, by virtue of opening up new target spaces, provide new chemical classes with anti-Mtb properties. All the host processes utilised by the pathogen could be considered as potential targets for host-directed interventions. Typically, pathogens tend to target central modules of host cellular processes (Dyer, Murali, & Sobral, 2008; Russell, 2011; Sheedy & Divangahi, 2021). These are also tightly regulated spaces, which can be amenable for modulation by pharmaceutical agents. Targeting host processes has the additional advantage that direct bacterial resistance to these processes would not be possible; hence, one could argue that these could reduce the risk of emergence of drug resistance.
Phenotypic assays that quantify the bacterial survival inside the host cells have direct relevance to the disease biology and show a strong ‘chain of translatability’, a term used to describe the shared mechanistic basis between the phenotypic assay and the disease model with the disease in humans (Moffat et al., 2017). While several phenotypic screens have been performed in Mtb and its surrogates such as Mycobacterium bovis BCG, and fewer screens performed in macrophage infection models, direct comparison of target spaces and hit compounds between the whole Mtb screen and macrophage-based screens are needed. Such a comparison is crucial to (a) provide information on the relevance and accessibility of Mtb targets inside macrophages and (b) provide evidence for new targets inside macrophages. Sundaramurthy et al. screened the same FDA-approved compound library against M. bovis BCG inside macrophages and directly against the bacteria. The results show only a partial overlap in the hits, suggesting that target spaces are distinct under the two conditions (Sundaramurthy et al., 2013). Similar results were obtained in larger compound libraries screened by the Russell (VanderVen et al., 2015) and Soldati (Trofimov et al., 2018) groups. Thus, both loss of activity against bacterial targets and gain of new target spaces characterize the infected host cell model. The infected macrophage system thus represents a screening platform that encompasses a broad and disease-relevant target range (VanderVen, Huang, Rohde, & Russell, 2016) irrespective of whether the target is of bacterial or host origin.
5 HOST-BASED CHEMICAL SCREENING PLATFORMS – CURRENT STATUS AND RECENT ADVANCES
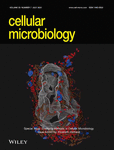
Several macrophage cells from mouse and human origins have been used as host cell system for phenotypic screening (Table 1). Such relatively simple infection models, while offering a significant ‘step-up’ to Mtb culture-based phenotypic screening, nevertheless have some disadvantages. A major critique of the system is that under in vivo infection conditions, rarely if at all is a single infected cell type seen. While macrophages are the most well-studied infection systems, other immune cells such as dendritic cells and neutrophils, and non-immune cells such as epithelial cells and mesenchymal stem cells get infected (Bussi & Gutierrez, 2019; Huang, Nazarova, et al., 2018). Thus, additional target spaces or distinct biology and host–pathogen interactions could manifest in these cell types (Figure 1). Hence, it is of considerable importance to understand and target other cell types. However, knowledge of their biology and interaction with Mtb is limited, compared to the macrophage infection model. In the recent years, new strategies have been adopted that could overcome these issues, while increasing the complexity of screening assays. The recent development of the deconstructed granuloma (dgr) model from Russell lab is an example (Huang, Kushner, et al., 2018). Similarly, in vitro granuloma systems could in principle be used as screening platform (Elkington et al., 2019; Fitzgerald, Abendaño, Juste, & Alonso-Hearn, 2014; Kapoor et al., 2013). Organoid models offer another opportunity (Fonseca, Rodrigues, Olsson, & Saraiva, 2017). Whole animal screening, which encompasses all the aspects discussed above, is a feasibility, thanks to the zebra fish infection model with Mycobacterium marinum. Finally, other orthogonal approaches could be attractive, for example, the use of amoeba model systems could combine the advantages of a single cell, relatively simpler infection model, with the in vivo context of the whole organism (Figure 1). In the sections below, we will detail the different categories mentioned here and recent advances in each of them for development of phenotypic assays, screening and identification of anti-mycobacterial compounds.
| Host system | Mycobacteria | Detection | Readout | Number of compounds | Library source | Reference |
|---|---|---|---|---|---|---|
| J774A.1 | M. tuberculosis (H37Rv-GFP) | GFP | Automated imaging | 2079 | Broad Institute bioactives collection, Kinase inhibitors (Dana Farber Cancer Institute) | Stanley et al., 2014 |
| J774A.1 | M. tuberculosis CDC1551 | mCherry | Fluorescence plate reader | 340,000 | Vertex Pharmaceuticals, synthetic small molecules and natural products | VanderVen et al., 2015 |
| RAW 264.7 | M. bovis BCG | Luminescent reporter strain of M. bovis BCG (BCG-lux ) | Luminescence measurement (RLU) | 214 | FDA approved drugs without antibacterial effects | Schiebler et al., 2015 |
| RAW 264.7 | M. tuberculosis (H37Rv-GFP) | GFP | Automated imaging | 56,984 | Synthetic compounds | Christophe et al., 2009 |
| RAW 264.7 | M. tuberculosis (H37Rv-GFP) | GFP | Automated imaging | 121,156 | Synthetic compounds | Pethe et al., 2013 |
| THP1 | M. tuberculosis | tomatoRFP | Automated imaging | 400 | Ontario Institute for Cancer | Shapira et al., 2020 |
| Research Kinase Inhibitor library | ||||||
| THP1 | M. abscessus | Red fluorescence protein (RFP) | Automated imaging | 317 | UNC library | Richter et al, 2019 |
| Human primary MDM | M. bovis BCG | GFP | Automated imaging | 2000 | FDA-approved compounds, Microsource Spectrum Discovery Collection | Sundaramurthy et al., 2013 |
| MRC5 lung fibroblast cells | M. marinum | Cell viability | MIC assay, plate reader | 28,000 | ChemBridge and CBCS primary screening set | Tükenmez et al., 2019 |
| Deconstructed Granuloma, DGr | M. tuberculosis Erdmann | initial infection - mCherry, ex-vivo challenge (mKO)/P606′::mKO-tetON | Fluorescence plate reader | 10,000 | Bioactive compounds | Huang et al., 2018; Huang, Nazarova, Tan, Liu, & Russell, 2018 |
| Zebrafish | M. marinum | tdTomato | Imaging | 1200 | Prestwick Library | Matty et al., 2019 |
| hPSC-derived macrophage-like cells (iMACs) | M. tuberculosis H37Rv- GFP | GFP | Automated imaging | 3716 | Prestwick Library, NIH | Han et al., 2019 |
| Acanthamoeba castellanii | M. marinum | GFP | Fluoresence plate reader, kinetic measurement | 168 | GSK-TB library | Trofimov et al., 2018 |
| BV2 microglia | M. marinum | GFP | Time lapse imaging | |||
| Acanthamoeba castellanii | M. marinum/L. pneumophila | GFP | Fluorescence plate reader, kinetic measurements | 1255 | Custom made Chemically Highly Diverse Pathway-Based Library of Compounds (designed) | Hanna et al., 2020 |
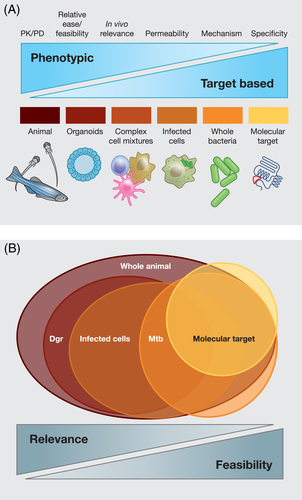
Figure 1B shows a schematic outlining the potential overlaps of putative target spaces in the various levels of screening modalities outlined above. For instance, targets that are validated at a molecular level in vitro might lose relevance in the context of Mtb due to acquisition of resistance or possible redundancies in the system. Another assumption is that at all the levels required – whole bacteria, cellular, tissue and animal – the bacterial target is relevant, essential and accessible. While this might be true for really strong targets such as cell wall synthesis and protein synthesis, these also represent target spaces that have already been exhaustively searched by industry and academic groups (Bahuguna & Rawat, 2020; Huang, Kushner, et al., 2018). The repeated identification of similar target spaces in such assays attest to this, suggesting that these ‘low-hanging’ spaces have been saturated. As discussed above, host cell-based screens provide a comprehensive platform that encompasses bona-fide bacterial targets that are relevant and accessible in the host, in addition to new class of bacterial targets that are active in hosts (and hence missed in whole bacterial phenotypic screens) as well as host targets that are relevant in the control of infection. Thus, while whole bacterial phenotypic screens offer a relative ease of use, host cell-based screens offer a more comprehensive target space. As the levels of complexity of the different host cell systems increase as we move from simple infection model to complex cell mixtures, organoid model and whole animal screens, the comprehensiveness of target space and the direct relevance to the disease, that is the ‘chain of translatability’, increase as well (Figure 1B). Thus, a trade-off between feasibility and relevance is necessary in making decisions on optimal phenotypic assays and screening strategies.
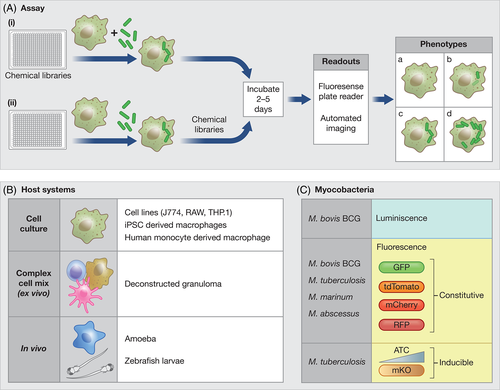
In the following sections, we will focus on the different components of the host cell-based screens (Figure 2A) and cover the recent advances made in each of the components. In almost all cases, the compounds to be screened are added after the bacterial uptake since the intent is to identify compounds that affect intracellular bacterial growth and not uptake. Table 1 summarises the features of different host system-based chemical screens performed to identify compounds with anti-mycobacterial activity.
The murine macrophage cell lines, RAW 264.7 and J774A.1, are the most commonly used in vitro infection models. Christophe et al. screened a library of 56,984 synthetic compounds in RAW 264.7 cells infected with M. tuberculosis (H37Rv-GFP) in a high throughput (384 well plate) format. The compounds were pre-plated in screening plates and RAW 264.7 cells infected with Mtb H37Rv-GFP in suspension were dispensed into individual wells. Plates were imaged after 5 days using a fully automated platform (EVOscreen-MarkIII) inside BSL3. The image analysis pipeline detected the GFP bacteria and SYTO 60 stained macrophages and scored for the percentage of infected macrophages. The screen identified 135 active compounds with potent intracellular anti-mycobacterial efficacy and no host cell toxicity. Among these, dinitrobenzamide derivatives (DNB) were validated to have effect on intracellular bacterial growth as well as it was active against the XDR strains. Additionally, the screen identified a bacterial enzyme, the decaprenyl-phosphoribose epimerase DprE1/DprE2, as a new target for antimycobacterial inhibitors (Christophe et al., 2009).
Another study from Pethe et al. screened 121,156 compounds and discovered 106 active hits, which included a class of imidazopyridine amide (IPA) compounds that block Mtb growth by targeting the respiratory cytochrome bc1 complex. The IPA compound Q203 was shown to have high potency at even lower dosage against MDR and XDR M. tuberculosis clinical isolates in culture broth medium and mouse tuberculosis infection model (Pethe et al., 2013). The screen followed similar methodology as described previously by Christophe et al. (2009). In both the cases, a ‘no wash’ strategy was used with compounds pre-plated into the assay plate (Figure 1A i). This approach has two significant advantages. First, it eliminates the need for compound dispensation, plate washing and associated instrumentation/robotics inside BSL3, thus simplifying the process significantly. Second, it could minimise potential plate to plate variations in terms of infection since a pre-infected cell population is seeded into each well.
Vander Ven et al. performed a high throughput screen of a proprietary compound library having 340,000 synthetic small molecules and natural products. The screen was performed in M. tuberculosis CDC1551 expressing mCherry-infected J774 macrophages (VanderVen et al., 2015). The screen quantified the Mtb-mCherry fluorescence signal after 6 days of infection using an Envision Multilabel plate reader, resulting in 1359 confirmed hits. Further, they identified bacterial metabolic enzyme targets that are involved in cholesterol metabolism out of which two compounds were found to inhibit the HsaAB enzyme complex, which is important in completing degradation of the cholesterol A/B rings, an essential bacterial target inside host cells.
While these screens used large-scale unbiased compound libraries, other groups have adapted an approach of using smaller focused libraries. Stanley et al. screened ~2000 small molecules including known kinase inhibitors in J774A.1 cell line (Stanley et al., 2014). Macrophages were infected with M. tuberculosis H37Rv-GFP, followed by compound addition and incubation for 3 days. Cells were fixed and imaged on ImageXpress Micro high-throughput microscope (Molecular Devices). The screen identified 133 unique hits that restrict intracellular bacterial growth and validated previously described targets as well as identification of potentially novel factors important for infection including EGFR, serotonin transporter, serotonin and dopamine receptor and sodium channels. In an independent screen, Schiebler et al. screened 214 FDA approved drugs, which do not have known anti-bacterial effects and identified compounds which activates mTOR-independent autophagy mechanism in RAW 264.7 cells infected with M. bovis luminescent reporter strain. The drugs carbamazepine and valproic acid were shown to stimulate the autophagic killing of M. tuberculosis within primary human macrophages in subsequent secondary assays (Schiebler et al., 2015).
The human monocyte-derived macrophage cell line, THP1, has also been used as host system. Unlike the murine cell lines, THP1 is a monocyte cell line that requires an additional activation step for differentiation to macrophages. Shapira et al. screened 400 compounds (Shapira et al., 2020) in Mtb-infected THP1 cells. The intracellular bacteria were measured using tomato RFP signal by high content imaging using CellInsight CX5 High Content platform and Thermo Fisher Scientific TM HCS StudioTM Cell Analysis Software. The screen identified 32 kinase inhibitors that hamper the mycobacterial growth inside macrophages. The screen validated the bactericidal property and specificity of the compound DDUG against Mtb. Secondary assays using RNAi mediated knockdown validated the kinase CHK2 as involved in the anti-Mtb activity of DDUG.
The THP-1 infection system has also been used in the screen against intracellular Mycobacterium abscessus (Richter, Shapira, & Av-gay, 2019), a recalcitrant pathogenic non-tuberculous mycobacteria (NTM) that is intrinsically resistant to several antibiotics active against Mtb (Gutierrez & Somoskovi, 2014). The intracellular M. abscessus growth was measured using red fluorescence protein (RFP) in an automated high-content microscopy platform with low resolution imaging. Two libraries having 568 antibiotics and 317 human kinase inhibitors, respectively, were screened in the study. The screen identified two compounds with distinct bacterial growth inhibition and the kinase inhibitor screen resulted in yielding three compounds with an inhibitory effect on mycobacterial growth. An interesting aspect of this study was the use of two different infection models. In addition to THP-1, the authors also used the amoeba Dictyostelium discoideum to confirm one of the hit compounds (Richter et al., 2019). Such confirmatory approaches in orthogonal models are important to identify conserved host cell-type independent targets.
The screens mentioned above use the relatively simple macrophage cell line models. While cell lines are convenient to use and manipulate and are certainly good starting point for screens, factors such as continual passage and non-uniformity in passage numbers raise questions on the physiological relevance. Few other screens on intracellular Mtb survival have been performed using macrophage systems that are either iPSC derived, or from primary human origin. Han et al. modified a protocol for generating homogeneous populations of macrophage-like cells from human embryonic stem cells (hPSC-derived macrophage-like cells [iMACs])(Han et al., 2019). They successfully scaled up the protocol to perform a host directed screen of ~3,000 chemicals in an Mtb-GFP infection assay. The screen methodology involved automated fluorescence microscopy imaging using a 20× magnifying objective (Operetta, PerkinElmer) inside BSL3. Primary screen identified 120 hits and secondary screen and validation in intracellular and extracellular Mtb dose-response assays confirmed a novel anti-Mtb compound, 10-DEBC, that also showed activity against drug-resistant strains.
Sundaramurthy et al. used human monocyte-derived macrophages from buffy coats as host cell system and screened FDA-approved compound library in an M. bovis BCG-GFP infection assay (Sundaramurthy et al., 2013). Monocytes were isolated from buffy coats and seeded into 384 well plates and differentiated in situ using hM-CSF, followed by infection with M. bovis BCG-GFP. Plates were washed extensively after infection to remove extracellular bacteria and compounds were added subsequently. 48 hours post infection, plates were fixed and imaged in automated confocal system, Opera, at high resolution. In contrast to some of the earlier screens, this screen involved extensive processing at the level of individual wells in 384 well plate. Robust assay development and use of automated robotic platforms ensured uniformity of macrophage differentiation, infectivity and cell adherence post washing across wells and plates. The screen utilised high resolution imaging and multiparametric image analysis for identifying and validating three hits, Nortriptyline, prochlorperazine edisylate and Haloperidol as host acting anti-TB compounds (Sundaramurthy et al., 2013).
A different screening strategy with an indirect readout was used by Tukenmez et al., who used non-phagocytic fibroblasts cells as model. Fibroblasts were incubated with M. marinum and screened for compounds that reduced mycobacteria-induced cell death (Tükenmez et al., 2019). 28,000 compounds were screened in a calorimetric MIC assay using plate reader, 49 initial compounds were identified that improves the cell survival after infection. 11 compounds were further validated in a similar assay in Mtb. Further validation is required to assess the suitability of this approach as a screening strategy.
In complete contrast to the above screens, Huang et al. developed a novel host platform that utilises deconstructed granuloma for drug screening (Huang, Kushner, et al., 2018), a new and much welcome development in the field. This platform attempts to integrate the relative ease and reproducibility of an in vitro infection system in a multi-well screening format with the complexity and multiple cell types of an in vivo infection scenario (see below). In this assay, mice are infected with Mtb expressing mCherry for 2 weeks and single cell suspensions generated from infected mouse lungs and seeded into screening compatible plates containing the compounds to be screened. The single cell suspensions contain Mtb infected cells, but the signals from them are not sensitive enough for screening. Hence, a second round of infection, this time with Mtb expressing mKOrange (mKO) under an inducible promoter, is necessitated. The inducible promoter system provides a higher detection sensitivity (Huang, Kushner, et al., 2018; Huang, Nazarova, et al., 2018). Using this assay, Huang et al. screened 10,000 bioactive compounds based on fluoresence readout from plate reader and 16 compounds were identified as hits.
A whole organism screen in zebra fish larvae was performed by Matty et al. to identify non–anti-microbial compounds for host-directed therapy (Matty et al., 2019). Zebrafish larvae were infected with M. marinum expressing tdTomato. Three infected and one uninfected larvae were arrayed per well in a 96 well plate and screened with Prestwick Library (1,200 compounds), and imaged 5 days post infection. Total fluorescence area and mean fluorescent intensity were used as readouts. The screen identified 9 non-anti-microbial hits. Extensive follow-up assays identified and validated Clemastine as a compound with strong host-directed antimycobacterial effect.
The use of Amoeba spp, notably Acanthamoeba castellanii and D. discoideum, as host for mycobacteria infection assays (Kicka et al., 2014; Steinert, 2011) offers a neat host-pathogen system that combines the ease of use of simple cell culture model while retaining the in vivo nature of a free living organism. Soldati lab has published a series of screens infecting the amoebae with M. marinum expressing GFP and screening targeted chemical libraries. They have used fluorescent plate reader-based measurement of fluorescent intensity post infection as readout (Hanna et al., 2020; Trofimov et al., 2018). Interestingly, they have adapted a comparative approach by performing multiple screens against the same chemical library in different host (A. castellanii, D. discoideum, as well as mammalian cells) as well as pathogen (M. marinum and Legionella pneumophilia) systems. Such comparisons are shedding light on both overlaps and differences in target spaces (Hanna et al., 2020; Trofimov et al., 2018). Indeed, such comparisons across host and pathogen systems offer much needed glimpses into the conserved nature, or otherwise, of target spaces across different host–pathogen systems.
6 OUTLOOK FOR HOST-BASED SCREENING PLATFORMS
6.1 Host systems
As discussed above, multiple platforms have been used to explore the host–pathogen interaction space for anti-Mtb drug discovery (Table 1). Most screens have used macrophage cell lines (Christophe et al., 2009; Pethe et al., 2013; Schiebler et al., 2015; Stanley et al., 2014; VanderVen et al., 2015). The ease of use and maintenance are understandable reasons for this popular choice. In recent years, significant advances have been made to encompass a broader spectrum of host systems to reflect better physiological relevance, such as human primary macrophages (Sundaramurthy et al., 2013; Sundaramurthy et al., 2014) or iPSC-derived macrophages (Han et al) (Figure 2B). While this system has the advantages of primary cells, it is a mono-culture of macrophages and does not completely capture the target spaces possible during in vivo infections. Recently, Huang et al. have developed a system called deconstructed granuloma (Dgr) where ex vivo lung-infected cells from Mtb infected mice are used as host system for screening (Huang, Kushner, et al., 2018; Huang, Nazarova, et al., 2018). This system has the advantages of retaining the in vivo influences and Mtb-induced alterations during in vivo environments. Moreover, because multiple cell types are involved, it represents a more physiologically relevant and encompassing platform for chemical screening. It will be of obvious advantage if the Dgr protocol can work directly on primary infected cells; however, since infection events are relatively rare in the timescales of in vivo infection (2 weeks), a second round of infection is needed for robust detection sensitivity. The reinfection is performed with Mtb expressing mKO under a tet-inducible promoter. This system allows detection of fluorescence specifically associated with live Mtb, thus providing higher sensitivity and specificity. It will be interesting to further develop the protocol to an imaging format and couple it with sub-cellular and cellular markers to see if potential differences in cellular and sub-cellular milieus of Mtb in vivo can be exploited for drug discovery. The Dgr assay thus elegantly combines the relevance and influences of an in vivo infection with the ease and scalability of in vitro platforms. However, a drawback of the method could be the loss of spatial cues during the preparation of single cell suspensions. Additionally, due to the necessity of a reinfection, the phenotype is read from the second round of infection. It might be advantageous to read out the effect of drug treatment on primary infected bacteria rather than the reinfected Mtb. Thus, new assays with improved detection sensitivities of primary infected cells could be interesting.
The zebrafish infection with M. marinum offers a true in vivo system that could retain spatial and temporal cues during infection. Protocols have been successfully established for drug screening for M. marinum survival in zebra fish larvae and drug screens performed (Matty et al., 2019; Takaki, Davis, Winglee, & Ramakrishnan, 2013) in this system. The zebrafish system has the obvious advantages of a true in vivo infection model, however achieving high throughput could be challenging since larvae have to be infected individually by injection of M. marinum. The recent development of a method to infect zebrafish larvae by incubating in media containing M. marinum (Dalton et al., 2017), or robotic injection (Spaink et al., 2013), if adapted successfully to a screening format with good assay metrics and reproducibility could alleviate the need for manual injection of individual larvae. Finally, the amoebae model (Hanna et al., 2020; Ouertatani-Sakouhi et al., 2017; Richter et al., 2019; Trofimov et al., 2018) that involves infection of single celled ameobae with either M. marinum or M. tuberculosis infection offers a neat host–pathogen interaction space that combines the advantages of in vitro platform and relevance of an in vivo system. Cytotoxicity assays performed in amoeba model compares favourably with the mammalian cells (Trofimov et al., 2018). Further, screening of similar compound libraries between amoeba/M. marinum infection with the more widely used macrophage/Mtb system shows conservation of some – but not all – hits (Trofimov et al., 2018), possibly due to constraints imposed by the free living lifestyle. The amoeba system can thus be a filter to identify universal mechanisms and conservation of intracellular target spaces.
6.2 Mycobacterial systems
BOX 1. Possible future strategies
Natural products library: Natural products have not been extensively screened for anti-TB effect inside host systems. These compounds represent new and previously untapped chemical spaces that can act against the expanded target range inside host cells.
Pro-infectives: One interesting category of compounds generally ignored in most screening campaigns is the compounds that increase the bacterial survival. While it is understandable that these pro-infective compounds will have limited value, if at all, in drug discovery, they could be potential discovery tools to understand the mechanisms restricting intracellular mycobacterial survival.
Physiology reporter strains: The new generation of Mtb physiology reporter strains can be harnessed for intracellular screening to identify perturbations that alter the physiology of Mtb. Such an approach will enable identification of compounds that, while not necessarily killing Mtb, could alter the physiological status of the bacteria. Such approaches can have consequences for identifying drug tolerant sub-populations.
Comparative screening: Multiple host-mycobacteria systems can be screened against the same chemical library. Such an approach can provide insights into shared ‘universal’ mechanisms versus cell type restricted, or context specific, perturbations. Identification of such distinctions will have implications in drug discovery.
Machine learning for phenotype classification: High content image analysis is maturing to provide automated phenotype classification based on machine learning. Clever multiplexing of mycobacterial readouts with different host cellular/sub-cellular markers combined with high-resolution high-content imaging could enable rapid detection of distinct phenotype classes. Potential correlation of such classes with drug tolerance will have implications for drug discovery.
Most of the screens performed to identify compounds acting inside host system have used mycobacteria expressing a fluorescent protein, with reduction in the fluorescence being the major readout. Green fluorescent protein (GFP) expressing mycobacteria are the most common (Christophe et al., 2009; Han et al., 2019; Koo et al., 2008; Pethe et al., 2013; Stanley et al., 2014; Sundaramurthy et al., 2013), although screens have been performed with mycobacteria expressing red fluorescent protein (RFP)(Richter et al., 2019), mCherry (Huang, Kushner, et al., 2018; VanderVen et al., 2015) or tdTomato (Shapira et al., 2020) (Figure 2C). In all the cases, the fluorescent protein is expressed under a strong promoter. However, the fluorescent proteins that enable the detection of the bacterium could also present a potential caveat. The fluorescence does not go away immediately after the bacteria are killed (Hagedorn & Soldati, 2007). Since these proteins are expressed in high levels and are quite stable, scoring a reduction in fluorescence intensity could impact the sensitivity, resulting in higher rates of false negatives. Compounds showing subtle but important effects are thus likely to be missed. Another major limitation is that the advantages of imaging is somewhat negated by the fact that it is often difficult, if not impossible, to assign live/dead status to intracellular Mtb based on the fluorescent images.
One important advance is the generation of Mtb strains where fluorescent reporters are conditionally expressed upon induction. Huang et al. optimised assay conditions for high throughput screening using Mtb that expresses mKO under Tet inducible promoter and mCherry under a constitutive promoter (Huang, Kushner, et al., 2018; Huang, Nazarova, et al., 2018). In this system, mKO is expressed upon anhydro tetracycline (ATc) induction, hence active protein synthesis is required to visualise mKO fluorescence, indicating that the Mtb emitting the fluorescence reporter is alive. In their assay, infected cells are incubated with drugs for 24 h before addition of ATc to induce the expression of mKO. Dead bacteria can still be visualised by their residual mCherry expression, but will not express mKO. Thus, the mKO/mCherry ratio serves as a good metric to assess live/dead status for intracellular Mtb under different perturbation conditions. This approach significantly increases the sensitivity of the assay and provides scope for future screens and high content assays that can be multiplexed with host cellular and sub-cellular markers.
Several sophisticated Mtb reporter strains have been engineered in the last few years (Abramovitch, 2018; MacGilvary & Tan, 2018) that detect Mtb response to environmental conditions such as NO, hypoxia (Tan, Sukumar, Abramovitch, Parish, & Russell, 2013), redox (Bhaskar et al., 2014) and ions such as H+ (Abramovitch, Rohde, Hsu, & Russell, 2011; Johnson et al., 2015), chloride (Tan et al., 2013), potassium (MacGilvary, Kevorkian, & Tan, 2019), as well as Mtb physiological status such as replication (Sukumar, Tan, Aldridge, & Russell, 2014). These strains typically use a fluorescent protein under a promoter responsive to the respective conditions and are providing a wealth of information on the environmental conditions Mtb experiences during infection. Identification of condition-specific perturbation is now realistic, for example by screening a panel of reporter strains against a drug library. Furthermore, multiplexing these assays with host cellular and sub-cellular markers could identify niches having potentially differential susceptibilities for chemical perturbations.
6.3 Read out strategies
Typically, screens – both chemical and genetic – tend to have relatively simple readouts, with hit selection based on metrics such as a Z score that provides the distance from the negative control of a single parameter being the major criteria. For such simple readouts, plate readers detecting fluorescence from GFP – or mCherry – expressing bacteria is often the method of choice (Huang, Kushner, et al., 2018; Huang, Nazarova, et al., 2018; VanderVen et al., 2015). The emergence of automated imaging platforms has resulted in more sophisticated readouts that are based on imaging the bacteria inside infected cells. Several analysis platforms – usually custom developed – are used to automatically detect host cells and bacteria from thousands of images. Automated imaging for chemical screens for intracellular Mtb in live cells enforces additional demands because of the requirement of biosafety-level 3 laboratory. If infected cells are to be imaged without fixation, the imager must be located inside the biosafety level 3 laboratory space, a significant constraint in terms of space and costs. While some screens are performed under such conditions (Christophe et al., 2009; Han et al., 2019; Pethe et al., 2013), other screens have used surrogates of Mtb that does not require BSL3 (Richter et al., 2019; Sundaramurthy et al., 2013), or image the plates after fixation (Stanley et al., 2014; Sundaramurthy et al., 2013).
Although multiple features can be extracted from images, only few parameters such as the total fluorescence intensity of bacteria have been used to define hits (Christophe et al., 2009; Han et al., 2019; Pethe et al., 2013). However, deep analysis of multiple parameters from images can reveal systems properties that are not accessible to ensemble measurements (Bray et al., 2016; Collinet et al., 2010). Such approaches can be used to understand better the nature of chemical perturbations and gain insights into the mechanism of action of hit compounds already at the level of primary screen (Sundaramurthy et al., 2013). As an example of such an approach, multiple features of the infected macrophage such as the total number of cells, fraction of infected cells, area and other morphological features of the macrophage, number of bacteria per infected cell, intensity, area and morphological features of the bacteria within the macrophage were extracted and categorised into host and bacterial features. Compounds showing similar profiles were grouped and averaged profiles compared with genetic profiles from a genome-wide siRNA screen on endocytosis. Using this approach, the mechanism of action of compounds belonging to specific clusters was successfully predicted and experimentally confirmed (Sundaramurthy et al., 2013, 2014).
The role of heterogeneity in the host–pathogen interface and its influence on pathogenesis outcomes at tissue, cellular and sub-cellular is becoming apparent in recent years (Avraham et al., 2015; Claudi et al., 2014; Sachdeva, Goel, & Sundaramurthy, 2020). Even under non-perturbed conditions, it is not unusual to see different infection outcomes at an individual cell level at both cellular (Avraham et al., 2015; Avraham & Hung, 2016; Sachdeva et al., 2020) and tissue (Claudi et al., 2014) levels. How the heterogeneity at single cell level influences population outcomes and what the primary drivers of the heterogeneity are remain outstanding questions. The extensive use of imaging in assessing the outcomes of chemical perturbations in host-mycobacteria infection systems, as outlined here, invites the incorporation of single cell readouts into analysis strategies, thus encompassing cellular heterogeneity parameters into further analysis considerations. For instance, similar net bacterial fluorescence from two different chemical perturbations could be an outcome of either more cells infected with fewer bacteria or fewer infected cell having more bacteria. The latter case could indicate a sub-population showing distinct phenotype. Harnessing the full power of high content image analysis, including automated phenotype classification from images, will be crucial for such analyses.
7 CONCLUSIONS AND PERSPECTIVES
With a combination of emerging technologies and recent advances in different domains such as complex host systems, reporter Mtb strains and sophisticated high content readouts and analysis platforms, the time appears ripe for meaningful multiplexing. Additional multiplexing is feasible by including sub-cellular markers such as lysosomes or autophagosomes in a macrophage screen set up, or including celltype specific markers in a deconstructed granuloma screening set up to further classify phenotypes in multiparametric space based on cell type specific- or sub-cellular localisation specific- metrics. Moreover, distinct alterations in Mtb physiological states upon different chemical perturbations can be captured in multi-parametric space by using the new generation of Mtb reporter lines in chemical screening platforms and combining it with host subcellular and cell type-specific markers. Such powerful approaches can identify subtle or context specific phenotypes and perturbations that could have significant impact on distinct sub-populations of Mtb. Identification of such perturbations will be hugely relevant, given the importance of distinct Mtb sub-populations that are non-replicating and tolerant, with differential susceptibility for anti-microbial drugs. The importance of successfully sterilising every last niche of Mtb cannot be overstated. While no single assay is representative of the true complexities of Mtb life style, combination of chemical screening strategies outlined here could open up new possibilities in the realisation of this ambitious goal.
ACKNOWLEDGEMENTS
We acknowledge the support of Department of Atomic Energy, Government of India, under Project Identification No. RTI 4006 and NCBS-TIFR, core funds. NS acknowledges fellowship from Council of Industrial and Scientific Research (CSIR).
CONFLICT OF INTEREST
The authors declare they have no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during the current study