EGFR/FAK and c-Src signalling pathways mediate the internalisation of Staphylococcus aureus by osteoblasts
Funding information: National Natural Science Foundation of China, Grant/Award Number: 81772366
Abstract
Internalisation of Staphylococcus aureus in osteoblasts plays a critical role in the persistence and recurrence of osteomyelitis, the mechanisms involved in this process remain largely unknown. In the present study, evidence of internalised S. aureus in osteoblasts was found in long bone of haematogenous osteomyelitis in mice after 2 weeks of infection. Meanwhile, eliminating extracellular S. aureus by gentamicin can partially rescue bone loss, whereas the remaining intracellular S. aureus in osteoblasts may be associated with continuous bone destruction. In osteoblastic MC3T3 cells, intracellular S. aureus was detectable as early as 15 min after infection, and the internalisation rates increased with the extension of infection time. Additionally, S. aureus invasion stimulated the expression of phosphor-focal adhesion kinase (FAK), phosphor-epidermal growth factor receptor (EGFR) and phosphor-c-Src in a time-dependent way, and blocking EGFR/FAK or c-Src signalling significantly reduced the internalisation rate of S. aureus in osteoblasts. Our findings provide new insights into the mechanism of S. aureus internalisation in osteoblast and raise the potential of targeting EGFR/FAK and c-Src as adjunctive therapeutics for treating chronic S. aureus osteomyelitis.
1 INTRODUCTION
Osteomyelitis is an inflammation process of bone and bone marrow caused by infecting microorganisms (Lew & Waldvogel, 2004). It is often secondary to open trauma, surgical reduction of fracture or haematogenous dissemination (Taki, Krkovic, Moore, Abood, & Norrish, 2016). The most common pathogen associated with osteomyelitis is Staphylococcus aureus, accounting for 34–59% of culture-positive cases (Jiang et al., 2015; Ma et al., 2018). Chronic osteomyelitis occurs if acute osteomyelitis is not treated appropriately, it is characterised by persistence of microorganisms and low-grade inflammation (de Mesy et al., 2017; Jerzy & Francis, 2018). The treatment of chronic osteomyelitis remains a great challenge for orthopaedic surgeons.
Persisting bacterial biofilm on foreign material and submicron canaliculi of osteocyte are the common causes for a chronic state of osteomyelitis (de Mesy et al., 2017; Tottrup et al., 2016). Recent works have demonstrated that internalisation of S. aureus by non-professional phagocytes essentially contributes to development of chronic and recrudescent infections (Hamza et al., 2013; Josse, Velard, & Gangloff, 2015; Loffler, Tuchscherr, Niemann, & Peters, 2014). The intracellular S. aureus may escape from host immune response and the action of antibiotics, thereby serves as bacterial pool and reservoir for persistent infections (Hamza et al., 2013; Kahl, Becker, & Loffler, 2016). Researchers have been exploring drugs that can effectively eliminate intracellular S. aureus, such as rifampicin, clindamycin, etc. However, due to the destruction of bone caused by infection, the amount of antibiotics penetration into infection lesions is very limited (Tottrup et al., 2016). Antibody–antibiotic conjugate have been used to kill intracellular S. aureus, but it is effective only when released into the proteolytic environment of the phagolysosome (Lehar et al., 2015). Therefore, inhibiting the internalisation of S. aureus by osteoblasts in the early stage of infection may be a new method for prevention and treatment of chronic osteomyelitis (Josse et al., 2015; Shi & Zhang, 2012).
Non-phagocytic cells such as epithelial cells, endothelial cells and osteoblasts can internalise S. aureus, but the internalisation rates is substantially different, suggesting that different types of cells internalise S. aureus by different molecular mechanisms (Strobel et al., 2016). Besides, inactivated S. aureus can also be ingested by osteoblasts, indicating that the internalisation of S. aureus is an active process involving host cells (Hudson, Ramp, Nicholson, Williams, & Nousiainen, 1995). Studies have shown that protein kinases such as focal adhesion kinase (FAK) and Src kinase are significantly activated during internalisation of S. aureus in kidney cell line 293T and epithelial cells, and inhibition of FAK and Src lead to decrease in S. aureus internalisation (Agerer et al., 2005; Agerer, Michel, Ohlsen, & Hauck, 2003; Richter et al., 2016). However, it has been largely unknown how S. aureus is internalised into osteoblasts.
Studies have demonstrated the over activation of epidermal growth factor receptor (EGFR) signalling in S. aureus-infected bone (Chen, Yao, et al., 2018; Mendoza Bertelli et al., 2016). Epidermal growth factor receptor (EGFR) is a key molecule regulating cell survival (Liu et al., 2019; Zhang et al., 2011). Crosstalk among EGFR, Src and FAK has been attributed to various cellular behaviours (Chen, Oh, et al., 2018; Pentassuglia & Sawyer, 2013). Considering the link between S. aureus internalisation and FAK and Src in 293T and epithelial cells, and the functional association of FAK and Src pathway with EGFR signalling, we postulate that EGFR/Src and EGFR/FAK signalling mediate the internalisation of S. aureus by osteoblasts. Here, we have found that S. aureus internalises into osteoblasts as early as 15 min after infection, and progressively increased with the extension of infection time in vitro. Furthermore, we demonstrated that EGFR/FAK and c-Src signalling are essential for internalisation of S. aureus in osteoblasts.
2 RESULTS
2.1 Staphylococcus aureus induces loss of osteoblasts
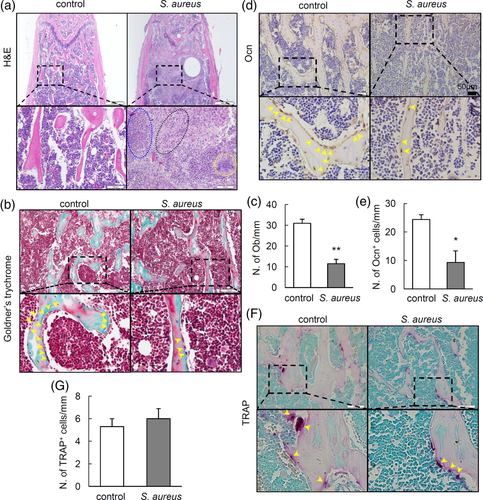
To analyse the effect of S. aureus infection on osteoblasts and bone formation, we infected 10-week old male mice with S. aureus by intravenously injection. Femurs were harvested for histomorphological analysis. The development of osteomyelitis in infected bone was verified by histological examination. As shown in haematoxylin and eosin (H&E) staining (Figure 1a), severe destructions in trabecular bone structure were observed at Week 2 after S. aureus infection. Additionally, intensive infiltration of inflammatory granulocytes, necrosis and marrow fibrosis were observed in infected femur. To detect the changes of osteoblasts on the surface of trabecular bone, Goldner's trychrome staining was performed. Results showed diminished numbers of osteoblast on the surface of trabecular bone (Figure 1b,c). To further confirm the loss of osteoblast induced by S. aureus, immunostaining of the osteocalcin (Ocn), a marker for osteoblast, was performed in S. aureus-infected femur. Results showed significantly reduced number of Ocn+-stained cells on the surface of trabecular bone (Figure 1d,e). Since bone homeostasis is maintained by a balance between bone formation by osteobalsts and bone resorption by osteoclasts. We therefore performed tartrate-resistant acid phosphatase (TRAP) staining to assess the changes of osteoclasts activity in infected femur. However, we did not observe much difference in the number of TRAP+ cells in bone between two groups (Figure 1f,g). Together, these results indicate that S. aureus infection may mainly induce loss of osteoblasts at early stage of infection.
2.2 Internalisation of S. aureus may be partially associated with osteoblasts lost
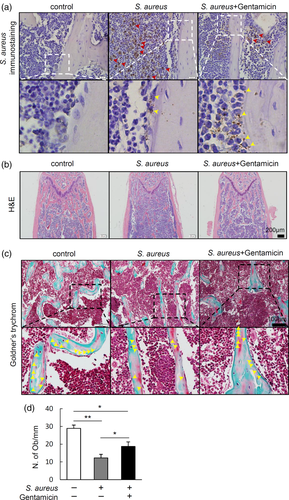
To investigate the distribution of S. aureus in bone marrow, we performed immunohistochemical staining to detect S. aureus in infected tibia. Results showed that most of the S. aureus distributed in the bone marrow cavity, importantly, there were some intracellular localisation of S. aureus in osteoblasts in S. aureus-infected group (Figure 2a). Since gentamicin had poor killing effect on the intracellular S. aureus in vivo even at high dosage (10 mg/kg) (Sandberg, Hessler, Skov, Blom, & Frimodt-Møller, 2009), we treated S. aureus-infected mice with gentamicin at the dosage of 0.1 mg/kg which had been report to significantly reduce S. aureus burden in mice (Li et al., 2008), to explore the role of the intracellular S. aureus in bone destruction. Results showed that gentamicin treatment significantly suppressed the amount of S. aureus in bone marrow, but had no much effect on S. aureus internalised in osteoblasts (Figure 2a). H&E staining was performed to assess the pathological changes in femurs. Results showed thinner trabecular bone in S. aureus-infected mice compared with that in control mice, while gentamicin treatment partially rescued the loss of trabecular bone induced by S. aureus infection (Figure 2b). To assess the effect of intracellular S. aureus on the osteoblasts number, we performed Goldner's trychrome staining to quantify osteoblasts on the surface of trabecular bone. Results showed that gentamicin treatment partially restored the loss of osteoblasts induced by S. aureus infection in bone, additionally, compared with control mice, S. aureus-infected mice has significantly less osteoblasts even after gentamicin treatment (Figure 2c,d). Together, these data indicate that intracellular S. aureus in osteoblasts might be a major cause for the loss of osteoblasts and bone.
2.3 Kinetics of S. aureus internalisation by MC3T3 cells
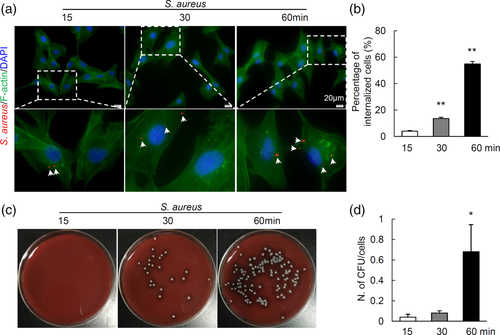
To determine the internalisation kinetics of S. aureus, osteoblastic MC3T3 cells were incubated with S. aureus. Immunofluorescent staining showed that intracellular S. aureus was detectable as early as 15 min after infection (Figure 3a). Quantification results showed that the internalisation rates increased with the extension of infection time (Figure 2b). In addition, culture of intracellular S. aureus colonies confirmed increased internalisation, the number of bacterial colony-forming units (CFU) per cells was significantly increased by 60 min after infection (Figure 3c,d). The above data indicate that S. aureus can be internalised by MC3T3 cells after infection in vitro, and the internalisation rates increased with the extension of infection time.
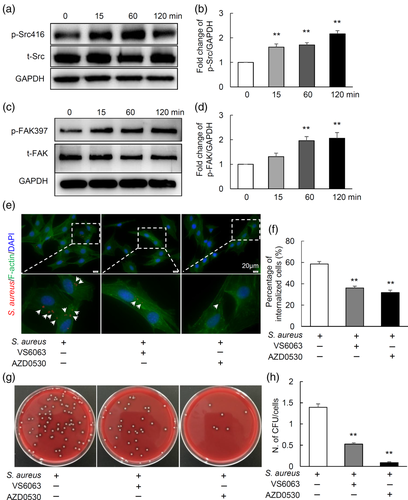
2.4 FAK and c-Src signalling pathways mediate the internalisation of S. aureus by MC3T3 cells
It was reported that FAK and Src signalling pathways have been involved in internalisation of S. aureus in kidney cell line 293T and endothenial cells (Agerer et al., 2003; Agerer et al., 2005; Richter et al., 2016). To elucidate the signalling pathways by which osteoblasts internalise S. aureus, the protein expression of FAK and Src were analysed in MC3T3 after S. aureus infection. As shown in Figure 4a–d, the expression of phospho-Src (p-Src) and phospho-FAK (p-FAK) were significantly increased in time-dependent way, indicating that the activation of c-Src and FAK pathway in MC3T3 cells might be associated with the internalisation of S. aureus. To further test whether c-Src and FAK signalling are required for internalisation of S. aureus, MC3T3 osteoblastic cells were pre-treated with inhibitors of FAK (VS6063) or c-Src (AZD0530) signalling for 1 hr, then were infected by S. aureus. After 1 hr infection, cells were treated with lysostaphin and gentamicin for 1 hr to kill extracellular bacteria, washed with PBS for three times, and finally fixed for immunofluorescence or lysed for agar plate culturing to show intracellular bacteria. Interestingly, both VS6063 and AZD0530 could strongly inhibit internalisation of S. aureus, as shown in Figure 4e,f, the control group had 59% internalisation rate, while VS6063 and AZD0530 significantly decreased the rate to 36% and 32%, respectively. Consistent with the aforementioned results, culture and quantification of intracellular bacteria results showed a remarkable reduced number of bacterial CFUs by VS6063 (0.5 CFUs/cells) and AZD0530 (0.09 CFUs/cells) treatments compared to control (1.4 CFUs/cells) (Figure 4g,h). Together, these data demonstrate that FAK and c-Src signalling mediate the internalisation of S. aureus by osteoblastic cells.
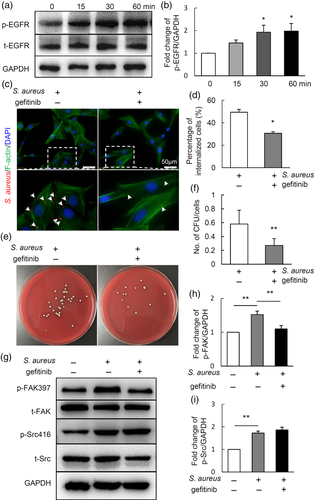
2.5 EGFR/FAK signalling may mediate the internalisation of S. aureus by MC3T3 cells
A recent report showed that EGFR is a host-entry cofactor triggering hepatitis B virus internalisation (Iwamoto et al., 2019). We then detected the changes of EGFR signalling in MC3T3 in response to S. aureus infection using western blot. Results showed that the expression of phosphorylated EGFR (p-EGFR) was significantly increased by 15 min after S. aureus infection, and progressively increased with the extension of infection time (Figure 5a,b). To further explore whether the EGFR signalling mediates the internalisation of S. aureus by osteoblasts, MC3T3 osteoblastic cells were pre-treated with gefitinib, an inhibitor for EGFR signalling, for 1 hr, followed by S. aureus infection and internalisation for 1 hr. Next, cells were treated with lysostaphin and gentamicin for 1 hr to kill extracellular bacteria, finally fixed for immunofluorescence or lysed for agar plate culturing to show intracellular bacteria. Results showed that gefitinib treatment significantly suppressed the percentage of internalised cells compared with control (Figure 5c,d). Consistently, the intracellular bacteria colonies were significantly reduced after being treated with gefitinib (Figure 5e,f). To further clarify the potential linkage among EGFR, c-Src and FAK pathways in S. aureus internalisation, MC3T3 osteoblastic cells were pre-treated with gefitinib followed with S. aureus infection. As shown in Figure 5g,i, the expression of p-FAK and p-Src were increased after being infected by S. aureus, interestingly, gefitinib treatment blocked the upregulation of p-FAK induced by S. aureus infection but had no effect on the level of p-Src. All the above data suggest that EGFR/FAK signalling and c-Src mediate the internalisation of S. aureus in osteoblastic cells.
3 DISCUSSION
The internalisation and survival of S. aureus in host cells allow bacteria to evade host immune response and antibiotics treatment, thereby play significant role in progress of chronic S. aureus osteomyelitis. In the present study, we provide strong evidence that S. aureus internalised by osteoblasts in vivo and in vitro. Next, we found that S. aureus activates EGFR/FAK and c-Src signalling in osteoblasts. Further, we demonstrated that blocking EGFR/FAK or c-Src signalling inhibits internalisation of S. aureus in osteoblasts. Our findings may contribute to the understanding of the pathogenesis of chronic S. aureus osteomyelitis and provide a possible therapeutic approach for concomitant drugs therapy of chronic S. aureus osteomyelitis.
It was reported that S. aureus osteomyelitis induced extensive loss of cortical and trabecular bone through activation of osteoclast-mediated bone resorption (Putnam et al., 2019) or by loss of osteoblast (Hou et al., 2019; Zhu et al., 2019). Loss of osteoblasts may be due to S. aureus virulence factors and inflammatory factors, which can induce apoptosis and pyroptosis (Hou et al., 2019; Josse et al., 2016; Zhu et al., 2019). Our present study showed that haematogenous infection of S. aureus for 2 weeks resulted in diminished osteoblasts and colonisation of S. aureus in osteoblasts in vivo. Eliminating extracellular S. aureus by gentamicin can partially rescue bone loss, indicating that the remaining intracellular S. aureus in osteoblasts may be associated with the loss of osteoblasts and continuous bone destruction, because internalised S. aureus in osteoblasts can protect it from immune cells and antibiotics that cannot penetrate the cells (Valour et al., 2015), and induce apoptosis in osteoblasts as well (Tucker, Reilly, Leslie, & Hudson, 2000).
Prior works have described the internalisation of S. aureus in MC3T3 cells at approximately 108 CFU bacteria after 45 min of infection (Ellington et al., 1999; Tucker et al., 2000), here our work further delineate time-dependent internalisation of S. aureus in MC3T3 cells at low concentration of bacteria (105 CFU). S. aureus infection may activate FAK and Src phosphorylation in branchial epithelial cells and kidney cell line 293T, and targeted pharmacological inhibition of these pathways result in a significantly reduction of intracellular S. aureus (Agerer et al., 2005; Richter et al., 2016). In line with the above observation, our data show that S. aureus infection activates the phosphorylation of FAK and c-Src in osteoblast. Additionally, we demonstrate the greatest activation of FAK and c-Src by 60 min after infection, and the maximal internalisation rate of S. aureus correlates well with the maximal phosphorylation level of FAK and c-Src. Furthermore, blocking FAK or c-Src inhibits S. aureus internalisation, demonstrating that internalisation of S. aureus in osteoblasts was specifically linked to the activity of FAK and c-Src signalling. Previous study has documented the critical role of actin microfilaments in the internalisation process of S. aureus (Ellington et al., 1999). Considering that FAK and Src family kinases are main signalling required to stabilise the actin microfilaments (Frame, 2004; Siesser et al., 2008), our study raises the possibility of FAK/Src/microfilaments system in initiating the internalisation of S. aureus by osteoblasts.
Fibronectin-binding proteins (FnBPs) in the cell-wall of S. aureus is an important component which can bind to extracellular matrix protein fibronectin, thereafter, the FnBP-Fn-α5β1 integrin interaction may initiate the internalisation of S. aureus into epithelial cells and endothelial cells (Ilic et al., 2004; Josse et al., 2015). It is reported that expression of fibronectin can be regulated by FAK and EGFR signalling, the deficiency of either FAK or EGFR signalling was demonstrated to result in downregulated expression of fibronectin (Hsu, Chang, Chang, Chang, & Chen, 2017; Ilic et al., 2004). Our data showed that blocking EGFR/FAK signalling suppresses S. aureus internalisation. It can be inferred from the above work that EGFR/FAK may participate in initiating S. aureus internalisation by stimulating the expression of fibronectin in osteoblasts.
EGFR signalling is critical for the survival, proliferation as well as differentiation osteoblastic cells (Liu et al., 2019; Zhang et al., 2011). Here our work shows a new role of EGFR signalling that it can mediate the internalisation of S. aureus by osteoblasts. A recent study report that hepatitis B virus can be internalised into cells with the endocytotic relocalisation of EGFR (Iwamoto et al., 2019). Although our results show that EGFR/FAK signalling may be involved in the internalisation of S. aureus into osteoblasts, additional further studies are warranted to determine the process of S. aureus internalisation by activating EGFR/FAK signalling.
Recent studies have demonstrate that activation of both c-Src and EGFR signalling are required for internalisation of virus or Chlamydia pneumoniae by endocytosis (Bannach et al., 2020; Hänsch et al., 2020; Lingemann et al., 2019). Our data also clearly indicate the importance of c-Src and EGFR signalling in internalisation of S. aureus in osteoblasts. As c-Src is a non-receptor tyrosine kinase that can transactivate and prolong EGFR-mediated downstream signalling (Donepudi & Resh, 2008), it is possible that c-Src and EGFR signalling activate the internalisation of S. aureus synergistically in osteoblasts.
Together, the present work reveals that internalisation of S. aureus is closely associated with osteoblast loss. Additionally, we demonstrate that S. aureus internalises into osteoblastic cells by activating EGFR/FAK and c-Src signalling in vitro. Although the mechanism for EGFR/FAK and c-Src associated S. aureus internalisation remains to be elucidated, the finding could be instructive that targeting these pathways may be a potential adjunctive therapeutics in the treatment of chronic S. aureus osteomyelitis.
4 EXPERIMENTAL PROCEDURES
4.1 Bacterial strain and culture
A clinical S. aureus strain was isolated from osteomyelitis patient and was identified using PHOENIX 100 (Becton Dickinson Microbiology System). For preparation of S. aureus for in vivo and in vitro experiments, S. aureus were cultured in tryptic soy broth in tubes overnight at 37°C under shaking conditions. Cultures were then harvested by centrifugation at 2500g for 10 min. The pellets were washed three times and resuspended with PBS. Next, the bacteria concentration was adjusted to an optical density of 0.5 at 600 nm, approximately equal to 1 × 108 CFU/ml.
4.2 Staphylococcus aureus osteomyelitis mice model
Ten-week old C57BL/6 male mice were obtained from the Laboratory Animal Center of Southern Medical University (Guangzhou, China). All experimental procedures were performed in accordance with protocols approved by the Animal Care and Use Committee at the Southern Medical University. The animals were housed with a 12 hr dark/12 hr light cycle, and the food and water were provided ad libitum. To evaluate the role of intracellular S. aureus in bone destruction, mice were randomly divided into three groups: two S. aureus-infected group with or without gentamicin treatment, and one control group. Control mice were injected with 100 μl PBS via caudal vein. For S. aureus-infected group, mice were received intravenous injection of 1× 106 CFU of S. aureus in 100 μl PBS via caudal vein. Besides, those S. aureus-infected mice with gentamicin treatments (0.1 mg/kg every other day) were injected intraperitoneally at Day 6 after infection. At Week 2 after infection, tibias and femurs were harvested for histochemistry, immunohistochemistry and immunofluorescence analysis.
4.3 Histochemistry and immunohistochemistry staining
Tibias and femurs of mice were dissected free from soft tissues, fixed in 4% paraformaldehyde for 48 hr, decalcified in 0.5 M ethylenediaminetetraacetic acid (EDTA, pH 8.0) for 1 week, and then embedded in paraffin. Samples were cut into 4-μm-thick sections longitudinally for H&E staining, Goldner's trychrome staining and TRAP staining. To assess the number of osteoblasts on the surface of trabecular bone, Goldner's trychrome staining was performed using a Goldner's trychrome staining kit (Cat. G3550, Solarbio, China) following manufacturer's instructions. To detect the osteoclasts activity on the surface of trabecular bone, TRAP staining was performed using a leukocyte acid phosphatase kit (Cat. 387A-1, Sigma-Aldrich) according to manufacturer's directions. The number of osteoblasts per millimetre of bone surface (N. of Ob/mm) and the number of TRAP+ cells per millimetre of bone surface (N. of TRAP+/mm) were quantified.
For immunohistochemistry staining, after de-paraffinising and re-hydrating sections, antigen retrieval was performed by 0.5% hyaluronic acid at 37°C for 15 min. Next, endogenous peroxidase was quenched with 3% H2O2 for 15 min in dark. After being blocked in serum for 30 min, sections were incubated with primary antibodies at 4°C overnight. Rabbit anti-S. aureus (ab20920, Abcam), rabbit anti-osteocalcin (Ocn) (ab93876, Abcam) were used as the primary antibodies. The next day, after three times washing in PBS, sections were incubated with biotinylated secondary antibody for 1 hr at room temperature, followed by incubating with avidin-conjugated horseradish peroxidase complex using VECTASTAIN ABC HRP kit (Cat. PK-4001, Vector Laboratories) according to the manufacturer's protocol. Finally, peroxidase activities of sections were revealed with 3,3′-diaminobenzidine substrate (Vector Laboratories). Images were acquired with an optical microscope (CX31, Olympus, Hamburg, Germany). The number of Ocn+ cells per millimetre of bone surface (N. of Ocn+/mm) was quantified.
4.4 Cell culture infection and treatments
Osteoblastic MC3T3-E1 (GNM15, Shanghai, Cell bank of typical culture preservation Committee of Chinese Academy of Sciences, China) cells were cultured in α-MEM supplemented with 10% FBS. To investigate whether S. aureus can internalises into osteoblasts and to quantify the ratio of bacterial internalisation, MC3T3 cells were seeded in the six-well plates at a density of 1 × 105 cells/well. Two days later, cells were infected with S. aureus at a concentration of 1 × 105 CFU/ml. At 15, 30 and 60 min after infection, MC3T3 cells were treated with 20 μg/ml of lysostaphin and 50 μg/ml of gentamicin for 1 hr to kill extracellular bacteria. To visualise the internalisation of S. aureus in the cells, wash the cells with PBS for three times, then fixed with 4% paraformaldehyde for immunofluorescence staining of S. aureus. To determine the number of CFU of the internalised bacteria, cells were lysed by treating with 0.1% Triton X-100 in PBS for 10 min to release all intracellular bacteria, followed by plating on blood agar plates and incubating overnight at 37°C.
To detect the effect of S. aureus infection on the signal pathways in osteoblasts, MC3T3 cells were seeded in six-well plates at density of 1 × 105 cells/well. When cells reached 90% confluence, cells were infected with S. aureus at a concentration of 1 × 105 CFU/ml, proteins were harvested at the indicated time points after infection for western blot.
To detect the role of signalling pathway in internalisation of S. aureus in osteoblasts, MC3T3 cells were pre-treated with 5 μM AZD0530 (S1006, Selleck), an inhibitor for Src signalling, 5 μM VS6063 (S7654, Selleck), an inhibitor for FAK signalling, or 5 μM gefitinib (S1025, Selleck) for 1 hr, followed by S. aureus infection. After 1 hr infection, cells were then fixed with 4% paraformaldehyde followed by immunofluorescence analysis or lysed for quantification of internalised S. aureus CFU numbers.
4.5 Immunofluorescent staining of intracellular bacteria
After killing the extracellular bacteria with lysostaphin (20 μg/ml) and gentamicin (50 μg/ml) and three times washing with PBS, MC3T3 cells were fixed with 4% paraformaldehyde for 20 min. After being permeabilised by PBS with 0.1% Triton X-100 solution (0.1% PBST) for 10 min, cells were blocked with 5% BSA for 30 min, followed by incubation with primary antibodies against S. aureus (Cat. ab20920, Abcam) for 2 hr at room temperature. Next, cells were washed three times, followed by incubation with goat anti-rabbit IgG(H + L) (Cat. HA1016, Hangzhou HuaAn Biotechnology). In addition, cells were incubated with Phalloidin-ifluor™ 488 Conjugate (Cat. 23115, AAT Bioquest) to show the outline of cells. Cell nuclei was stained blue in DAPI mounting medium (Cat. S2110, Solarbio, Beijing, China). Images were acquired with a fluorescence microscopy (BX63, OLYMPUS). To quantify the ratio of S. aureus internalisation in MC3TC osteoblastic cells, four different microscopic fields under 4 × 10 magnification were randomly acquired for each sample, the ratio of the number of cells with S. aureus to the total cell number was quantified.
4.6 Quantification of intracellular bacteria load
To quantify live intracellular S. aureus, extracellular bacteria were eliminated by lysostaphin (20 μg/ml) and gentamicin (50 μg/ml) treatment as mentioned above. After being washed with PBS for three times, cells were lysed by 0.1% PBST to release all intracellular bacteria, serial dilutions of the cell lysates were plated on blood agar plates and incubated overnight at 37°C. The relative number of bacterial CFU was calculated by dividing the number of CFU by the total number of MC3T3 cells.
4.7 Western blot
MC3T3 cells were lysed using lysis buffer containing proteinase inhibitors and phosphatase inhibitors (Cat. KGP250, KeyGEN BioTECH, Jiang Su, China). Proteins were quantified with Bicinchoninic Acid Protein Assay Kit (Cat. 23,225, Thermo Fisher Scientific). Proteins (30 μg/lane) were resolved by electrophoresis on SDS-PAGE, and then were transferred to 0.45 μm pore PVDF Immobilon-P membrane (Cat. IPVH00010, Merck Millipore, Germany). After blocking with 5% skimmed milk in TBS with 0.05% Tween for 1 hr, the membranes were incubated with primary antibodies overnight at 4°C. The primary antibodies include rabbit anti-phospho-Src (Cat. 2101S, Cell Signaling Technology), rabbit anti-Src (Cat. 2108S, Cell Signaling Technology), rabbit anti-phospho-FAK (Cat. bs-3,159, Bioss, Beijing, China), rabbit anti-FAK (Cat. 12,636-1-AP, Proteintech), rabbit anti-phospho-EGFR (Cat. ET1606-44, HuaAn Biotechnology, Hangzhou, China), rabbit anti-EGFR (Cat. ET1603-37, HuaAn Biotechnology, Hangzhou, China) and rabbit anti-GAPDH (Cat. ET1601-4, Hangzhou HuaAn Biotechnology). After three washing steps with TBST, the membranes were incubated with horseradish peroxidase-conjugated secondary antibody (Cat. SC-2357, Santa Cruz Biotechnology) at room temperature for 1 hr. Finally, the protein signals were developed by an enhanced chemiluminescence kit (Cat. WBKLS0500, Millipore), and the images were acquired with chemiluminescence instrument (Guangzhou Ewell Bio-Technology Co. Ltd., China).
4.8 Statistical analysis
For cell culture experiments, all results were obtained from experiments being repeated independently at least three times. The data of each group were presented as mean ± SEM. For comparisons between two groups, unpaired Student's t-test. For multiple comparisons, one-way analysis of variance (ANOVA) with Bonferroni post hoc test was used. p-Value <.05 was considered to be statistically significant difference. All statistical analyses were performed using SPSS 20.0 software (SPSS, Inc., Chicago, IL).
ACKNOWLEDGEMENT
This work was supported by National Natural Science Foundation of China (No. 81772366).
CONFLICT OF INTEREST
No competing financial interests exist.
AUTHOR CONTRIBUTIONS
Xianrong Zhang designed the experiments; Zhiguo Ji, Jianwen Su and Yilong Hou performed the experiments; Xianrong Zhang, Zhiguo Ji and Bin Yu analysed the data, Zhiguo Ji drafted the manuscript; Xianrong Zhang supervised the experiments, revised and approved the manuscript.