Rickettsial pathogen uses arthropod tryptophan pathway metabolites to evade reactive oxygen species in tick cells
Funding information: National Institute of Allergy and Infectious Diseases, Grant/Award Number: R01AI130116
Abstract
Reactive oxygen species (ROS) that are induced upon pathogen infection plays an important role in host defence. The rickettsial pathogen Anaplasma phagocytophilum, which is primarily transmitted by Ixodes scapularis ticks in the United States, has evolved many strategies to escape ROS and survive in mammalian cells. However, little is known on the role of ROS in A. phagocytophilum infection in ticks. Our results show that A. phagocytophilum and hemin induce activation of l-tryptophan pathway in tick cells. Xanthurenic acid (XA), a tryptophan metabolite, supports A. phagocytophilum growth in tick cells through inhibition of tryptophan dioxygenase (TDO) activity leading to reduced l-kynurenine levels that subsequently affects build-up of ROS. However, hemin supports A. phagocytophilum growth in tick cells by inducing TDO activity leading to increased l-kynurenine levels and ROS production. Our data reveal that XA and kynurenic acid (KA) chelate hemin. Furthermore, treatment of tick cells with 3-hydroxyl l-kynurenine limits A. phagocytophilum growth in tick cells. RNAi-mediated knockdown of kynurenine aminotransferase expression results in increased ROS production and reduced A. phagocytophilum burden in tick cells. Collectively, these results suggest that l-tryptophan pathway metabolites influence A. phagocytophilum survival by affecting build up of ROS levels in tick cells.
1 INTRODUCTION
Ticks are important vectors that transmit several pathogens to humans and wide range of animals (Dumler et al., 2005; Foley, Rejmanek, Fleer, & Nieto, 2011). In the United States, Anaplasma phagocytophilum, the agent of human granulocytic anaplasmosis (HGA), originally termed as human granulocytic ehrlichiosis (HGE), is primarily transmitted by Ixodes persulcatus complex ticks including Ixodes scapularis in the Eastern region and Ixodes pacificus in the Western region (Dumler et al., 2005; Foley et al., 2011). Within ticks, A. phagocytophilum is not transovarially transmitted but can be maintained transstadially in different developmental stages (de la Fuente et al., 2010; Rar & Golovljova, 2011). Upon entry into ticks through a blood meal, A. phagocytophilum modulates several intracellular molecular signalling pathways in the vector host (Alberdi, Cabezas-Cruz, Prados, & Rayo, 2019; Khanal, Taank, Anderson, Sultana, & Neelakanta, 2018; Sultana et al., 2010; Taank et al., 2017; Turck, Taank, Neelakanta, & Sultana, 2019). Some of the molecular pathways affected include manipulation of immune response, remodelling of the cytoskeleton, metabolism, inhibition of apoptosis, and redox pathways (Alberdi et al., 2019; Rikihisa, 2010). In response to the pathogen infection, tick cells may exhibit many strategies to fight against bacterial propagation. One of the defence mechanisms from host cells in response to bacterial pathogen infection is the production of reactive oxygen species (ROS) (Sena & Chandel, 2012). The role of ROS in host defence is mainly mediated by nicotinamide adenine dinucleotide phosphate (NADPH) oxidases (NOXs) in phagosomes that lead to direct killing of pathogens (Holzerováa & Prokischa, 2015; Sena & Chandel, 2012).
Anaplasma phagocytophilum has evolved strategies to prevent NOX-mediated ROS production by inhibiting superoxide synthesis in both human and murine neutrophils (Rikihisa, 2010). Recently, it was reported that the redox system in ticks acts to limit A. phagocytophilum infection (Alberdi et al., 2019). ROS also acts as a generic molecule that could bring together other active and reactive partially reduced O2 metabolites including oxygen and hydroxyl radicals (O2•– and •OH respectively) (Alberdi et al., 2019). These later molecules can be defined as true free radicals that can undergo redox reactions by interacting with surrounding molecules forming stable non-radical conditions (Alberdi et al., 2019). Whereas, hydrogen peroxide (H2O2) is more a pro-oxidant non-radical agent (Novo & Parola, 2008). The process on how A. phagocytophilum interacts with other free radicals is poorly understood.
The regulation of ROS is critical for its ability to participate in physiological process of the cell (Sena & Chandel, 2012). These regulatory events for ROS are controlled at several levels including negative feedback mechanisms, mitochondrial localization and function of antioxidant enzymes (Sena & Chandel, 2012). A number of active metabolites from kynurenine pathway were shown to act as peroxyl radical scavengers and antioxidants (Badawy, 2017). The kynurenine pathway is the primary route for tryptophan degradation (Badawy, 2017). The metabolites in the kynurenine pathway have different anti- and pro-oxidant properties that are essential in the maintenance of cell homeostasis (Han, Beerntsen, & Li, 2007). Among the kynurenine pathway metabolites, xanthurenic acid (XA) was shown to act as an antioxidant in Aedes aegypti midgut during digestion of blood meal (Lima et al., 2012) and kynurenine was shown to prevent Toxoplasma gondii growth through induction of apoptosis (Majumdar et al., 2019). The 3-hydroxyl-l-kynurenine (3-HK), another tryptophan metabolite, is shown to stimulate ROS production (Amaral & Outeiro, 2013; Han et al., 2007). However, in another study, authors suggested that 3-HK has powerful antioxidant property that leads to scavenging of peroxyl radicals (Christen, Peterhans, & Stocker, 1990). The 3-HK can also initiate the nicotinamide metabolic pathway leading to the production of many biologically active metabolites (Badawy, 2017). Kynurenic acid (KA) is also shown to act as a potential endogenous antioxidant through its role on scavenging hydroxyl radicals, superoxide anion and peroxynitrite (Breda, Sathyasaikumar, Sograte, Notarangelo, & Estranero, 2016; Campesan et al., 2011; Fujigaki, Yamamoto, & Saito, 2017). Furthermore, tryptophan 2,3 dioxygenase (TDO) and indolamine 2,3 dioxygenase (IDO), the two enzymes that initiate l-tryptophan catabolic pathways use superoxide anion as a cofactor (Xu, Liu, & Fu, 2018). TDO and IDO can efficiently clear free radicals much more than that of superoxide dismutase enzyme (Xu et al., 2018).
Our previous study reported involvement of tryptophan pathway in the establishment of A. phagocytophilum infection in ticks mainly through XA and kynurenine aminotransferase (KAT), an enzyme important in the production of XA and KA (Taank et al., 2017). The purpose of the present study is not only to investigate the effect of other tryptophan pathway metabolites on A. phagocytophilum infection in tick ISE6 cells but also to establish the consequence of redox state and ROS production on bacterial growth in these cells.
2 RESULTS
2.1 Ixodes scapularis and I. ricinus have conserved pathway for NAD(P)+ biosynthesis from the l-tryptophan pathway
Nicotinamide adenine dinucleotide (NAD) exists either as NAD+ (oxidised form) and NADH (reduced form). The NAD+ is produced by salvage pathway by recycling preformed components such as nicotinamide to NAD+ or by de novo synthesis from l-tryptophan (Magni et al., 2004). We searched tryptophan catabolic pathway gene orthologs in phylum Arthropoda using three steps. First, we retrieved nucleotide and annotated protein sequences of I. scapularis genes involved in the tryptophan pathway from the information available on KEGG database (Figure 1a). Each amino acid sequence was used as a query to search orthologs from other arthropods in National Center for Biotechnology Information (NCBI) and VectorBase databases. Secondly, we have used nucleotide sequence for each ortholog as a query in BLASTn search at NCBI against genomes of other arthropods. Finally, each annotated amino acid sequence retrieved from the first two searches was blasted against NCBI conserved domain to predict conserved domain(s). The results that were retrieved from only complete genomes were considered in the analyses. We have used the sequences from Chordata, and Mollusca, as an out-group, to allow the comparison with upper eukaryotic species (Figure 1b). In mammals, the kynurenine pathway is initiated via the catalysis of l-tryptophan to N-formylkynurenine by tryptophan 2,3-dioxygenase (TDO, KEGG enzyme entry number EC: 1.13.11.11) and Indolamine dioxygenase (IDO, EC: 1.13.11.52). The organisms in phylum Arthropoda have lost IDO and this enzyme seems to be conserved only in upper eukaryotes and mammals, making Arthropoda depending on TDO to catalyse the first reaction. Duplication of TDO is seen in some species including Homoptera Acyrthosiphon pisum, and the lepidoptera Plutella xylostella and Helicoverpa armigera (Figure 1b). Arylformamidase (KYF) (EC: 3.5.1.9) is a hydrolase that catalyses the transformation of N-formylkynurenine to l-kynurenine (L-Kyn). This enzyme is also involved in the glyoxylate and dicarboxylate metabolism pathway making it as not specific to the tryptophan metabolism. Arylformamidase is lost in Arachnida Tetranychus urticae, Dermatophagoides pteronyssinus, Sarcoptes scabiei and Leptotrombidium delicense (Figure 1b). Arylformamidase is duplicated in the Hymenoptera Pseudomyrmex gracilis, the Coleoptera Tribolium castaneum and Dendroctonus ponderosae (Figure 1b).
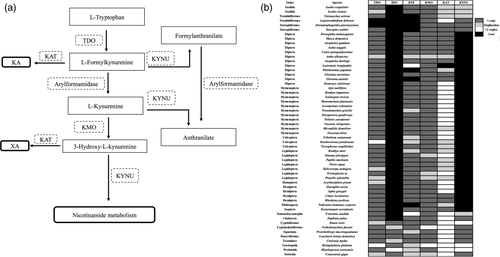
Through the l-kynurenine catabolic pathway, three enzymes use l-kynurenine as a substrate to initiate three different pathways (Figure 1a). Kynurenine aminotransferase (KAT, EC: 2.6.1.7) initiates the synthesis of Kynurenate. Kynureninase (KNYU, EC: 3.7.1.3) initiates Anthranilate pathway, and kynurenine 3-monooxygenase (KMO, EC:1.14.13.9) catalyses the hydroxylation of l-kynurenine to 3-HK (Figure 1a). KAT can then use 3-HK as a substrate to initiate the xanthurenate pathway (Figure 1a). KMO seems to be conserved in all arthropods (Figure 1b). Lepidoptera species like Danaus plexippus, Trichoplusia ni and Plutella xylostella have duplicate copies of KMO (Figure 1b). KAT seems to be under duplication in some arthropods (Figure 1b). However, the number of kat gene copies varied among arthropods. Some arthropods (e.g. Ixodid and Phlebotomus papatasi) have two copies and others (e.g. Arachnida, Leptotrombidium deliense) have up to 10 copies of kat genes. However, most of the organisms belonging to order Diptera and Sarcoptiformes have only one copy of kat gene (Figure 1b). KNYU also acts upstream to l-kynurenine and catalyses the cleavage of N-formylkynurenine to formylanthranilate (Figure 1a). Arylformamidase can then catalyse the transformation of the substrate to Anthranilate (Figure 1a). With the available genome information, we were able to observe that KNYU is found only among Acari species including I. scapularis and I. ricinus, which seem to be under duplication (two copies). KNYU is also the key enzyme that links the catabolism of l-tryptophan pathway to nicotinamide metabolism and the synthesis of NAD+ and NADP+ co-factors through the catabolism of 3-hydroxy-l-kynurenine and the initiation of Quinolinate pathway (Badawy, 2017). Absence of KNYU in a large group of arthropods suggests that the synthesis of NAD+ and NADP+ co-factors in these organisms occurs possibly from nicotinic acid (salvage pathway) rather than from quinolinic acid (main pathway).
Bioinformatic analysis of proteins that participate in nicotinamide pathway (Figure S1) suggests that large number of arthropods use different strategies to synthetize NAD+ and NADP+. Ixodes scapularis and I. ricinus are able to initiate the synthesis from nicotinamide, niacin or quinolinate pathways (Badawy, 2017). The other arthropod species are undergoing either loss or gain of genes involved in the nicotinamide pathway, including duplication events for some genes. As an example, Aedes aegypti, Anopheles gambiae, Culex quinquefasciatus, Lutzomyia longipalpis and Phlebotomus papatasi are only able to use niacin to initiate complete NAD+ and NADP+ synthesis (Figure S1). Aedes albopictus, Anopheles darlingi and Glossina morsitans are able to use the complete pathway from both nicotinamide and/or niacin (Figure S1). In summary, bioinformatics analysis indicates that I. scapularis and I. ricinus have complete enzymatic panel that may result in rapid scavenging and cleavage of l-tryptophan metabolites leading to the maintenance of kynurenate, xanthurenate and NAD(P)+ pool. Our analysis also indicated that large number of arthropods lacks KNYU that could result in increased accumulation of kynurenate, xanthurenate from L-tryptophan pathway. We believe that blood-feeding arthropods that lack KYNU may depend on the acquisition of vitamin B3 group for NAD(P)+ biosynthesis.
2.2 Tryptophan catabolism pathway genes are up-regulated in I. scapularis ticks and ISE6 tick cells upon A. phagocytophilum infection
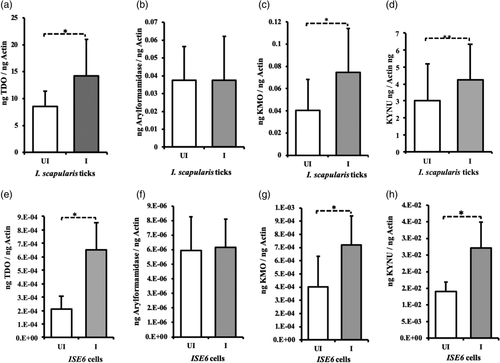
Our previous studies showed that A. phagocytophilum up-regulates expression of kat, a gene critical in l-tryptophan catabolism pathway (Khanal et al., 2018; Taank et al., 2017). In this study, we investigated whether A. phagocytophilum modulates expression of other genes in l-tryptophan pathway. QRT-PCR was performed with RNA samples extracted from uninfected and A. phagocytophilum-infected unfed nymphs (Figure 2a–d) or tick cells (Figure 2e–h). QRT-PCR analysis revealed significant (p < .05) up-regulation of tdo (Figure 2a,e), kmo (Figure 2c,g) and knyu (Figure 2d,h) gene transcripts in both A. phagocytophilum-infected unfed ticks (Figure 2a–d) and tick cells (Figure 2e–h) in comparison with respective uninfected controls. However, no significant (p > .05) difference in the expression of arylformamidase transcripts was noted between A. phagocytophilum-infected unfed ticks (Figure 2b) or tick cells (Figure 2f) in comparison with their respective uninfected controls (Figure 2b,f). Taken together, results from this study and from our previous study (Taank et al., 2017) indicate that most of the l-tryptophan catabolism pathway genes are up-regulated upon A. phagocytophilum infection of ticks or tick cells.
2.3 Xanthurenic acid and kynurenic acid act differently on A. phagocytophilum growth in tick cells
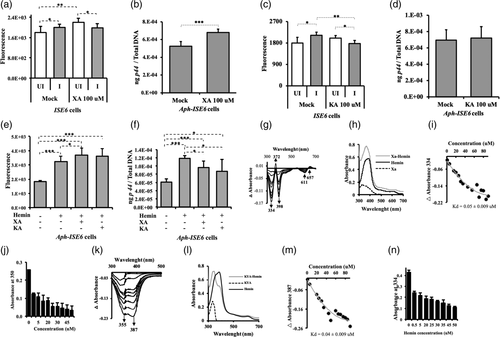
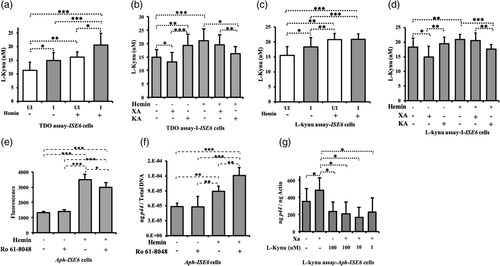
An antioxidant property for arthropod XA has been previously reported in mosquitoes (Lima et al., 2012). In addition, our previous study showed that exogenous addition of XA increases A. phagocytophilum loads in tick salivary glands and ISE6 cells (Taank et al., 2017). The observation of induced gene expression of many genes involved in the l-tryptophan catabolism pathway upon A. phagocytophilum infection of ticks or tick cells (Figure 2), prompted us to investigate the effects of XA and KA on bacterial growth and on the production of ROS. Treatment of ISE6 tick cells with different concentrations of XA or KA (0.1–100 μM) revealed no changes in cell morphology at 4 or 24 hr post infection (p.i.) in comparison to mock-treated cells (Figure S2). After 24 hr p.i., tick cells were harvested and split into two equal portions, one was subjected to ROS quantification and the other portion for DNA extraction. In mock-treated group, significantly (p < .05) increased ROS levels were noted in A. phagocytophilum-infected tick cells in comparison with the levels noted in uninfected control (Figure 3a). However, upon 100 μM XA treatment, significantly (p < .05) reduced ROS levels were observed in A. phagocytophilum-infected tick cells in comparison with levels observed in uninfected control (Figure 3a). No differences in the ROS levels were noted between A. phagocytophilum-infected XA-treated and mock-treated groups (Figure 3a). Treatment of tick cells with 0.1, 1 or 10 μM concentration of XA showed no significant (p > .05) differences in the ROS levels between uninfected and A. phagocytophilum-infected groups (Figure S3A). QRT-PCR analysis revealed significantly (p < .05) increased A. phagocytophilum burden in tick cells upon treatment with 0.1, 1 or 100 μM concentration of XA in comparison with mock-treated control (Figures 3b and S3B). Upon 100 μM KA treatment, significantly (p < .05) reduced ROS levels were observed in A. phagocytophilum-infected tick cells in comparison with levels observed in KA-treated uninfected control (Figure 3c). As like the observations noted with mock treatment, significant (p < .05) difference in ROS levels was noted upon treatment with lower concentrations (0.1, 1 μM) of KA in uninfected or A. phagocytophilum-infected cells (Figure S3C). Significant (p < .05) increase in bacterial burden was noted in 0.1 μM KA-treated A. phagocytophilum-infected cells in comparison with mock-treated control (Figure S3D). However, the treatment of tick cells with other concentrations of KA (1, 10, 100 μM) had no significant (p > .05) effect on bacterial growth in comparison with mock-treated control (Figures 3d and S3D). These results indicate that XA supports multiplication of A. phagocytophilum associated with reduction in the ROS level and the antioxidant effects from KA do not support bacterial multiplication in tick cells. In addition, these results suggest that the effect of XA and KA on A. phagocytophilum growth is concentration dependent and independent of ROS level.
2.4 Hemin supports A. phagocytophilum multiplication in tick cells
Ixodes scapularis lacks the pathway to synthesise heme (Perner et al., 2016). Therefore, these ticks are depending on the haemoglobin in the host blood meal to acquire heme (Narasimhan et al., 2014). Hemin is an organic compound that can be formed from processed red blood cells in the blood meal. Previous report shows that upon A. phagocytophilum infection, expression of genes involved in tick hemoglobinolytic and heme transport pathways was up-regulated (Villar et al., 2016). A study has shown that heme has both pro-oxidant and cytotoxic effects (Yachie, Varga, Vercellotti, & Eaton, 2019). We therefore investigated whether exogenous treatment of hemin has any effect on A. phagocytophilum growth and ROS production. The ISE6 tick cells were treated with 100 μM of hemin for 4 hr. Additionally, tick cells were treated with same concentrations of XA or KA along with hemin (100 μM). Anaplasma phagocytophilum-infected mock-treated cells were used as controls. Cells were infected 4 hr after treatment and harvested at 24 hr p.i. to evaluate ROS levels and bacterial burden. Significantly (p < .05) high levels of ROS were noted in hemin-treated A. phagocytophilum-infected tick cells in comparison with the levels noted in A. phagocytophilum-infected mock-treated control (Figures 3e and S3E). Anaplasma phagocytophilum-infected cells treated with hemin along with XA or KA also showed significant (p < .05) higher levels of ROS in comparison with the levels noted in A. phagocytophilum-infected mock-treated control (Figure 3e). Furthermore, significantly (p < .05) increased levels of ROS were observed in A. phagocytophilum-infected tick cells treated with both hemin and XA in comparison with the levels noted in A. phagocytophilum-infected tick cells treated with hemin only (Figure 3e). Interestingly, a significant (p < .05) increase in A. phagocytophilum loads were noted in hemin-treated A. phagocytophilum-infected tick cells in comparison with the loads noted in mock-treated A. phagocytophilum-infected cells (Figure 3f). However, significantly (p < .05) reduced bacterial loads were evident in A. phagocytophilum-infected tick cells treated with hemin along with XA or KA in comparison with the loads noted in A. phagocytophilum-infected cells treated only with hemin (Figure 3f). These results indicate that hemin alone, like XA, supports A. phagocytophilum growth in tick cells but in combination with XA or KA affects bacterial growth.
2.5 XA and KA chelate hemin
The observation of reduced A. phagocytophilum loads in tick cells upon treatment with hemin along with XA or KA (Figure 3f) suggested that the later metabolites could chelate hemin. We monitored the hemin titration and binding to XA or KA in separate experiments by UV–visible spectroscopy as reported (Soto et al., 2012). Hemin exhibits absorbance in the UV–Vis spectrum due to a Soret band near 400 nm (Comer & Zhang, 2018). When 5 μM of XA was titrated with incremental concentration of hemin, we observed the Soret band shifting to 364 nm with a significant increase in its amplitude (Figure 3g). The difference spectra at pH 8.0 (△ absorbance) between hemin and hemin binding to XA revealed a Soret band at 334, 372, 398, 611 and 657 nm (Figure 3g,h). The △ absorbance at 334 nm showed a Bmax value at 55 μM for hemin followed by saturation suggesting specific binding between hemin and XA (Figure 3i). We have observed that XA binds hemin with good affinity (equilibrium dissociation constant, KD = 0.05 ± 0.009 μM). Furthermore, we measured the absorbance from free XA at 350 nm. The results showed reduction in the Bmax value for XA with increase in concentration of hemin suggesting binding between both molecules and availability of less free XA (Figure 3j). We also monitored KA binding to hemin. We observed a shift in the hemin absorbance to 343 upon KA binding (Figure 3k). The difference in the spectra at pH 8.0 (△ Absorbance) between hemin and hemin binding KA revealed a Soret band at 355 and 387 nm (Figure 3k,l). The △ absorbance at 387 nm showed a Bmax value at 55 μM for hemin then saturation was reached suggesting specific binding of this molecule to KA (Figure 3m). Furthermore, our results revealed that KA binds hemin with good affinity (KD = 0.04 ± 0.009 μM). The absorbance from free KA was monitored at 334 nm. The results showed reduction in the Bmax value for KA as the concentration of hemin increased, suggesting binding between these molecules and availability of less free KA (Figure 3n). These results indicate that both XA and KA are potent hemin chelators.
2.6 Hemin, XA and KA modulate tryptophan dioxygenase activity
Tryptophan dioxygenase (TDO) is the first rate-limiting enzyme in l-tryptophan pathway (Badawy, 2017). We therefore determined if hemin, XA or KA modulate TDO activity in tick cells. The enzymatic assay to assess the level of TDO activation was performed using colorimetric method as previously reported (Gibney et al., 2014). Cells were harvested at 24 hr p.i. and processed for this assay. Significantly (p < .05) increased TDO activity was observed in A. phagocytophilum-infected cells in comparison with the levels noted in uninfected control (Figures 4a and S4A). Upon hemin treatment (100 μM), significantly (p < .05) increase in TDO activity was observed in both uninfected and A. phagocytophilum-infected tick cells in comparison with respective control groups without treatment (Figures 4a and S4A,B). Furthermore, significantly (p < .05) reduction in TDO activity was observed in A. phagocytophilum-infected cells upon treatment with XA (100 μM) in comparison with the activity noted in A. phagocytophilum-infected mock-treated cells (Figure 4b). In contrast, significantly increased TDO activity was noted in A. phagocytophilum-infected cells upon treatment with KA (100 μM) in comparison with the activity noted in mock-treated cells (Figure 4b). However, in the presence of hemin, significantly reduced TDO activity was noted in A. phagocytophilum-infected cells upon KA treatment in comparison with activity noted in A. phagocytophilum-infected cells treated with hemin alone (Figure 4b). Even though not significant (p > .05), reduced TDO activity was observed in hemin+XA-treated A. phagocytophilum-infected cells in comparison with hemin-alone treated cells (Figure 4b). Collectively, these results not only indicate that infection or hemin treatment induces TDO activity but also reveal that XA and KA inhibit hemin-mediated impact on TDO activity.
2.7 Hemin, XA and KA modulate l-kynurenine levels in tick cells
We then investigated the impact of TDO activity on the pool of l-kynurenine in tick cells. We performed the experiment similar to the assay used to assess TDO activity, and l-kynurenine level was measured without hydrolyzing N-formyl kynurenine to l-kynurenine (Alegre, López, & González, 2005). This enzymatic assay gives the pool of l-kynurenine accumulated in cells at 24 hr p.i. Similar to the observation noted in TDO assay, the l-kynurenine assay shows that A. phagocytophilum infection and hemin treatment significantly (p < .05) increase the level of l-kynurenine in tick cells (Figures 4c and S4C). In both uninfected and A. phagocytophilum-infected hemin-treated cells, a significant (p < .05) increase in the amount of l-kynurenine was noted compared with the levels observed in the respective mock-treated cells (Figures 4c and S4D). Upon XA treatment, significantly (p < .05) reduced l-kynurenine levels were noted in A. phagocytophilum-infected tick cells in comparison with the levels noted in mock-treated control (Figure 4d). However, upon KA treatment, no significant (p > .05) differences in the l-kynurenine levels were noted between KA-treated A. phagocytophilum-infected tick cells in comparison with mock-treated A. phagocytophilum-infected tick cells control (Figure 4d). A significantly reduced l-kynurenine levels were noted in hemin + KA group in comparison with the levels noted in hemin-alone-treated A. phagocytophilum-infected tick cells (Figure 4d). However, no significant differences in the l-kynuerenine levels were observed between hemin-alone treated and hemin+XA-treated A. phagocytophilum-infected tick cells (Figure 4d). Taken together, these results indicate that infection or hemin treatment induces l-kynurenine levels, whereas, the presence of KA inhibits hemin-mediated effect on L-kynurenine levels.
2.8 Exogenous addition of l-kynurenine affects XA-mediated A. phagocytophilum growth in tick cells
l-Kynurenine is the substrate used by KMO to synthetize the 3-hydroxy-l-kynurenine (3-HK) (Figure 1a). It has been reported that 3-HK can scavenge peroxyl radicals (Thomas, Witting, & Stocker, 1996). However, it is also reported that auto-oxidation of 3-HK leads to the hyper production of ROS that damages cellular lipids, proteins, and DNA (Zhuravlev, Zakharov, & Shchegolev, 2016). Recently, it was shown that overexpression of KMO creates a metabolic environment during which the cell exhibits increased tolerance for exogenous 3-HK-mediated cellular injury (Wilson et al., 2016). Using the selective KMO-inhibitor (Ro-61-8048), we checked the effect of KMO inhibition on ROS production and A. phagocytophilum growth in tick cells. Our results show that in the absence of hemin, treatment of A. phagocytophilum-infected cells with Ro-61-8048 (100 μM) had no significant (p < .05) impact on ROS (Figure 4e) or bacterial levels (Figure 4f) compared with their respective controls. Whereas, in the presence of hemin, treatment of A. phagocytophilum-infected cells with Ro-61-8048 showed significant reduction in ROS level (Figure 4e) and significant increase in bacterial loads (Figure 4f) compared with their respective control groups. These results suggest that hemin-mediated ROS induction is due to the cleavage of l-kynurenine to 3-HK by KMO. Furthermore, in the absence of hemin, treatment of A. phagocytophilum-infected tick cells with different doses of l-kynurenine (1, 10, 100 μM) along with XA resulted in significantly (p < .05) reduced bacterial burden in comparison with levels noted upon treatment with XA alone (Figure 4g). These results support the conclusion that XA mediates A. phagocytophilum growth by reducing TDO activity that leads to the decreased accumulation of l-kynurenine in tick cells. The overall data suggest that the level of conversion of l-kynurenine to 3-HK impacts A. phagocytophilum multiplication in tick cells.
2.9 Exogenous treatment with 3-hydroxyl l-kynurenine affects A. phagocytophilum growth in tick cells
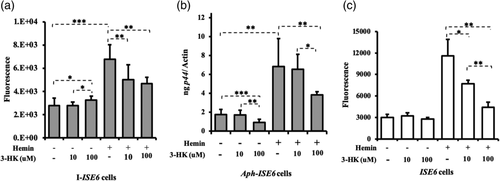
Next, we investigated if the accumulation of 3-HK that results from the increased flux in the kynurenine pathway could influence bacterial growth. We treated A. phagocytophilum-infected tick cells with two different concentrations of 3-HK (10 and 100 μM) in the absence or presence of hemin. The results showed dose-dependent response in ROS production (Figure 5a) and effect on bacterial burden (Figure 5b). In hemin non-treated cells, we noted significantly (p < .05) increase in ROS production in A. phagocytophilum-infected tick cells upon treatment with 100 μM of 3-HK compared with the levels noted with 10 μM of 3-HK or mock treatment (Figure 5a). In contrast, significantly (p < .05) reduced bacterial burden was noted upon treatment with 100 μM of 3-HK in comparison with the burden noted upon treatment with 10 μM of 3-HK or mock-control (Figure 5b). In the presence of hemin, treatment with 100 μM 3-HK significantly (p < .05) reduced ROS production (Figure 5a) and bacterial burden (Figure 5b) in A. phagocytophilum-infected tick cells in comparison with their respective infected mock-treated controls. Even though ROS levels were significantly low (Figure 5a), no significant difference in the bacterial burden (Figure 5b) was noted upon treatment with 10 μM 3-HK. The observation of reduced bacterial loads, even when ROS levels were low at 24 hr p.i., suggests that 3-HK could induce ROS at earlier time point resulting in clearance of bacteria from tick cell. To test whether 3-HK induces ROS at earlier time point, we performed an experiment with uninfected tick cells and measured ROS production after 3 hr addition of 3-HK. In the absence of hemin, no effect of 3-HK on ROS production was noted in uninfected tick cells at both tested (10, 100 μM) doses (Figure 5c). However, in the presence of hemin, significantly (p < .05) reduced ROS levels were noted upon treatment with 3-HK at both tested doses (10, 100 μM) in comparison with the untreated control (Figure 5c). The overall results indicate that hemin could lead to rapid elimination of 3-HK leading to scavenging of ROS. However, in the absence of hemin, accumulation of 3-HK could lead to its auto-oxidation and thereby increases ROS levels.
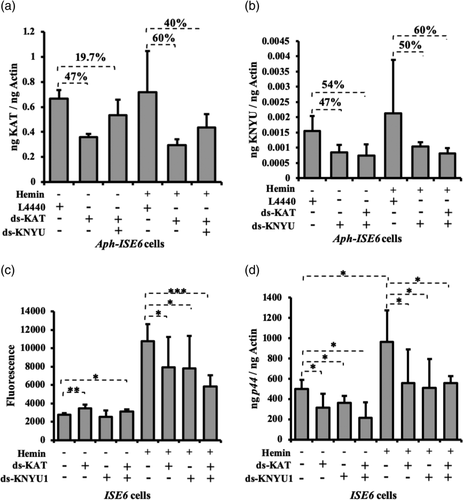
2.10 Knockdown of KAT and kynureninase leads to the inhibition of A. phagocytophilum growth
Our previous study showed that knockdown of kat gene expression by RNAi leads to significantly reduced bacterial burden in tick cells (Taank et al., 2017). In this study, we determined whether knockdown of kat has any impact on ROS production. In addition, the observation of reduced A. phagocytophilum burden upon 3-HK treatment suggested testing the effect of kynureninase knockdown on ROS production and bacterial burden in tick cells. Knockdown efficiency for each targeted gene is shown in Figures 6a,b. In the absence of hemin, significantly (p < .05) increased ROS levels were noted in kat-dsRNA-treated A. phagocytophilum-infected cells in comparison with the levels noted in mock-treated A. phagocytophilum-infected cells (Figure 6c). However, no significant differences in ROS levels were noted between knyu-dsRNA-treated A. phagocytophilum-infected cells and mock-treated A. phagocytophilum-infected cells (Figure 6c). However, double knockdown of both kat and knyu gene expression resulted in significantly increased ROS levels in kat + knyu-dsRNA treated A. phagocytophilum-infected cells in comparison with the mock-treated control (Figure 6c). In the presence of hemin, knockdown of gene expression for kat or knyu or both resulted in significantly (p < .05) reduced ROS levels in comparison with the mock-treated control (Figure 6c). QRTPCR analysis revealed significantly reduced bacterial burden upon knockdown of gene expression for kat or knyu or both in comparison with mock-control in presence or absence of hemin (Figure 6d). These results suggest that metabolism of 3-HK by KAT and KYNU is critical for A. phagocytophilum survival in tick cells.
3 DISCUSSION
Over the last decade, several studies have implicated the role of tryptophan metabolism in many physiological pathways (Badawy, 2017; Davis & Liu, 2017). Almost 99% of the ingested tryptophan not used for protein synthesis is catabolised in the kynurenine pathway (Davis & Liu, 2017). Initial studies were focused on the characterisation of kynurenine pathway in the biogenesis of nicotinamide adenine dinucleotide (NAD) (Badawy, 2017). However, recent studies link kynurenine pathway with neurodegenerative diseases, tumour proliferation, inflammation, immunity and depression (Badawy, 2017; Davis & Liu, 2017; Höglund, Øverli, & Winberg, 2019; Zhuravlev et al., 2016). Furthermore, l-tryptophan pathway was also reported to have a role during infections caused by virus like HIV (Lyons, Tovar-y-Romo, Thakur, McArthur, & Haughey, 2014) and bacteria like Rickettsia conorii (Feng & Walker, 2000) and A. phagocytophilum (Taank et al., 2017). The l-tryptophan pathway leads to accumulation of many active metabolites (Sas & Szab, 2018). KA and quinolinic acid are most studied due to their neuroactive roles in brain. TDO, IDO, 3-HK, XA and 3-hydroxyanthranilic acid have roles in redox system (Reyes Ocampo et al., 2014). Even though most of the experiments in this study were performed with tick cells and might not reflect events in live ticks, we believe that this study provides important evidence that indicates the impact of l-tryptophan pathway metabolites on the survival of A. phagocytophilum in its vector host.
Bioinformatic analysis of the Arthropoda genes involved in l-tryptophan and nicotinamide pathways showed that I. scapularis and I. ricinus have conserved the link between the kynurenine and nicotinamide pathways through duplication of KNYU enzyme. We believe that this would facilitate the two tick species to synthetize NAD(P)+ from the accumulation of bioactive kynurenine metabolite. The presence of complete enzymatic panel of genes in I. scapularis and I. ricinus ticks suggests that l-tryptophan is rapidly cleaved to other downstream metabolites including KA, XA and NAD(P)+ in these ticks. KNYU enzyme seems to be lost in many arthropods including blood-feeding arthropods suggesting that these species depend on niacin and nicotinamide to insure the pool of NAD+ and NADP+ co-factors. The loss of KNYU in many arthropods suggests increased levels of l-kyn and 3-HK that subsequently may lead to more accumulation of XA in them. This notion supports previous study that reported observation of abundant amounts of XA in Aedes aegypti mosquito (Lima et al., 2012). We noted that arthropods have lost IDO and retained TDO in them. Even though TDO has low affinity to l-tryptophan compared with IDO, it can efficiently catabolise this metabolite (Badawy, 2017). IDO, unlike that of TDO, can be inhibited at high l-tryptophan concentration (Badawy, 2017). l-Tryptophan is an essential amino acid. Therefore, acquisition of this amino acid is completely dependent on the diet (Lindseth, Helland, & Caspers, 2015). It is reasonable to hypothesise that substrate inhibition of IDO by l-tryptophan might have led to the loss of this gene in arthropods.
Anaplasma phagocytophilum is transstadially maintained in different developmental stages of ticks. During moulting, ticks may elicit ROS response to control bacterial replication in unfed stage. We therefore used unfed ticks to analyse the expression of genes involved in l-tryptophan pathway. The observation of increased expression of all arthropod genes involved in l-tryptophan pathway suggests that A. phagocytophilum infection activates this pathway in both ticks and tick cells. We monitored the production of ROS in each experiment due to the reason that the metabolites from the kynurenine pathway are associated with the redox state of the cell (Sas & Szab, 2018). Our data indicated that XA and KA metabolites act differently on ROS production and bacterial growth. XA allows bacterial growth and protect cells by not allowing build-up of ROS upon A. phagocytophilum infection. At high concentration, KA reduces ROS levels without having an impact on A. phagocytophilum growth. However, at low concentration, KA induces both ROS and bacterial growth. Previous studies have shown that XA and KA act as antioxidants, removing reactive-oxygen and -nitrogen and enhance cell survival against free radical damage (Xu et al., 2018). KA is a competitive inhibitor of the glycine co-agonist site of the N-methyl-d-aspartate (NMDA) receptor (Campesan et al., 2011; Castellano-Gonzalez et al., 2019). KA was reported to inhibit the release of glutamate in a dose-dependent manner (Campesan et al., 2011). In a recent study reported that ROS production by activated murine neutrophils upon bacterial infection is mediated by glutamate release through the action on NMDA receptor. The release of neurotoxic quantities of the neurotransmitter glutamate is shown to activate neuronal NADPH oxidase leading to the injurious production of superoxide (del Arroyo et al., 2019). Whereas, the inhibition of NMDA receptor leads to the reduction of ROS production (del Arroyo et al., 2019). Anaplasma phagocytophilum is totally dependent on exogenous acquisition of l-glutamine or l-glutamate (Huang, Wang, Kikuchi, Kumagai, & Rikihisa, 2007). From our study, it seems that at high concentration, KA may act on NMDA receptor for the retention of l-glutamate. This hypothesis is also supported from previous observation that reported decrease in l-glutamate in A. phagocytophilum-infected ISE6 cells (Cabezas-Cruz, Espinosa, Obregón, Alberdi, & de la Fuente, 2017).
The observation of increased ROS levels and reduced bacterial growth upon treatment of tick cells with hemin in combination with XA or KA suggests that tryptophan metabolites are controlling the action of hemin in cells. Our study also showed that hemin is chelated by XA or KA. This binding could block the functional activity of XA or hemin in promoting bacterial growth in tick cells. We found that A. phagocytophilum infection, hemin and KA induce TDO activity and XA inhibits TDO activity. The reduced TDO activity noted upon treatment of tick cells with hemin in combination XA or KA could be due to the chelating property of tryptophan metabolites with hemin. TDO is synthetized as an apoenzyme that interacts with heme to become heme-containing holoenzyme. Hemin is also used by TDO as a cofactor (Badawy, 2017). Ticks lack complete pathway to synthesise heme (Perner et al., 2016). Therefore, the observation of increased TDO activity upon hemin treatment of tick cells is not surprising. Collectively, our data suggest that hemin, XA and KA have synergistic effect on l-tryptophan pathway to modulate bacterial growth and ROS levels.
Our data suggest that hemin-mediated influence on A. phagocytophilum growth is associated with increased levels of l-kynurenine. l-Kynurenine is cleaved by three enzymes including KAT, KNYU and KMO (Badawy, 2017). However, KMO is considered as the major route for the kynurenine pathway because of its high affinity for l-kynurenine than other enzymes (Castellano-Gonzalez et al., 2019; Fujigaki et al., 2017). Kynurenine oxidised by KMO results in the formation of 3-HK, a metabolite that can stimulate production of reactive oxygen species (Campesan et al., 2011; Han et al., 2007). The observation of reduced ROS levels and increased bacterial burden upon KMO inhibition in the presence of hemin could be due to low 3-HK levels. These data indicate that hemin-mediated induction of ROS production could be due to conversion of l-kynurenine to 3-HK. The observation of reduced TDO activity upon XA treatment and reduced bacterial burden upon l-kynurenine treatment suggests that XA supports A. phagocytophilum growth by reducing amounts of l-kynurenine through inhibition of TDO activity upstream of the l-tryptophan pathway.
A study has reported that 3-HK has both pro-oxidant and antioxidant properties (Reyes Ocampo et al., 2014). Micromolar concentrations as low as 1 μM are noted to be toxic to cultured striatal neurons (Okuda, Nishiyama, Saito, & Katsuki, 1996). However, we noted that ISE6 cells were resistant to 3-HK at different concentrations (Figure S2). The resistance of tick cells to toxicity induced by 3-HK suggests importance of this molecule in several physiological aspects of these cells. In the presence of hemin, 3-HK seems more likely to protect tick cells from cytotoxicity possibly by reducing ROS levels. 3-HK was reported as a scavenger of superoxide anion in insects and during infection and inflammatory disease in human derived cells (Christen et al., 1990; Goshima, Wadano, & Miura, 1986). The auto-oxidation of 3-HK leads to the production of unstable xanthommatin that is rapidly oxidised to hydroxanthommatin (Atherton, Dillon, & Gaillard, 1993; Reyes Ocampo et al., 2014). Hydroxanthommatin is more stable and can react with superoxide anion (Atherton et al., 1993; Reyes Ocampo et al., 2014). It is also reported that haemoglobin and hematin (hemin) mediate auto-oxidation of 3-HK to hydroxanthommatin (Ishii, Iwahashi, Sugata, & Kido, 1992). Therefore, it is reasonable to hypothesise that the observed inhibition in ROS levels upon 3-HK treatment of tick cells could be due to auto-oxidation of this metabolite mediated by hemin.
In mosquitoes, oxidation of 3-HK leading to xanthommatin production is important for eye pigmentation (Li, Beerntsen, & James, 1999). However, similar role for 3-HK in ticks is not known. The observation of reduced bacterial loads in tick cells upon 3-HK treatment in the absence of hemin could be due to increased ROS levels that might be deleterious for bacterial growth. Our results suggest that the metabolism of 3-HK is important for A. phagocytophilum survival in tick cells. Higher levels of ROS observed in kat-dsRNA treated cells but not in knyu-dsRNA-treated cells in the absence of hemin suggests that KAT is more efficient to metabolise 3-HK than KNYU. The observation of reduced bacterial burden in kat-dsRNA-treated cells or kynu-dsRNA-treated cells or in cells treated with both dsRNAs in the presence or absence of hemin suggests that KAT and/or KNYU are important for A. phagocytophilum survival in tick cells. The influence of KAT and KYNU on bacterial growth seems to be independent of ROS levels. We previously reported that KAT (Taank et al., 2017) is critical for A. phagocytophilum survival in tick cells. In this study, we show that KNYU activity is also essential for A. phagocytophilum survival in tick cells. Moreover, these findings highlight the importance of 3-HK metabolism in the survival of A. phagocytophilum in tick cells.
The metabolism of 3-HK is associated with the tricarboxylic acid cycle (TCA cycle). KAT and KYNU cleave l-kynurenine resulting in the formation of l-glutamate and l-alanine respectively. Genome analysis of many rickettsial pathogens has reported absence of alanine and glutamate synthesis pathways (Dunning Hotopp et al., 2006). However, in I. scapularis, all genes required for the synthesis of glutamate and alanine from alpha-ketoglutarate and pyruvate, respectively, are present (Cabezas-Cruz, Espinosa, et al., 2017). Studies have shown that A. phagocytophilum decreases glutamate and alanine levels upon infection in ticks (Cabezas-Cruz, Espinosa, et al., 2017; Villar et al., 2018). The observation of reduced bacterial burden upon knockdown of KAT and/or KNYU suggests that production and the catabolism of 3-HK and other metabolites in l-tryptophan pathways are critical for the supply of glutamate and alanine to A. phagocytophilum metabolism. Glutamate is a glucogenic amino acid that can be transformed to alpha-ketoglutarate that aids in bacterial TCA cycle (Cabezas-Cruz, Alberdi, Valdés, & Villar, 2017). Glutamate is also the precursor of arginine (Cabezas-Cruz, Espinosa, et al., 2017), another amino acid that cannot be synthetized by A. phagocytophilum (Dunning Hotopp et al., 2006). As A. phagocytophilum was reported to have incomplete TCA pathway, exogenous supplementation of l-glutamate and glutamine from cells is essential for the bacterial growth (Huang et al., 2007). Interestingly, it was reported that A. phagocytophilum inhibits tick TCA cycle (Cabezas-Cruz, Alberdi, et al., 2017). Authors have suggested that TCA cycle inhibition allows A. phagocytophilum to prevent the entry of glutamine and glutamate to the TCA cycle via alpha-ketoglutarate (Cabezas-Cruz, Alberdi, et al., 2017). Our study suggests that l-tryptophan and kynurenine pathways appear as an alternative route for A. phagocytophilum to get glutamate and alanine for its TCA cycle. Taken together, based on the results from current and previous studies, we propose a mechanistic model on the role of l-tryptophan metabolites and hemin in influencing redox state of tick cells during A. phagocytophilum infection (Figure 7).
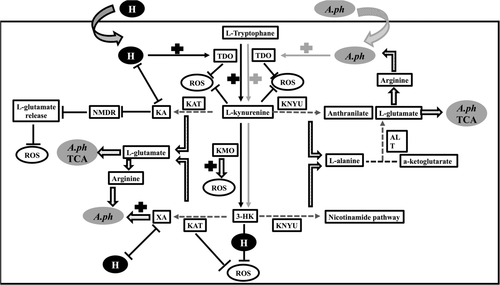
In summary, A. phagocytophilum and hemin activates l-tryptophan pathway in ticks that leads to accumulation of bioactive metabolites. Even though metabolites from l-tryptophan pathway play different roles in influencing the redox state of the cells, XA seems to allow A. phagocytophilum survival through inhibition of TDO activity that subsequently affects l-kynurenine levels and build-up of ROS. 3-HK metabolism seems to be essential for A. phagocytophilum survival in tick cells. This study not only highlights the role of l-tryptophan pathway and hemin in A. phagocytophilum survival in tick cells but also provides a comprehensive view on the molecular changes in the redox state of the cells during vector–pathogen interactions.
4 EXPERIMENTAL PROCEDURES
4.1 Bacterial isolates, ticks and tick cell line
Anaplasma phagocytophilum isolate NCH-1 was used throughout this study and referred as Aph in figures. Escherichia coli JM109 strain was used as a cloning host for generating different pGEM-T plasmids. Ticks used in this study were obtained from continuously maintained colony at the Department of Entomology, Connecticut Agricultural Experiment Station (New Haven, CT). The I. scapularis tick cell line ISE6 was maintained as previously described (Taank et al., 2017). Tick rearing was conducted in an incubator at 23 ± 2°C with 95% relative humidity and a 14/10-hr light/dark photoperiod regiment.
4.2 Mice and ethics statements
C3H/HeN mice (4–6 weeks, females, CharlesRiver Laboratories) were used in this study. Uninfected or A.phagocytophilum-infected unfed nymphs were generated by feeding larvae on uninfected or A. phagocytophilum-infected mice and then moulted to nymphs as previously described (Taank et al., 2017). All animal studies were carried out following the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institute of Health. The animal protocol (permit number: 16–017 and 19-009) approved by the Old Dominion University Institutional Animal Care and Use Committee (Animal Welfare Assurance Number: A3172-01) was used in this study. Acepromazine was used as a tranquilliser that was administered into mice prior to handling.
4.3 RNA or DNA extractions and quantitative real-time PCR (QRT-PCR) analysis
Total RNA or DNA extraction from ISE6 tick cells or ticks and QRT-PCR was performed as previously reported (Taank et al., 2017) using Aurum Total RNA mini kit (Bio-Rad) and Qiagen DNeasy blood and tissue kit (Qiagen). The cDNA was prepared from RNA using Bio-Rad cDNA synthesis kit (Bio-Rad). QRT-PCR reactions were performed using iQ-SYBR Green Supermix (Bio-Rad) as described (Khanal et al., 2018; Taank et al., 2017). Oligonucleotides used in the study are mentioned in Table S1. As an internal control and to normalise the amount of template, I. scapularis beta-actin was quantified using oligonucleotides as mentioned in Table S1. Total DNA or known amounts of actin was also used as an internal control to normalise amount of template used in each reaction. In QRT-PCR reactions, the standard curve was generated using sixfold serial dilutions starting from 10−2 to 10−7 ng of known quantities of respective fragments.
4.4 dsRNA synthesis
The dsRNA against kat transcript was generated as previously described (Taank et al., 2017). kynu 1 dsRNA was first generated by amplifying the gene sequence from cDNA obtained from ticks using primers listed in Table S1. PCR products were cloned into the pGEM-T vector (Promega, Madison, WI), according to the manufacturer's instructions. The recombinant plasmid was transformed and propagated in E. coli JM109, and these plasmids were sequenced at Eurofins USA and used as templates to amplify the corresponding gene fragments with primers that contain T7 promoter sequence overhangs (Table S1). The dsRNA for each gene was synthesised using the MEGAscript RNAi Kit (Ambion Inc.) following manufacturer's instruction.
4.5 Reagent and stock solution preparations
XA, KA, 3-HK and Ro-61-8,048 were obtained from Sigma Aldrich. Hemin was purchased from MP Biomedicals (Fisher Scientific). l-kynurenine was obtained from Thermoscientific. Stocks (10 mM) of XA, KA, hemin or Ro-61-8048 were made by dissolving corresponding weight in 1 mL 0.5 N NaOH solutions. The volume was then increased to 10 mL by adding 1× PBS. The solution was then filtered through 0.45 μm filter. Serial dilution of 1:10 was made in 1× PBS to obtain smaller concentrations. The highest concentration of the vehicle (NaOH) used in the experiment was 0.005 N contained in 1 mM solution of each compound. Mock solution was prepared to contain 0.005 N of NaOH and used in all experiments as control. l-kynurenine stock solution of 50 mM was made by dissolving required weight in 1 mL 0.5 M HCL. The volume was then increased to 10 mL by adding 1× PBS. The solution was filtered through 0.45 μm filter. Two serial dilutions of 1:10 and 1:5 were made in 1× PBS to obtain 1 mM solution of l-kynurenine (with 0.001 M HCL final concentration). Mock solutions that contained 0.005 N NaOH and 0.001 M HCL were made and used in experiment with l-kynurenine. The other stock solution of l-kynurenine was made from 1:10 dilution in 1× PBS. Mock solution was first tested on ISE6 cells. Treatment revealed no change in cell morphology and media colour at 24 and 48 hr post-treatments (Figure S2). Finally, the 10 mM stock solution of 3-HK was dissolved in 1× PBS, and lower concentration solutions were made by serial dilution of 1:10.
4.6 Tick cell line experiments and reactive oxygen species measurement
Strain A. phagocytophilum NCH-1 was maintained in human promyelocytic cell line (HL-60, American Type Culture Collection), and cell-free bacteria isolated from these cells were used for in vitro infection of ticks cells as described (Thomas & Fikrig, 2007). Tick cells (3 × 105) were seeded onto 12-well plates and incubated for 24 hr. In experiment involving only XA or KA, tick cells were treated with different concentrations of XA or KA (1, 10, 100 μM). Equal volumes of mock solution (corresponding to 100 μM volume) were added to control cells. Where hemin is involved, cells were seeded into two groups with or without hemin. For the group without hemin, cells were treated with 100 μM of XA or KA and equal volumes of mock solution (corresponding to 100 μM volume) as control. For the groups with hemin treatment, cells were treated as above, then, 100 μM final concentration of hemin was added to each well. The mock group was also treated with 100 μM final concentration of hemin and an equal volume of mock solution (corresponding to the volume for 100 μM concentration stocks). To allow the comparison between the two groups with or without hemin, equal volume of mock solution (corresponding to 100 μM volume) was added to the cells without hemin. After 4 hr of treatment, tick cells were infected with A. phagocytophilum and incubated for 24 hr. Cells were then harvested and divided into two equal portions, one was subjected to ROS quantification and the other portion for RNA or DNA extractions to measure mRNA transcripts of tick genes or A. phagocytophilum load respectively. Similar methodology was used when Ro-61-8048, l-kynurenine or 3-HK was analysed in the experiments with or without XA or KA. ROS quantification was measured using DCFDA/H2DCFDA-Cellular ROS Assay Kit (Abcam) following the manufacturer's recommendations. Fluorescence was measured using fluorimeter (Tecan) with excitation at 495 nm and emission at 529 nm.
4.7 Tick cell line silencing experiments and microscopy
dsRNA knockdown was performed as previously described (Taank et al., 2017). Briefly, gene expression silencing experiments in tick cells were performed with lipofectamine transfection reagent (ThermoFisher Scientific). 4 × 105 tick cells were plated in L-15B300 medium on 12-well plates and incubated for 24 hr followed by addition of dsRNA mixed with lipofectamine reagent. 2× L15-B300 medium was added after 6 hr and plates were further incubated for 16 hr. Cell-free A. phagocytophilum isolated from HL60 cells was added after 24 hr post-transfection and tick cells were incubated for additional 24 hr and processed for ROS quantification or for analysis of bacterial loads. Microscopic images of tick cells upon treatment with different metabolites were collected with EVOS FL microscope. Representative images are shown in Figure S2. Silencing efficiency and bacterial burden were measured as described (Khanal et al., 2018; Taank et al., 2017).
4.8 TDO assay and l-kynurenine assay
The enzymatic assay was modified from a method previously described study (Gibney et al., 2014). Briefly, tick cells were seeded onto 12-well plates at a density of 2 × 105 cells/well. Treatment of the tick cells with different metabolites was performed as described above. Following the infection, uninfected and A. phagocytophilum-infected tick cells were harvested at 24 hr pi. Tick cells were homogenised in 1× PBS, and 10 μL 30% (v/v) trichloroacetic acid was added to each well. Cells were incubated for 30 min at 50°C to hydrolyze N-formylkynurenine to kynurenine. After incubation, cells were centrifuged at 3000× g for 10 min, and 100 μL of the supernatant was transferred to 96-well flat-bottom plate and mixed with equal volume of Ehrlich's reagent (in glacial acetic acid) and absorbance was measured at 480 nm. A standard curve was generated with defined concentrations of l-kynurenine (0–400 μM; Thermofisher). N-formylkynurenine can be hydrolysed to l-kynurenine by heating. This reaction represents TDO activity. Kynurenine concentrations in the supernatants were determined by comparing with the standard curve. TDO activity in the supernatants is presented as μM of l-kynurenine. For the l-kynurenine assay, cells were processed as above but without hydrolysing the N-formylkynurenine to kynurenine.
4.9 Hemin titration
Heme titrations were performed by difference spectroscopy at room temperature and aerobically using TECAN spectrophotometer as described (Soto, Fontanesi, Myers, Hamel, & Barrientos, 2012). Briefly, we used 5 μM of XA or KA and hemin in 50 mM Tris buffer pH 8.0. Hemin was dissolved in 5% DMSO to minimise its oligomerization. A baseline was selected by scanning samples from 300 to 700 nm. The samples used were 50 mM Tris buffer pH 8.0 in the reference cuvette and 50 mM Tris buffer pH 8.0 with 5 μM of XA or KA in the sample cuvette. Once the baseline was set, hemin (at equal concentration) was added to both reference and sample cuvettes. For this assay, we used low concentration of XA or KA (5 μM) and increasing concentrations of hemin (5–90 μM). We first scanned for XA or KA in the sample cuvettes and compared the readings from the reference cuvette containing buffer only (50 mM Tris pH:8.0) in the wavelength range from 300 to 700 nm. This number was considered as a baseline reading. Furthermore, hemin (with increasing concentrations) was added to both the samples and to the reference cuvettes. The obtained readings reflect only the binding of XA or KA with hemin and not the absorbance of the free hemin. The data obtained from the heme-XA or heme-KA titrations were plotted in Microsoft Excel 2016 and fit to an Equation (Y = Bmax × X/Kd + X) that describes a single binding site.
4.10 Statistics
Statistical significance in the datasets was analysed using Microsoft Excel 2016. Data were presented as mean and standard deviation. Lines on the top of histogram represent standard deviation. For data to compare two means, the non-paired Student t test was performed. p values of <.05 were considered significant in all analyses. Wherever necessary, statistical test and p values used are shown.
ACKNOWLEDGMENTS
This study was supported by funding from the National Institute of Allergy and Infectious Diseases (NIAID) and the National Institutes of Health (NIH) (Award number: R01AI130116) to G. N.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.