Caenorhabditis elegans mounts a p38 MAPK pathway-mediated defence to Cutibacterium acnes infection
Xiaowen Huang and Wen Pan contributed equally to this study.
Abstract
Cutibacterium acnes is capable of inducing inflammation in acne and can lead to a chronic prostatic infection. The diverse pathogenicity among different strains of C. acnes has been presented, but simple appropriate animal models for the evaluation of this bacterium are lacking. In this study, the nematode Caenorhabditis elegans was used as an invertebrate infection model. We revealed that C. acnes type strain ATCC 6919 caused lethal infections to C. elegans in solid and liquid culture media (p < .0001). Compared with the strain ATCC 6919, the antibiotic-resistant strain HM-513 was more virulent, resulting in reduced survival (p < .0001). Four different C. acnes strains killed worms with a p value of less than .0001 when provided to C. elegans at 4.8 × 108 CFU/ml. The infection model was also employed to explore host defence responses. An increase in numerous immune effectors in response to C. acnes was detected. We focused on nine C-type lectins, including: clec-13, clec-17, clec-47, clec-52, clec-60, clec-61, clec-70, clec-71 and clec-227. The induced expression of these C-type lectin genes was down-regulated in mutant worms deficient in the p38 mitogen-activated protein kinase (MAPK) pathway. Meanwhile, PMK-1 (MAPK) was phosphorylated and activated at the onset of C. acnes infection. By monitoring the survival of mutant worms, we found that PMK-1, SEK-1 (MAPKK) and TIR-1 (MAPKKK) were critical in responding to C. acnes infection. C. elegans pmk-1 and tir-1 mutants exhibited higher mortality to C. acnes infection (p < .0001). In conclusion, C. elegans serves as a simple and valuable model to study C. acnes virulence and facilitates improvements in understanding of host innate immune responses.
1 INTRODUCTION
The Gram-positive, anaerobic, slow-growing bacterium Cutibacterium acnes (formerly Propionibacterium acnes) is responsible for recrudescent or chronic acne, and can also affect implants, such as cardiovascular devices and prosthetic joints (Achermann, Goldstein, Coenye, & Shirtliff, 2014; Lee, Byun, & Kim, 2019). More than 50% of C. acnes isolates are resistant to topical macrolides, making treatment challenging (Leheste et al., 2017; Portillo, Corvec, Borens, & Trampuz, 2013; Van Boeckel et al., 2014).
The study of C. acnes pathogenesis is particularly challenging, in part because there is no facile animal model to study host–pathogen interactions (Fischer et al., 2013; Shinohara et al., 2013). Although rabbits and HR-1 mice can be inoculated with C. acnes via intradermal injection, these models usually require 2–4 weeks for the manifestation of comedones and inflammatory papules (Jang, Lee, Lee, Kim, & Lee, 2015; Wang et al., 2017). Similarly, the rat model of prostatic C. acnes infection is technically challenging and associated with significant cost (Olsson et al., 2012). Caenorhabditis elegans is a simple and genetically tractable model host for studying numerous bacterial and fungal pathogens (Zhang et al., 2017). Compared to traditional laboratory animal species, worms have a short life cycle, which can reduce the time of experimentation, and have been used extensively to study microbiology, immunology, neurobiology, and to investigate preclinical drugs (Kim et al., 2018; Marsh & May, 2012).
Employing an invertebrate model could help address some challenges, such as cost, ethical considerations, time and extensive specialised training posed by the mammalian models. In this work, we describe the C. elegans–C. acnes infection models, which can be used to evaluate the pathogenesis of C. acnes and host-elicited defences. The infection also manifests molecular responses from the host, which can be interpreted due to the wealth of C. elegans genetic information available (Pukkila-Worley, 2016).
Previous studies have already examined the transcriptional response in C. elegans to an array of infecting pathogens. The nematode C. elegans usually responds to pathogens by producing particular proteins, such as C-type lectins, CUB-2 containing proteins, hydrolase, lysozyme, GST, ShH-like toxin and DUF274 (Schulenburg, Hoeppner, Weiner, & Bornberg-Bauer, 2008; Shivers, Youngman, & Kim, 2008). Mammalian C-type lectins participate in both innate and adaptive immune responses during microbial infection (Brown, Willment, & Whitehead, 2018; Mayer, Raulf, & Lepenies, 2017). The nematode has 261 C-type lectin coding genes, which are generally assumed to be involved in pathogen defences. clec-39 and clec-49 were proven to be critical for host immunity during bacterial infection (Miltsch, Seeberger, & Lepenies, 2014). However, the exact function of each C-type lectin in the defence system of C. elegans remains unknown.
In this study, we systematically construct the C. elegans–C. acnes infection model both in solid and liquid media formats to examine the isolate virulence, and investigate C. elegans defence responses. We measure C. elegans survival after infection with four C. acnes isolates, which provides a model to interrogate pathogen virulence and host defences. Overall, we provide evidence of C-type lectins defending against C. acnes infection and reveal the response is dependent on the conserved p38 MAPK pathway.
2 RESULTS
2.1 C. acnes cause a lethal infection to C. elegans on solid media
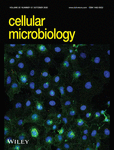
In laboratory settings, C. elegans typically consume Escherichia coli cells (Revtovich, Lee, & Kirienko, 2019). The normal nematode food source can be exchanged for alternate microbes. After ingestion of C. acnes, the nematode gut was distended (Figure 1a) compared to E. coli fed worms (Figure 1b), indicating the accumulation of C. acnes cells within the nematode intestinal tract.
The lifespan of N2 C. elegans was measured and compared to worms fed with heat-killed E. coli (HK-E. coli). In the solid media assay, the nematode lifespan was diminished after exposure to the lawn of C. acnes strain ATCC 6919 on brain heart infusion (BHI) agar plates, compared to HK-E. coli (p < .0001) (Figure 1c). The assay was confirmed using another C. elegans mutant strain, glp-4;sek-1, which is widely used in infection-related research and has previously proven useful in bacterial-C. elegans screening assays due to the shortened timescale to achieve an infection and the lack of progeny production (Rajamuthiah et al., 2014). As shown in Figure 1d, the median survival of the C. acnes infected glp-4;sek-1 worms was 2 days, which is shorter than that of HK-E. coli fed nematodes (p < .0001) and of N2 worms infected by C. acnes strain ATCC 6919 (median survival = 4 days).
The killing efficiency is a result of microbial virulence factors that directly mediate pathogenicity within the host (Crousilles et al., 2015). We next employed the C. elegans glp-4;sek-1 mutant worms to test the virulence of various C. acnes clinical isolates, which included two antibiotic-resistant strains HM-513 and HM-522, and one health-related strain, HM-554. As shown in Figure 1e, we found that all three C. acnes isolates tested were pathogenic to C. elegans and killed the worms within 1 week. Compared to the type strain ATCC 6919, isolate HM-513 demonstrated superior killing efficiency (p < .0001).
2.2 C. elegans nematodes are susceptible to C. acnes infection in liquid media
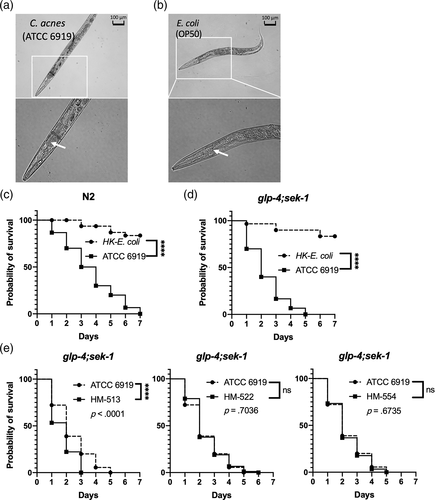
In the C. elegans model, infection dynamics can be different between solid and liquid media (Huang, Li, Xi, & Mylonakis, 2014; Pukkila-Worley, Ausubel, & Mylonakis, 2011). To this end, we endeavoured to evaluate whether C. elegans is also susceptible to C. acnes in a liquid assay format. The previously examined four strains of C. acnes (HM-513, HM-522, HM-554 and ATCC 6919) were evaluated by liquid assays, while heat-killed E. coli was used as a control. Unlike the lawn of bacterial pathogen that was used in the solid media assay, the liquid assay required the appropriate bacterial concentration in the assay system. Thus, we first tested the killing efficacy at various concentrations of C. acnes type strain ATCC 6919 to determine the optimum condition. As shown in Figure 2a, the nematode survival rate was determined after infection for 5 days with four different concentrations of C. acnes: 6 × 107 CFU/ml, 1.2 × 108 CFU/ml, 2.4 × 108 CFU/ml and 4.8 × 108 CFU/ml.
At the lower concentrations, 6 × 107 CFU/ml and 1.2 × 108 CFU/ml, C. elegans did not advance mortality. But at the median level of 2.4 × 108 CFU/ml, most of the tested nematodes died within the 5-day test period (p = .0002). For the highest concentration, all worms were killed by C. acnes strain ATCC 6919 (p < .0001).
We then evaluated worm survival after infection with other C. acnes strains at the same concentrations (Figure 2b). In the liquid assay, all four strains of C. acnes (HM-513, HM-522, HM-554 and ATCC 6919) caused significant mortality of C. elegans nematodes in a dose-dependent manner. C. acnes strain HM-513 killed nematodes at a faster rate (p = .0028) corresponding to data from the solid killing assay. The above results indicated that the strain HM-513 was more virulent and adept at killing nematodes.
Once the liquid assay was established, it was employed to evaluate antibiotics used for acne vulgaris clinical management. Tetracycline, an active antibiotic to C. acnes (Zhanel, Critchley, Lin, & Alvandi, 2019), was used in the liquid killing assay as a positive control. SYTOX Orange dye specifically stained dead worms by penetrating the damaged cell membrane. As shown in Figure 2c, tetracycline effectively prolonged nematode survival after C. acnes ATCC 6919 infection, indicated by the lack of SYTOX staining. Obtaining the transmitted and fluorescent images and quantifying with image analysis software ImageJ permitted evaluation of worm survival in this liquid assay (Figure 2d).
2.3 C. acnes infection induces antimicrobial peptides expression in C. elegans
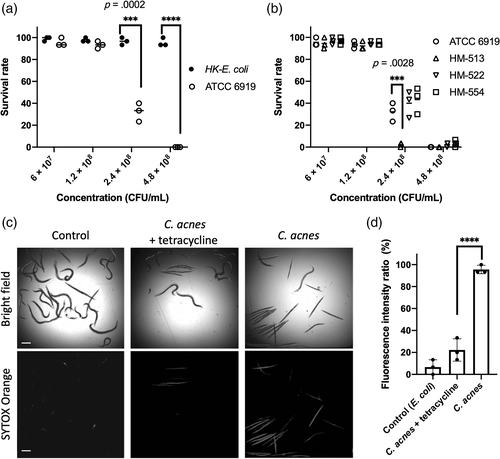
To explore the nematodes defence response against C. acnes, we assessed the expression of an antimicrobial molecule panel that included C-type lectins, lysozymes, antibacterial factors and infection-related genes (Liu et al., 2018; Shivers et al., 2008). Using quantitative PCR, an array of 61 candidate immune response genes were examined against the infection of C. acnes type strain ATCC 6919 (Figure 3a). Ten genes in five gene classes has elevated expression greater than twofold, including C-type lectins [clec-38 (4.72-fold), clec-52 (20.83-fold), clec-60 (49.68-fold), clec-70 (33.76-fold)], caenacins [cnc-1 (2.26-fold), cnc-4 (2.96-fold) and cnc-11 (9.03-fold)], cathepsin B-like cysteine proteases [cpr-2 (12.99-fold)], lysozymes [ilys-3 (283.19-fold), lys-5 (18.86-fold)] and caenopores [spp-12 (3.29-fold)].
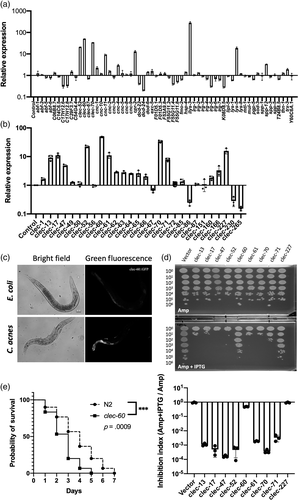
Focusing on C-type lectins, the quantitative PCR was used to screen a set of 41 C-type lectin genes (Supporting Information Table S2). As shown in Figure 3b, of all C-type lectin genes examined here, 68.3% (28 out of 41) were detectably expressed following the infection with C. acnes, and among them, nine C-type lectin genes were induced more than two folds, including clec-13, clec-17, clec-47, clec-52, clec-60, clec-61, clec-70, clec-71 and clec-227. In contrast, clec-86 (4.05-fold), clec-230 (3.52-fold) and clec-265 (6.52-fold) were downregulated greater than two folds upon pathogen exposure. Furthermore, we confirmed the increase of clec-60 expression using clec-60::GFP reporter worms, and observed the induction by C. acnes in the upper portion of the intestine compared with exposure to E. coli control (Figure 3c).
In the subsequent functional analysis, we attempted to purify the recombinant C-type lectins. However, there were no detectable recombinant proteins in the case of IPTG induction (data not shown). We found that the host E. coli BL21(DE3) cells have exhibited growth inhibition after the induction. As shown in Figure 3d, the antimicrobial efficacy of the nine C-type lectin proteins was assessed against E. coli. After the induction with IPTG, the C-type lectin genes transformed-E. coli strain BL21(DE3) cells developed fewer bacterial colonies compared with the vector control. Seven out of the nine C-type lectins were found to inhibit the growth of E. coli, suggesting C-type lectin proteins present antimicrobial activity.
We used C. elegans clec-60 mutant worms to investigate the effect of CLEC-60 in the defence response of C. elegans against C. acnes. The C. elegans clec-60 mutant worms (strain tm2319) were fed with C. acnes strain ATCC 6919 for 7 days. We observed that the median survival of clec-60 mutants was shortened to 3 days compared with 4 days of wild-type N2 worms following exposure to C. acnes (p = .0038), which indicated that clec-60 mutant was susceptible to C. acnes-mediated killing (Figure 3e). It depicts the expression of clec-60 is not only in response to C. acnes infection, but also required to promote the worm survival against C. acnes.
2.4 C. acnes activates the p38 MAPK pathway in C. elegans
The MAPK pathway is one of the most conserved pathways in host immunity. Mammalian p38 (MAPK) is activated in response to C. acnes infection (Li et al., 2015). Thus, we evaluated whether the p38 MAPK pathway was activated in C. elegans defence against C. acnes, which was measured by analysing the protein levels of PMK-1 (p38 homologue) and activated PMK-1 (phosphorylation of p38, p-p38). Here, we used the antibodies against human proteins to detect the orthologues in C. elegans (Inoue et al., 2005). As shown in Figure 4a, anti-GAPDH was included as an internal reference for immunoblotting. Nematodes deficient with gene pmk-1 were infected and used here to display the specific bands of protein PMK-1 and phosphorylated PMK-1 in the wild-type N2 worms. We found that the protein level of activated PMK-1 (p-p38) was significantly increased at the onset of C. acnes infection. The increased prevalence of phosphorylated PMK-1 revealed the activation of p38 MAPK pathway during the onset of C. acnes infection.
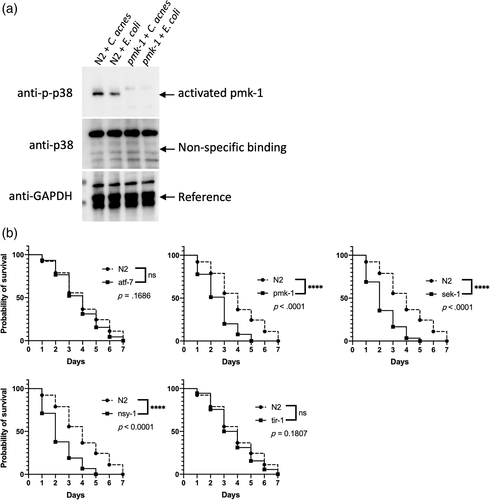
In C. elegans, PMK-1 (MAPK) is regulated by SEK-1 (MAPKK) and NSY-1 (MAPKKK). TIR-1 is one of the upstream components of MAPK signalling cascades, while ATF-7 is the down-stream component and mutant, atf-7, pmk-1, sek-1, nsy-1 and tir-1, worms were evaluated. We found that pmk-1, sek-1 and nsy-1 mutant worms were more sensitive to C. acnes. However, worms with defective mutation in ATF-7 and TIR-1 did not exhibit markedly decreased survival in the presence of C. acnes (Figure 4b).
As indicated in Figure 4b, the significant killing of pmk-1 (MAPK), sek-1 (MAPKK) and nsy-1 (MAPKKK) mutant worms induced by C. acnes demonstrated the involvement of these signalling molecules in the host defence. However, the upstream member TIR-1 and down-stream member ATF-7 had no obvious correlation with the reaction of nematodes to C. acne, which indicates some other transcription factors and MAPKKK's upstream signalling molecules mediate the response of anti-C. acnes infection.
2.5 The regulation of C-type lectin genes is dependent on the p38 MAPK pathway
In our current study, there are nine C-type lectin genes induced specifically related to C. acnes infection (Figure 3b). As C. acnes can activate p38 MAPK pathway by increase the phosphorylation level of protein PMK-1, we wondered whether those genes were under the control of the p38 MAPK pathway. We continued to examine the expression profile of those nine C-type lectin genes in p38 MAPK pathway mutant worms by using quantitative PCR. As shown in Figure 5, the expression of those C-type lectin genes was significantly changed in the mutant worms during infection with C. acnes compared to wild-type N2 worms.
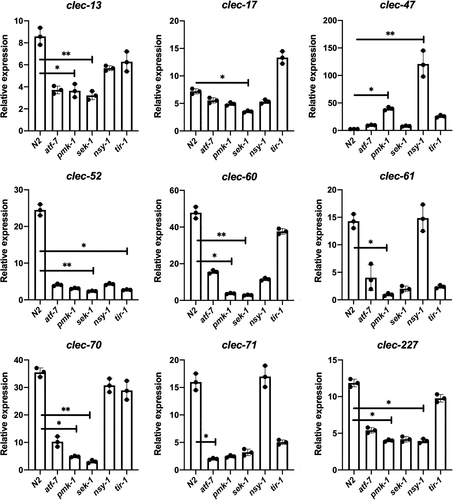
We observed that the pmk-1 (MAPK) mutant exhibited reduced expression of clec-13, clec-60, clec-61, clec-70 and clec-227 as compared to the levels found in the wild-type N2 worms. For the deletion of sek-1 (MAPKK), the expression levels of clec-13, clec-17, clec-52, clec-60 and clec-70 were significantly diminished. Regarding the nsy-1 (MAPKKK) mutant worms, down-regulation of expression was observed for clec-227. C-type lectins appear to be atf-7 and tir-1 independent. Interestingly, after the deletion of pmk-1 (MAPK) or nsy-1 (MAPKKK) gene, clec-47 was significantly up-regulated in a large magnitude, which indicated clec-47 might have a different role in the host immune response against C. acnes infection compared to other C-type lectins. Overall, the induced range of the nine C-type lectins was highly related to the p38 MAPK pathway.
3 DISCUSSION
In the current study, we have examined C. acnes in an invertebrate infection model. The infection conditions were systematically studied in both solid and liquid culture systems. Four different types of C. acnes were pathogenic to C. elegans. The antibiotic-resistant isolate HM-513 was the most virulent isolate to the nematode. Within the host, C. acnes infection was followed by the up-regulation of a set of immune-related genes. Nine C-type lectins genes were induced, and three genes were suppressed. The induced C-type lectins failed to be induced in p38 MAPK mutant worms, suggesting C-type lectins are p38 MAPK dependent during C. acnes infection. Further, PMK-1 is activated at the onset of C. acnes infection. This was demonstrated by p38 MAPK mutants, which exhibited greater susceptibility to C. acnes. Therefore, the p38 MAPK-dependent pathway plays an essential role in the defence against C. acnes infection.
In humans, the conditional pathogen C. acnes generally cause a self-limiting infection, which is not easy to mimic (Lim et al., 2018; Sivasankar et al., 2016). In 2013, a mouse model of chronic prostatic inflammation induced by C. acnes was developed using C57BL/6J mice, but it was preclinical and took 8 weeks for the entire observation (Shinohara et al., 2013). Jang et al. (2015) utilised four types of mice to construct the mammalian acne model. They found clinical inflammation was more prominent in HR-1 mice 2 weeks after the injection, which reflected the inflammatory responses of acne rather than the virulence of the anaerobe. Some other researchers indicated that the reaction levels of skin to C. acnes might be due to differing virulence among distinct C. acnes isolates (Fitz-Gibbon et al., 2013; Tomida et al., 2013). C. acnes strains have different pro-inflammatory potential related to TLR, PAR expressions, and cytokine production (Jasson et al., 2013). However, until now, there was little information available on the appropriate infection model for assessing C. acnes virulence in vivo. The presented results from the solid-media format demonstrate that C. acnes is pathogenic to C. elegans, and, together with the liquid-media format, establish a simple assay for evaluating the virulence of C. acnes.
Caenorhabditis elegans is considered as a genetically tractable host organism to dissect the virulence of pathogens, as well as host defence mechanisms (Marsh & May, 2012). Usually, the killing assay of nematodes is implemented on solid plates coated with a lawn of microorganisms, while the liquid-killing assay is utilised for chemical compound or drug screening (Rajamuthiah et al., 2014). We found that the killing of infected C. elegans was dependent on C. acnes assay inoculum concentration.
Due to the absence of specialised immune cells, C. elegans relies on its innate immune system, especially immune molecules, to defend against pathogens (Marsh & May, 2012; Shivers et al., 2008). Shivers and colleagues summarised five microarray studies, and presented that eight gene classes responded to pathogenic infections (Shivers et al., 2008). We analysed a set of these candidate infection-related genes. In our genetic screen of 61 infection-related genes, 10 genes belonging to five separate families were up-regulated prominently upon C. acnes challenge. Antimicrobial peptides caenacins, cathepsin B-like cysteine proteases, lysozymes and caenopores are well studied, while less information of C-type lectins has been reported (Dierking, Yang, & Schulenburg, 2016). We noticed the involvement of C-type lectins and reduced survival of clec-60 mutants upon C. acnes infection. After examining 41 C-type lectin genes, nine were induced, while three C-type lectins exhibited down-regulation, suggesting specific regulation of C-type lectin genes in nematodes against C. acnes.
Unique immune molecules responded to different pathogens by distinct host immune responses (Irazoqui et al., 2010; Shivers et al., 2008). Our pilot investigation on the function of nine C-type lectins only revealed their antimicrobial efficacy against E. coli. However, the recombinant proteins are indispensable for the functional study to check the bactericidal effect against C. acnes. There are 261 C-type lectins in C. elegans, and the current study just evaluated 15.7% (41 out of 261) of them. It could be more interesting to continue examining other C-type lectin genes during the infection of C. acnes.
In vertebrates, C-type lectin proteins are involved in defending pathogens, by acting as pattern recognition receptors (PRRs) or through their bactericidal activity (Brown et al., 2018; Mayer et al., 2017). Miltsch et al. reported a pivotal role for C-type lectin-like domain-containing proteins in C. elegans immunity (Miltsch et al., 2014). We observe antibacterial C-type lectin proteins conservation in nematodes. C-type lectin proteins also activate nematode immune responses to C. acnes.
C-type lectin proteins are p38 MAPK dependent via a phosphorylation event. p38 MAPK pathway promotes the expression of various antibacterial peptides as well as C-type lectin proteins of unknown function (Dierking et al., 2016). p38 pathway was exemplified by C. elegans strains deficient of the pmk-1, sek-1 and nsy-1 exhibiting increased susceptibilities to C. acnes. Indeed, C-type lectins in MAPK, MAPKK and MAPKKK mutants exhibited decreased expression while infected with C. acnes. However, the deletion of down-stream transcription factor ATF-7 cannot eliminate the induced expression of C-type lectins, suggesting that redundant signalling molecules, like SKN-1 or DAF-19, may also participate in the process. Infection with Pseudomonas aeruginosa leads to the induction of activated SKN-1 in the worm intestine (Papp, Csermely, & Sőti, 2012). Xie et al. reported that daf-19 could concert with atf-7 in response to the challenge of pathogens (Xie, Moussaif, Choi, Xu, & Sze, 2013). We intend to investigate further whether skn-1 and daf-19 mutant worms can affect C-type lectins expression and play roles in the C. acnes infection.
In conclusion, C. elegans is an excellent model organism for C. acnes infection, as it can provide an efficient killing assay, gene expression profile analysis and mutant worms for immune response studies. The p38 MAPK pathway is involved in the defence and regulates at least nine C-type lectins in nematodes during C. acnes infection. Further studies of C-type lectin recombinant proteins could be promising.
4 EXPERIMENTAL PROCEDURES
4.1 C. acnes strains and culture
Cutibacterium acnes strain ATCC 6919 was obtained from the American Type Culture Collection (ATCC, Manassas, VA). C. acnes strains HL043PA1 (HM-513), HL053PA1 (HM-522) and HL110PA3 (HM-554) were acquired from the Biodefense and Emerging Infections Research Resources Repository (BEI Resources, Manassas, VA), which were previously isolated at UCLA (Fitz-Gibbon et al., 2013; Tomida et al., 2013). HM-513 and HM-522 are antibiotic-resistant acne-associated ribotype IA isolates, and HM-554 is a ribotype II isolate from healthy human skin (Table 1) (Johnson, Kang, Barnard, & Li, 2016; Kolar et al., 2019). All C. acnes isolates were grown under anaerobic conditions (BD Diagnostic Systems) in BHI liquid and solid media (Sigma-Aldrich, St. Louis, MO) at 37°C for 5 days to reach the stationary phase. For routine experiments, the cells at the stationary phase were sub-cultured to the exponential growth phase before infecting nematodes. Typically, 50 ml of BHI broth was used, and bacteria were collected after centrifugation at 4,000g for 15 min at 4°C. The pelleted cells were washed three times in cold PBS or M9 buffer, and centrifuged as described before. Finally, C. acnes were suspended in PBS or M9 buffer, resulting in a concentration of 1 × 1010 CFU/ml for inoculum stock.
| Strain | Organism | Source | Description |
|---|---|---|---|
| ATCC 6919 | Cutibacterium acnes | ATCC | Type strain |
| HL043PA1 (HM-513) | Cutibacterium acnes | BEI Resources | Antibiotic-resistant acne-associated type IA-2 strain |
| HL053PA1 (HM-522) | Cutibacterium acnes | BEI Resources | Antibiotic-resistant acne-associated type IA-2 strain |
| HL110PA3 (HM-554) | Cutibacterium acnes | BEI Resources | Health-associated type II strain |
| OP50 | Escherichia coli | Lab inventory | Used as a feeder host for Caenorhabditis elegans |
| Bristol N2 | Caenorhabditis elegans | CGC | Wild-type |
| glp-4(bn2);sek-1(km4) | Caenorhabditis elegans | CGC | Temperature sensitive sterility and enhanced sensitivity to pathogens |
| clec-60(tm2319) | Caenorhabditis elegans | CGC | Knockout mutant worm |
| atf-7(gk715) | Caenorhabditis elegans | CGC | Knockout mutant worm |
| pmk-1(km25) | Caenorhabditis elegans | CGC | Knockout mutant worm |
| sek-1(ag1) | Caenorhabditis elegans | CGC | Knockout mutant worm |
| nsy-1(ag3) | Caenorhabditis elegans | CGC | Knockout mutant worm |
| tir-1(ok1052) | Caenorhabditis elegans | CGC | Knockout mutant worm |
| clec-60p::GFP(agEx39) | Caenorhabditis elegans | Lab inventory | Indicate the expression level of clec-60 by fluorescence |
4.2 Nematode strains and culture
Studies used the N2 wild type C. elegans strain unless otherwise specified. Mutant strains glp-4(bn2);sek-1(km4) (AU37), clec-60(tm2319) (CB6734), atf-7(gk715) (VC1518), pmk-1(km25) (KU25), sek-1(ag1) (AU1), nsy-1(ag3) (AU3) and tir-1(ok1052) (RB1085) were acquired from the Caenorhabditis Genetics Center (CGC, University of Minnesota, Minneapolis, MN). clec-60p::GFP(agEx39) was from our lab inventory (Table 1). Nematodes were reared and maintained at 16°C on nematode growth medium (NGM) agar plates with heat-killed (30 min at 65°C) Escherichia coli strain OP50 as the food source unless otherwise specified. Age-synchronised L4 larval or young adult worms were used for all experiments after washing three times with PBS or M9 buffer unless otherwise stated.
4.3 Solid media infection assay
Caenorhabditis elegans were infected with bacteria on solid media as previously described with minor modifications (Huang et al., 2014). All C. acnes isolates were grown to exponential phase. Ten microlitres of the bacterial culture was spread over a small area on 3.5 cm Petri dishes filled with BHI solid media and incubated at 37°C for 3 days under anaerobic conditions. Plates were then allowed to equilibrate to room temperature before use. BHI agar plates spread with heat-killed E. coli cells were used as the control.
Thirty age-matched worms (synchronised L4 or young adult) were transferred to either BHI plates with C. acnes cells or heat-killed E. coli and incubated at 25°C to observe the phenotypic changes after different time intervals exposures. The number of live and dead worms was scored. Worms were considered dead when they failed to respond to touch with a platinum wire pick. At each time point, the surviving worms were transferred to new assay plates. Three independent experiments were performed. The results assessed with Mantel–Cox log-rank test.
4.4 Liquid media infection assay
The time-course infection liquid-killing assay was performed as described previously with some modifications (Jayamani et al., 2015). In brief, synchronised L1 stage glp-4;sek-1 mutants were grown on NGM plates fed with heat-killed E. coli cells at 25°C for 35 hr, allowing them to reach the sterile L4 stage. Then 30 worms were transferred into each well in a 96-well plate format (Corning, NY) containing 20% BHI media in M9 buffer with the specified dilutions of C. acnes cells or heat-killed E. coli as a negative control. The assay plates were incubated at 25°C for 5 days. The culture wells were used as the system control, which contained 20% BHI media in M9 buffer and nematodes only. C. elegans survival was assessed on Day 5 by touching worms with a platinum wire. Only worms that responded to touch were considered alive. Three replicates were analysed.
Another method was applied to evaluate the killing efficacy by staining the dead worms in the assay plate with SYTOX Orange dye (Thermo Scientific, Wilmington, DE) as described previously with modifications (Rajamuthiah et al., 2014). Wells were washed with manual pipetting to remove bacteria, leaving 20 μl of M9 buffer with worms after the final aspiration. SYTOX Orange dye was added to each well at a final concentration of 0.7 mM. After incubating for 4 hr at 25°C, plates were imaged using an Image Xpress Micro automated microscope (Molecular Devices, San Jose, CA), capturing both transmitted light and TRITC (535 nm excitation, 610 nm emission) fluorescent images. All images were processed with ImageJ software (https://imagej.nih.gov/ij/, National Institutes of Health, Bethesda, MD).
4.5 Microscopy
A Zeiss Axiovert 200 microscope was used to view the worms. Nematodes were washed three times in M9 buffer, fixed with 1 mM levamisole hydrochloride solution (Sigma-Aldrich), and placed on 2% agarose pads to be viewed and imaged (Atakan et al., 2019). Visualisations were performed using the microscope with A-Plan objective lens (10× magnification) under the FITC and bright-field channel.
4.6 RNA extraction and complementary DNA (cDNA) synthesis
Treated nematodes were washed and harvested at the desired time points followed with freezing in liquid nitrogen. Total RNA was isolated with TRIzol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions with modifications. After adding TRIzol reagent, the freeze–thaw method was applied to break the worm cuticle. Chloroform was added then, total RNA was precipitated by adding isopropanol and was washed with 70% ethanol. The purified RNA was obtained by dissolving in RNase-free water and store at −80°C for future use.
The RNA concentration was determined by measuring absorbance at 260 nm with NanoVue UV–Visible Spectrophotometer (GE, Boston, MA). Ten micrograms total RNA of each sample was treated with DNase in the TURBO DNA-free Kit (Invitrogen) to remove the contamination of genomic DNA. Single-stranded cDNA was synthesised from RNA using the Verso cDNA Synthesis Kit (ThermoFisher Scientific, Waltham, MA) by following the manufacturer's instructions and then the cDNA was used as a template for the following PCR.
4.7 PCR and cloning of C-type lectin genes
Standard PCR amplification was carried out using Q5 High-Fidelity 2× Master Mix (NEB, Ipswich, MA) in a 25 μl reaction volume with the thermocycling conditions: 98°C for 30 s followed by 35 cycles of 98°C for 10 s, 60°C for 30 s and 72°C for 1 min and ending by an elongation cycle at 72°C for 5 min. The PCR primers used to amplify target sequences are listed in Table 2. The C. elegans cDNA was used as the template. After amplification, the DNA was digested with indicated restriction endonucleases and ligated into vector pET-51b (Sigma-Aldrich). The constructed prokaryotic expression plasmids were confirmed by DNA sequencing, which was done by GENEWIZ (Cambridge, MA).
| Gene | Restriction endonuclease | Forward primer | Reverse primer | Length (bp) |
|---|---|---|---|---|
| clec-13 | KpnI/SacI | cggGGTACCgATGCACACAATTTCCGCTCT | ccgGAGCTCcCGGTCTGTAAATCACAGAAG | 1,242 |
| clec-17 | KpnI/SacI | cggGGTACCgATGCAACTCCGTTTTGCACT | ccgGAGCTCcGCGTCTATAAACAATCGACG | 1,251 |
| clec-47 | KpnI/SacI | cggGGTACCgATGGGAATAATCACGTATCT | ccgGAGCTCcAGAATTCACTGCAATTTCGC | 483 |
| clec-52 | HindIII/NotI | cccAAGCTTATGTTTCGATCACTGCTCCT | ATTTGCGGCCGCATGAAGTGGTGCAATTTTAC | 927 |
| clec-60 | KpnI/SacI | cggGGTACCgATGAAACTCACATTTCTTAT | ccgGAGCTCcGTGTTTTACGGAAATTTCAG | 1,221 |
| clec-61 | KpnI/NotI | CGGGGTACCgATGGCAATGAAGTTTCTTCT | atttGCGGCCGCAATTTTTTCAGCTGTACAGT | 1,212 |
| clec-70 | BamHI/SacI | CGCGGATCCgATGCTGCTTCTCAAATTCAT | CGGGAGCTCcATCCGTATACGGTAGCCAAAAG | 1,401 |
| clec-71 | KpnI/SacI | cggGGTACCgATGATTCTAAAACTTTGTCT | ccgGAGCTCcATTTGAATACGGCAGCCAAT | 1,404 |
| clec-227 | KpnI/SacI | cggGGTACCgATGATCTGTGCTGTACTGAC | ccgGAGCTCcTTGAATGAACCAGACAATTG | 1,146 |
4.8 Analysis of gene expression
Gene expression was examined using iTaq Universal SYBR Green Supermix and a CFX96 Touch Real-Time PCR Detection System (Bio-Rad, Hercules, CA) with the following protocol: initial denaturation at 95°C for 30 s, followed by 40 cycles of 95°C for 5 s and 60°C for 30 s, then ending by a melt-curve analysis of 65–95°C with the instrument default setting. Detection primers are listed in Supporting Information Tables S1 and S2. Gene expression was normalised using housekeeping genes snb-1, actin and ama-1. Fold changes were calculated using a 2−ΔΔCq method, and compared to expression from the control group. Assays were performed with three independent experimental samples in triplicates. Differences in gene expression were assessed using one-way analysis of variance (ANOVA) followed by Dunnett's multiple comparisons test. A p value <.05 in all replicate experiments were considered statistically significant.
4.9 E. coli strain BL21(DE3) killing assay
The prokaryotic expression plasmids for C-type lectin were transformed into E. coli strain BL21(DE3) (Sigma-Aldrich) and cultured on LB plates with 100 μg/ml ampicillin (LA plates). Then, single colonies from each plate were suspended in 1 ml PBS and serially diluted 106-fold with PBS. The bacterial solution was spot-plated onto LA plates with or without 200 μM IPTG (Sigma-Aldrich). After incubating the plates at 37°C for 24 hr, colonies were enumerating. The experiment was performed in triplicate.
4.10 Immunoblotting
Worms and cells were homogenised in RIPA buffer (25 mM Tris–HCl pH 7.6, 150 mM NaCl, 5 mM EDTA, 1% NP-40, 1% sodium deoxycholate, 0.1% SDS) with Protease Inhibitor Cocktail and 1 mM PMSF (ThermoFisher Scientific). Then, samples were centrifuged at 10,000g for 20 min, and the supernatants were transferred to fresh tubes kept on ice. The 5× sample buffer was added after determining the protein concentration using a Pierce BCA Protein Assay Kit (ThermoFisher Scientific) according to the manufacturer's instructions. Proteins were electrophoresed on a 10% Mini-PROTEAN TGX Stain-Free Protein Gel (Bio-Rad) and transferred to a PVDF membrane, which was blocked with Invitrogen Membrane Blocking Solution (Invitrogen). For immunoblotting anti-phospho p38 and anti-p38 antibodies were purchased from Cell Signalling Technology (Danvers, MA). Anti-GAPDH antibody (Sigma-Aldrich) was used as a loading control. The membrane was developed with ECL system, and images were acquired with ChemiDoc Imaging Systems (Bio-Rad).
4.11 Statistical analysis
GraphPad Prism 8 (GraphPad Software, San Diego, CA) was used for all analyses. For the lifespan assay (survival assay), statistical tests for significant differences between survival curves were performed using the non-parametric log-rank (Mantel–Cox) test. The two-group analysis used unpaired t-test (two-tailed) or non-parametric Mann–Whitney U test. Comparisons of multiple groups were performed using one-way ANOVA and subsequent Dunn's multiple comparisons test. Data are expressed as mean ± SD. A p value of less than .05 in all replicate experiments was considered statistically significant.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Eleftherios Mylonakis and Kang Zeng supervised the project. Xiaowen Huang, Wen Pan, Wooseong Kim, Huiying Li, Kang Zeng and Eleftherios Mylonakis designed all experiments. Xiaowen Huang and Wen Pan performed and analysed all experiments. Xiaowen Huang, Wen Pan, Alexis White, Silei Li, Beth Burgwyn Fuchs and Eleftherios Mylonakis wrote the manuscript. Kiho Lee provided some comments.