Targeting E. coli invasion of the blood–brain barrier for investigating the pathogenesis and therapeutic development of E. coli meningitis
Funding information: National Institutes of Health, Grant/Award Numbers: AI113273, AI126176, AI84984, NS091102
Abstract
Escherichia coli is the most common Gram-negative bacillary organism causing neonatal meningitis. Escherichia coli meningitis remains an important cause of mortality and morbidity, but the pathogenesis of E. coli penetration of the blood–brain barrier remains incompletely understood. Escherichia coli entry into the brain occurs in the meningeal and cortex capillaries, not in the choroid plexus, and exploits epidermal growth factor receptor (EGFR) and cysteinyl leukotrienes (CysLTs) for invasion of the blood–brain barrier. The present study examined whether EGFR and CysLTs are inter-related in their contribution to E. coli invasion of the blood–brain barrier and whether counteracting EGFR and CysLTs is a beneficial adjunct to antibiotic therapy of E. coli meningitis. We showed that (a) meningitis isolates of E. coli exploit EGFR and CysLTs for invasion of the blood–brain barrier, (b) the contribution of EGFR is upstream of that of CysLTs, and (c) counteracting EGFR and CysLTs as an adjunctive therapy improved the outcome (survival, neuronal injury and memory impairment) of animals with E. coli meningitis. These findings suggest that investigation of host factors contributing to E. coli invasion of the blood–brain barrier will help in enhancing the pathogenesis and development of new therapeutic targets for E. coli meningitis in the era of increasing resistance to conventional antibiotics.
1 INTRODUCTION
Escherichia coli is the most common Gram-negative bacillary organism causing neonatal meningitis and neonatal E. coli meningitis remains an important cause of mortality and morbidity (Kim, 2003; Kim, 2008; Kim, 2010; Kim, 2012). Case fatality rates range from 5–25%, and approximately 25–50% of survivors sustain neurologic sequelae. The pathogenesis of E. coli meningitis, however, remains incompletely understood.
Several lines of evidence from human cases and experimental animal models of E. coli meningitis indicate that a high level of bacteremia is a prerequisite for E. coli penetration into the brain and that cerebral capillaries are the portal of entry into the brain (Dietzman, Fischer, & Schoenknecht, 1974; Kim et al., 1992). Since E. coli penetration into the brain occurred in the cerebral microvasculature (Kim et al., 1992), we used the blood–brain barrier model with human brain microvascular endothelial cells (HBMEC) to investigate E. coli invasion of the blood–brain barrier (Stins, Badger, & Kim, 2001; Stins, Gilles, & Kim, 1997). We have shown that meningitis isolates of E. coli invade HBMEC monolayer and the HBMEC invasion is correlated with the ability of E. coli to penetrate into the brain in the animal models of experimental hematogenous meningitis, which mimic the pathogenesis of E. coli meningitis in humans, for example, hematogenous penetration into the meninges (Huang et al., 1995, Huang et al., 1999, Wang, Huang, Wass, Stins, & Kim, 1999, Khan et al., 2002, Wang & Kim, 2002, Zhu et al., 2010, Zhu, Pearce, & Kim, 2010, Wang et al., 2016). These findings suggest that elucidation of the mechanisms involved in E. coli invasion of the blood–brain barrier is likely to enhance our knowledge on the pathogenesis of E. coli meningitis.
Antimicrobial therapy alone has limited efficacy in improving the outcome of E. coli meningitis (Kim, 1985; Kim, 2012). In addition, antimicrobial resistance has been increasingly recognised in meningitis isolates of E. coli, which include those producing CTX-M-type or TEM-type extended-spectrum β-lactamases, as well as those expressing KPC-2 carbapenemase and NDM-1 metallo-β-lactamase (Boyer-Mariotte et al., 2008; Johnson, Johnston, Clabots, Kuskowski, & Castanheira, 2010; Moissenet et al., 2010; Pouillot et al., 2012; Rogers, Sidjabat, & Paterson, 2011). These findings indicate the need for search of new strategy for treatment of E. coli meningitis.
Since E. coli invasion of the blood–brain barrier is a prerequisite for penetration into the brain (Kim, 2008), we hypothesize that targeting E. coli invasion of the blood–brain barrier represents a novel strategy for investigating the pathogenesis and therapy of E. coli meningitis. The feasibility of this hypothesis is shown in the current study, where pharmacological inhibition and gene deletion of the targets contributing to E. coli invasion of the blood–brain barrier was efficacious in inhibiting E. coli penetration into the brain. We examined whether counteracting the targets derived from investigating E. coli invasion of the blood–brain barrier represents a beneficial adjunct to antibiotic therapy in improving the outcome of animals with experimental hematogenous E. coli meningitis.
2 RESULTS
2.1 Escherichia coli entry into the brain without affecting the blood–brain barrier permeability
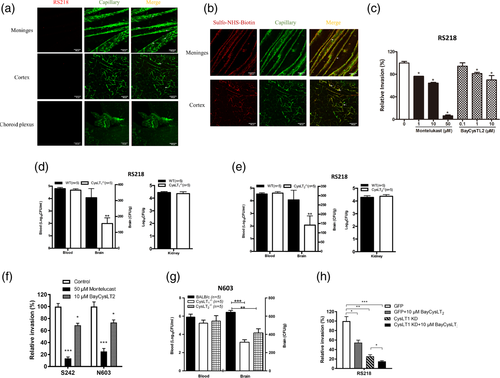
In the present study with the mouse model of experimental hematogenous E. coli meningitis, E. coli entry into the brain was shown to occur initially in the meningeal capillaries. This was shown by the demonstration that greater numbers of intravenously injected bacteria were found in the meningeal capillaries compared to the cortex capillaries at 1 hr following bacterial administration. A few bacteria were found outside the capillaries of the meninges and cortex (Figure 1a and Figure S1), indicating successful entry of E. coli into the brain at this time. There was no demonstration of E. coli in the choroid plexus at this time (Figure 1a and Figure S1). These findings demonstrate that the meningeal and cortex capillaries, not the choroid plexus, are the portal of circulating E. coli entry into the brain. It is important to note that intravascular small molecule tracer (i.e., Sulfo-NHS-biotin with m.w. of 443 Da) was confined to the meningeal and cortex capillaries and there was no extravasation (Figure 1b and Figure S1). These findings are consistent with those of our previous experiments in the infant rat model of experimental hematogenous E. coli meningitis, where the meningeal capillaries (capillaries in the subarachnoid space) are the entry sites of circulating E. coli into the brain (Kim et al., 1992) and E. coli entry into the brain occurred without affecting the blood–brain barrier permeability, as assessed by Cerebrospinal fluid (CSF) penetration of circulating albumin and extravasation of intravascular Evans blue dye (Kim, Wass, & Cross, 1997; Zhu, Maruvada, et al., 2010). Taken together, these findings indicate that E. coli penetration into the brain occurs without affecting the blood–brain barrier permeability and support the use of the blood–brain barrier model for investigating the pathogenesis of E. coli meningitis.
2.2 Escherichia coli exploits host CysLTs for penetration of the blood–brain barrier
The biosynthesis of cysteinyl leukotrienes (CysLTs) is initiated by cytosolic phospholipase A2α (cPLA2α) release of arachidonic acid from the outer nuclear membrane and conversion to LTA4 by 5-lipoxygenase (5-LO) (Peters-Golden & Henderson Jr., 2007). We have shown that E. coli penetration of the blood–brain barrier was inhibited by pharmacological inhibition and gene deletion of cPLA2α and 5-LO (Zhu, Maruvada, et al., 2010), but the role of CysLTs in E. coli passing through the blood–brain barrier remains incompletely understood. CysLTs exhibit their biological activities via interactions with their G-protein coupled receptors (GPCRs), including CysLT1 and CysLT2. The roles of CysLTs were examined by determining the effects of the CysLT1 antagonist (montelukast) and the CysLT2 antagonist (BayCysLT2) in E. coli invasion of HBMEC. Both antagonists exhibited a dose-dependent inhibition of E. coli invasion of HBMEC (Figure 1c). It is important to point out that exposure of HBMEC to E. coli and antagonists did not affect the HBMEC viability and monolayer integrity, as determined by live/dead staining (Molecular Probes), and the antagonists did not affect the bacterial growth (Stins et al., 2001). These findings suggest that E. coli is likely to exploit CysLT1 and CysLT2 for invasion of the blood–brain barrier.
The contributions of CysLT1 and CysLT2 to E. coli invasion of the blood–brain barrier were supported by the demonstration that E. coli entry into the brain was significantly less in CysLT1−/− and CysLT2−/− mice compared to strain-matched wild type mice (Figure 1d,e)). A magnitude of bacteremia is the primary determinant for circulating E. coli to penetrate into the brain (Huang et al., 1995; Kim et al., 1992), but the magnitudes of bacteremia did not differ significantly between the wild type and CysLT1−/− or CysLT2−/− animals (CFUs/ml of blood) (Figure 1d,e)). In contrast, the ability of E. coli to penetrate into the kidneys was similar between wild type mice and CysLT1−/− or CysLT2−/− mice (Figure 1d,e). These findings demonstrate that host CysLT1 and CysLT2 contribute to E. coli entry, specifically into the brain.
We next examined whether CysLT1 and CysLT2 contribution to E. coli penetration of the blood–brain barrier was relevant to TEM-52-extended spectrum β-lactamase producing meningitis isolates, strains N603 and S242 (Boyer-Mariotte et al., 2008; Moissenet et al., 2010). Both montelukast and BayCysLT2 exhibited significant inhibition of strains N603 and S242 invasion in HBMEC (Figure 1f). More importantly, the strain N603 exhibited significantly decreased entry into the brains of CysLT1−/− and CysLT2−/− mice compared to those of strain-matched wild type mice without affecting the magnitudes of bacteremia (Figure 1g). These findings demonstrate that E. coli exploitation of CysLT1 and CysLT2 for entry of the blood–brain barrier in vitro and in vivo includes antibiotic-susceptible and –resistant strains.
We next examined whether the contributions of CysLT1 and CysLT2 to E. coli invasion of the blood–brain barrier are redundant and/or additive to each other. This issue was examined by determining the effect of CysLT2 antagonist (BayCysLT2) in E. coli invasion in control and CysLT1 knockdown HBMEC. As expected, E. coli invasion was significantly less in HBMEC treated with BayCysLT2 and in CysLT1 knockdown HBMEC compared to vehicle-treated and control HBMEC, respectively (Figure 1h, Figure S2a). The addition of BayCysLT2 to CysLT1 knockdown HBMEC, however, exhibited significantly greater inhibition of invasion compared to targeting CysLT1 and CysLT2 individually (Figure 1h). These findings suggest that the contribution of CysLT1 to E. coli invasion of the blood–brain barrier is additive to that of CysLT2.
2.3 EGFR-cPLA2α-CysLTs contributes to E. coli invasion of the blood–brain barrier
We have shown that gefitinib (a selective inhibitor of EGFR) inhibited E. coli invasion of the blood–brain barrier (Wang et al., 2016). The role of EGFR in E. coli invasion of the blood–brain barrier was validated by using HBMEC with CRIPSR/Cas9 knockout of EGFR as well as by using EGFR knockout mice. Meningitis isolates of E. coli including antibiotic resistant strains N603 and S242 exhibited significantly less invasion in EGFR knockout HBMEC compared to control HBMEC (Figure 2a, Figure S2c). The role of EGFR in E. coli penetration into the brain was examined using the tamoxifen-inducible, endothelial-specific EGFR knockout mice. Endothelial-specific conditional EGFR knockout was induced by a 5-day intraperitoneal administration of tamoxifen. Two days later the mice were infected with E. coli. The bacterial counts recovered from the brains were significantly less in conditional EGFR knockout mice than in Tek-RFP-CreERT2 mice treated with tamoxifen as controls (Figure 2b), while the bacterial counts from the blood did not differ between the two groups. These in vitro and in vivo findings demonstrate that E .coli exploits EGFR for invasion of the blood–brain barrier.
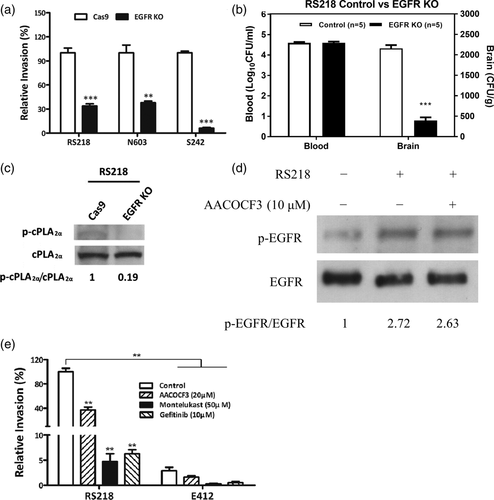
We next examined whether the contribution of EGFR to E. coli penetration of the blood–brain barrier is related to that of cPLA2α-CysLTs by determining the effect of EGFR knockout on cPLA2α activation in response to E. coli. cPLA2α activation in response to E. coli was inhibited in EGFR knockout HBMEC (Figures 2c and 3d). In contrast, EGFR activation in response to E. coli was not affected by inhibition of cPLA2α (Figure 2d). These findings indicate that the contribution of EGFR is upstream of that of cPLA2α-CysLTs in E. coli invasion of the blood–brain barrier.
2.4 Meningitis isolates of E. coli exploit EGFR-cPLA2α-CysLTs for invasion of the blood–brain barrier
Escherichia coli invasion of the blood–brain barrier is shown to be a biologically relevant trait for meningitis isolates, not for blood-isolates (Huang et al., 1995). We examined whether E. coli exploitation of EGFR-cPLA2α-CysLTs is relevant to meningitis and blood isolates, by determining the effect of pharmacological inhibition of EGFR, cPLA2α and CysLTs on HBMEC invasion by E. coli isolates from CSF and blood (strains RS218 and E412, respectively). Strain RS218 is a meningitis isolate and invasive in HBMEC, and strain E412 is a blood isolate and non-invasive in HBMEC (Huang et al., 1995). The invasin protein IbeA is shown to contribute to E. coli invasion of the blood–brain barrier, resulting in positive CSF culture (Huang et al., 1995). The presence of IbeA expression was demonstrated in meningitis isolates of E. coli strains RS218, S242 and N603, while the blood isolate strain E412 lacks the expression of IbeA (Figure S3). We have previously shown that positive CSF culture as well as positive brain culture after perfusion reflect bacterial entry into the brain, and there was no bacterial contamination from blood, as shown by no recovery of bacteria from blood after perfusion (Zhu, Maruvada, et al., 2010). This concept was shown in our animal model of experimental hematogenous E. coli meningitis where invasive meningitis isolate strain RS218 and non-invasive blood isolate strain E412 exhibited similar levels of bacteremia. Strain RS218, but not strain E412, however, exhibited positive brain cultures after perfusion (Figure S4). As expected, strain RS218 exhibited significantly greater invasion of HBMEC than strain E412. Pharmacological inhibition of cPLA2α, CysLT1 and EGFR inhibited RS218 invasion, but did not significantly affect E412 invasion (Figure 2e). These findings support that meningitis isolates, not blood isolates of E. coli are likely to exploit cPLA2α, CysLT1 and EGFR for invasion of HBMEC.
2.5 Sphingosine-1 phosphate receptor 2 (S1P2) contributes to E. coli invasion of the blood–brain barrier in vitro and in vivo
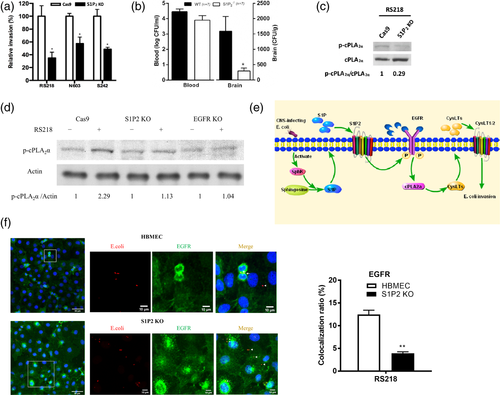
We have shown that that S1P2 is likely to participate in E. coli invasion of the blood–brain barrier, as shown by decreased E. coli invasion in HBMEC with pharmacological inhibition of S1P2 (Wang et al., 2016). The role of S1P2 in E. coli invasion was validated by the demonstration that E. coli invasion was significantly less in S1P2 knockout HBMEC (Figure 3a, Figure S2D). Biological relevance of decreased E. coli invasion in S1P2 knockout HBMEC was examined in S1P2−/− mice compared to strain-matched wild type mice. Escherichia coli strain RS218 penetration into the brain was significantly less in S1P2−/− mice than in the wild type animals without affecting the magnitudes of bacteremia (Figure 3b). These in vitro and in vivo findings demonstrate that S1P2 is the critical host factor contributing to E. coli penetration of the blood–brain barrier.
We showed that S1P2 is upstream of EGFR and EGFR is upstream of cPLA2α-CysLTs in E. coli invasion of the blood–brain barrier (Wang et al., 2016). We next showed that cPLA2α activation in response to E. coli was inhibited in S1P2 knockout HBMEC as well as in EGFR knockout HBMEC (Figure 3c,d). Taken together, these in vitro and in vivo findings demonstrate that meningitis isolates of E. coli exploit host S1P2, EGFR and cPLA2α-CysLTs for penetration of the blood–brain barrier and that S1P2 is upstream of EGFR-cPLA2α-CysLTs in E. coli invasion of the blood–brain barrier (Figure 3e). This concept is supported by the demonstration of E. coli strain RS218 co-localised with EGFR in control HBMEC, while such co-localization was significantly less in S1P2 knockout HBMEC compared to control HBMEC (Figure 3f).
2.6 Targeting E. coli penetration of the blood–brain barrier as a therapeutic adjunct for the treatment of E. coli meningitis
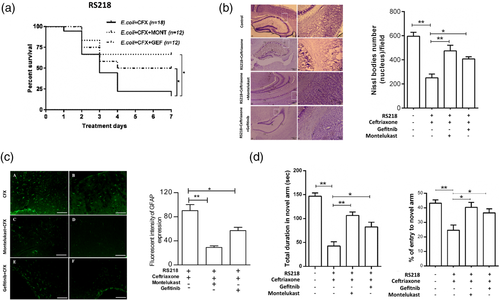
Antimicrobial therapy alone has limited efficacy in improving the outcome of E. coli meningitis (Kim, 1985; Kim, 2008; Kim, 2010; Kim, 2012). At present, no strategy exists for development of an improved therapy for E. coli meningitis. We, therefore, examined whether counteracting targets derived from investigating E. coli invasion of the blood–brain barrier might provide a novel approach for improving the outcome of E. coli meningitis by using pharmacological inhibitor/antagonist of EGFR and CysLT1 in experimental settings mimicking clinical scenario, for example, animals with experimental E. coli meningitis were treated with an antibiotic alone or in combination with inhibitor/antagonist, and animals were assessed for survival, neuronal injury and memory impairment. All infected animals receiving montelukast or gefitinib alone died, while animals with E. coli meningitis receiving ceftriaxone plus montelukast or gefitinib exhibited significantly greater survival compared to mice receiving ceftriaxone alone (Figure 4a). All surviving animals receiving the combination therapy exhibited less neuronal injury as assessed by Nissl stain (Figure 4b), less astrocyte activation (Figure 4c) and less memory impairment as assessed by Y-maze (Figure 4d) compared to mice receiving ceftriaxone alone.
3 DISCUSSION
Meningitis isolates of E. coli exhibit the ability to invade the blood–brain barrier in vitro (HBMEC monolayer) and in vivo (animals with experimental hematogenous meningitis) (Kim, 2008). We hypothesize that elucidating E. coli invasion of the blood–brain barrier is likely to enhance the knowledge on the pathogenesis and also help in development of therapeutic targets for E. coli meningitis.
In the present study, we showed that E. coli penetration into the brain occurred in the meningeal and cortex capillaries without affecting the blood–brain barrier permeability, for example, extravasation of even a small molecule tracer of 443 Da. These findings indicate that elucidation of E. coli invasion of the blood–brain barrier will enhance our knowledge on the pathogenesis of E. coli meningitis. We have shown using pharmacological inhibition that CysLT1 contributes to E. coli invasion of the blood–brain barrier (Wang et al., 2016; Zhu, Maruvada, et al., 2010). This concept was further expanded by the demonstration that CysLT1 as well as CysLT2 contribute to E. coli invasion of the blood–brain barrier. Pharmacological inhibition of CysLT1 and CysLT2 exhibited significantly decreased E. coli invasion of HBMEC and gene deletions of CysLT1 and CysLT2 resulted in significantly decreased E. coli penetration into the brain. More importantly, the contribution of CysLT1 to E. coli invasion of the blood–brain barrier was additive to that of CysLT2, as shown by significantly greater inhibition of E. coli invasion with targeting both CysLT1 and CysLT2 compared to individual GPCRs. We also showed that pharmacological inhibition of EGFR and S1P2 affected E. coli invasion of the blood–brain barrier (Wang et al., 2016). Pharmacological inhibition can point to specific targets of interest (e.g., montelukast for CysLT1, BayCysLT2 for CysLT2, gefitinib for EGFR), but not without any concern about off-target effects. This off-target issue was addressed by targeting specific genes such as gene knockout of EGFR and S1P2 in HBMEC as well as using gene knockout animals (e.g., CysLT1−/−, CysLT2−/−, EGFR−/−, S1P2−/−). We also showed that E. coli exploitation of CysLTs and EGFR for invasion of the blood–brain barrier is relevant to meningitis isolates, not to blood isolates. These findings demonstrate that meningitis isolates of E. coli exploit S1P2, EGFR, CysLT1 and CysLT2 for invasion of the blood–brain barrier. Additional studies are needed to elucidate the molecular basis for S1P2, EGFR, CysLT1 and CysLT2 contributions to E. coli invasion of the blood–brain barrier.
Of interest, the contributions of S1P2, EGFR and cPLA2α-CysLTs to E. coli invasion of the blood–brain barrier are inter-related, that is, S1P2 is upstream of EGFR, and EGFR is upstream of cPLA2α-CysLTs. This concept is shown by the demonstration that (a) gene knockout of S1P2 and EGFR abolished activation of cPLA2α, while cPLA2α inhibition did not affect EGFR activation in response to E. coli in HBMEC and (b) E. coli co-localization with EGFR was inhibited in S1P2 knockout HBMEC. These findings demonstrate that S1P2, EGFR and CysLTs form a distinct host cell signalling network contributing to E. coli invasion of the blood–brain barrier (Figure 3e) and elucidation of such network is likely to enhance our knowledge on the pathogenesis of E. coli meningitis.
Antimicrobial therapy alone has limited efficacy in improving the outcome of E. coli meningitis and we examined whether targets derived from investigating E. coli penetration of the blood–brain barrier might represent a beneficial therapeutic adjunct to antibiotic therapy of E. coli meningitis. This concept is supported by our demonstration that animals with E. coli meningitis who received the combination therapy of antibiotic and pharmacological inhibitor of EGFR or CysLT1 exhibited improved survival, less neuronal injury and less memory impairment compared to those receiving antibiotic therapy alone. Additional studies are needed to elucidate how counteracting targets derived from investigating E. coli invasion of the blood–brain barrier can be a beneficial therapeutic adjunct to antibiotic therapy of E. coli meningitis. These findings suggest that targets derived from investigating E. coli invasion of the blood–brain barrier can represent a novel strategy for improving the outcome of meningitis and that continued investigation of E. coli invasion of the blood–brain barrier will provide additional therapeutic targets for E. coli meningitis. Of particular relevance is the availability of EGFR inhibitors and CysLT1 antagonists, which are shown to be well tolerated in clinical trials (Funk, 2005; Jamal-Hanjani & Spicer, 2012; Montuschi, Sala, Dahlen, & Folco, 2007). It is important to note that use of such drugs as an adjunctive therapy for bacterial meningitis is likely to be brief (e.g., 6 days shown in the current study) and less likely to result in adverse effects, allowing ready translation into clinical trials.
Taken together, this is the first demonstration that meningitis isolates of E. coli exploit S1P2, EGFR and CysLTs for invasion of the blood–brain barrier in vivo and in vitro, and that S1P2-EGFR-CysLTs represents a novel target for investigating the pathogenesis and therapeutic development of E. coli meningitis.
4 EXPERIMENTAL PROCEDURES
4.1 Bacterial strains and cell culture
Cerebrospinal fluid (CSF) isolates of E. coli from neonates with meningitis were used, which include a prototype meningitis isolate strain RS218 and CTX-M-type or TEM-type extended-spectrum β-lactamases producing strains S242 and N603 (Boyer-Mariotte et al., 2008; Huang et al., 1995; Kim, 2008; Kim, 2010; Kim, 2012; Moissenet et al., 2010). These CSF isolates exhibited the ability to invade HBMEC, while a blood isolate of E. coli strain E412 was not invasive in HBMEC and used as a negative control (Huang et al., 1995). HBMEC were isolated and cultured as described previously (Stins et al., 1997; Stins et al., 2001).
4.2 Reagents and antibodies
Gefitinib, montelukast, BayCysLT2 and ceftriaxone were purchased from Cayman Chemical Company (Ann Arbor, MI). Arachidonyltrifluoromethyl ketone was from Biomol Laboratories (Plymouth Meeting, PA). Protein G Agarose Fast Flow beads and anti-phosphotyrosine (4G10) horseradish peroxidase (HRP)-conjugate antibody (1:1,000) were from EMD Millipore Corporation (Temecula, CA). DAPI, anti-mouse IgG Alexa Fluor 488-conjugated secondary antibody (1:500) and EZ-Link™ Sulfo-NHS-Biotin were from ThermoFisher Scientific (Waltham, MA). Anti-EGFR conjugated antibody and CD31 antibody were from Santa Cruz Biotechnology (1:1,000) (Santa Cruz, CA). cPLA2α and phospho-cPLA2α antibodies and anti-rabbit IgG HRP-conjugate antibody were from Cell Signalling Technology (Danvers, MA). Anti-actin antibody was from Sigma-Aldrich (1:3,000) (St. Louis, MO). Anti-S1P2 (1:500) antibody was from Proteintech (Rosemont, IL). The Texas Red® Streptavidin was purchased from Vector Laboratories (Burlingame, CA).
4.3 Bacterial invasion assays in HBMEC
The ability of E. coli strains to invade HBMEC monolayer was determined as previously described (Huang et al., 1995, Huang et al., 1999, Wang et al., 1999, Khan et al., 2002, Wang & Kim, 2002, Zhu, Maruvada, et al., 2010, Zhu, Pearce, & Kim, 2010, Wang et al., 2016). In some experiments, HBMEC were pre-treated with various inhibitors for 1 hr prior to addition of bacteria and then assayed for bacterial invasion. The results were calculated as percentages of the initial inoculums, (number of internalised bacteria/number of bacteria inoculated) × 100 and presented as relative invasion (percent invasion compared with invasion in the presence of the vehicle control). Each assay was performed in triplicate.
4.4 Construction of EGFR and S1P2 CRISPR knockout HBMEC
Cas9 gene was incorporated into the HBMEC by lentivirus produced by lentiCas9-Blast plasmid (Addgene #52962) in 293 T cells (Joung et al., 2017; Wang et al., 2016). The EGFR target DNA sequences as well as S1P2 target DNA sequences were cloned into LentiGuide-puro plasmid (Addgene #52963) (Joung et al., 2017) using primers listed in Table S1. The 293 T cells were transfected with 10 μg of the transfer plasmid LentiGuide-puro-EGFR or S1P2, 5 μg pVSV-G, 7.5 μg psPAX2, 100 μl of Plus Reagent (ThermoFisher Scientific), and 50 μl of Lipofectamine 2000 (ThermoFisher Scientific) to produce the CRISPR lentivirus targeting EGFR or S1P2. HBMEC-Cas9 cells were infected by CRISPR lentivirus targeting EGFR or S1P2, and selected by HBMEC media supplemented with 1 μg/ml puromycin. The single cells of EGFR or S1P2 CRISPR knockout HBMECs were sorted to 96-well tissue culture plate by flow cytometry and grown to confluence in HBMEC media supplemented with 1 μg/ml puromycin. The expressions of EGFR and S1P2 were verified by Western blotting with anti-EGFR and S1P2 antibodies, respectively.
4.5 Immunoprecipitation and western blotting
HBMEC were serum-starved overnight, incubated with E. coli at a multiplicity of infection (MOI) of 100 for indicated periods of time, lysed and processed for immunoprecipitation and Western blotting analysis as previously described (Wang et al., 2016).
4.6 Immunofluorescence in HBMEC
Control and S1P2 knockout HBMEC were grown on collagen-coated glass slide to confluence, incubated with RFP-RS218 at MOI of 1:100 at 37°C for 90 min. Cells were washed with PBS to remove the free, unbound bacteria and then fixed with 4% paraformaldehyde. The cells were washed by PBS and permeabilized with 0.25% Triton X-100 solution for 10 min, and blocked with 5% BSA for 1 hr. Cells were then incubated with anti-EGFR antibody, and incubated with Alexa Fluor 488-labelled secondary antibody, followed by nucleus staining with DAPI. The glass slide was mounted and visualised using fluorescence microscopy as previously described (Wang et al., 2016).
4.7 Immunostaining of E. coli with anti-IbeA antibody
Escherichia coli strains were harvested from overnight culture, washed with PBS and then fixed with 4% paraformaldehyde. Bacteria were washed by PBS and permeabilized with 0.25% Triton X-100 solution for 10 min, and blocked with 5% BSA for 1 hr. Bacteria were then incubated with anti-IbeA antibody (Huang et al., 1995), and incubated with Alexa Fluor 488-labelled secondary antibody, followed by nucleus staining with DAPI. The glass slide was mounted and visualised using a Zeiss LSM 700 confocal microscope.
4.8 Animal experiments
CysLT1−/−, CysLT2−/− and strain-matched wild type BALB/c mice, as well as S1P2−/− and strain-matched wild type C57BL/c mice, either male or female, between 4 and 6 weeks, were used as previously described (Maekawa, Kanaoka, Xing, & Austen, 2008; Wang et al., 2016; Zhu, Maruvada, et al., 2010). Each mouse received approximately 1 × 107 CFUs of E. coli in 50 μl sterile normal saline via tail vein injection. At 1 hr post-inoculation, the blood was collected for quantitative cultures. Subsequently, the mice were perfused with the mammalian Ringer solution until the perfused solution became colourless, and the brains and kidneys were removed, weighed, homogenised, and plated to determine the bacterial counts (CFUs/g of weight).
4.9 Construction of EGFR conditional knockout mice
The EGFR knockout mice are embryonically lethal, and the tamoxifen-inducible, endothelial-specific EGFR knockout mice were generated by crossbreeding floxed EGFR mice (Lee & Threadgill, 2009) with Tek-RFP-CreERT2 mice in the background of C57BL/c as described previously (Metzger & Chambon, 2001). EGFRfl/flmice were crossed with Tek-RFP-CreERT2 mice to generate EGFRfl/fl /Tek-RFP-CreERT2mice. Genotyping was performed by PCR of tail DNA with the Cre primers (Cre forward: 5′- CTA AAC ATG CTT CAT CGT CGG TC -3′; Cre reverse: 5′- TCT GAC CAG AGT CAT CCT TAG CG −3′) and Lox3 primers (Lox3 forward: 5′- CTT TGG AGA ACC TGC AGA TC -3′; Lox3 reverse: 5′- CTG CTA CTG GCT CAA GTT TC -3′). CreERT2 consists of Cre conjugated with a mutated oestrogen receptor (ERT2) that binds poorly to endogenous oestrogen but with high affinity to the oestrogen derivative tamoxifen (Metzger & Chambon, 2001). Endothelial-specific knockout of EGFR was induced by a 5-day intraperitoneal administration of tamoxifen (1 mg/kg). Two days later, the mice were infected with E. coli and the bacteria counts from the blood and brain were determined as described above. Tek-RFP-CreERT2 littermates treated with tamoxifen were served as controls.
4.10 Examination of entry sites for circulating E. coli penetration into the brain and assessment of the blood–brain barrier permeability
Each mouse received 1 × 108 CFUs of RFP-RS218 in 100 μl PBS via the tail vein as described above. At 1 and 12 hr later, animals were perfused with PBS followed by 2% paraformaldehyde (PFA). The brains and skullcap were removed, fixed overnight with 2% PFA at 4°C, and then re-hydrated in 1x PBS at 4°C for 3 hr. The brains were embedded in 3% agarose and cut into sections of 150 μm thickness using a vibratome (Leica). The meninges were carefully detached from the skullcap. To assess the blood–brain barrier permeability with E. coli penetration into the brain, 200 μl solution (20 mg/ml) of Sulfo-NHS-biotin (a low molecular weight tracer with m.w. of 443 Da) was intraperitoneally injected 10 min before intracardiac perfusion. Covalently immobilised Sulfo-NHS-biotin was visualised with fluorescent streptavidin, and extravasation is indicative of leakage from the brain microvessels. The brain sections and meninges were incubated with CD31 antibody diluted in 1x PBSTC (1x PBS + 1% Triton X-100 + 0.1 mM CaCl2) +5% BSA overnight at 4°C. Then they were washed three times with 1x PBSTC, and subsequently incubated overnight with different secondary antibodies or Texas Red streptavidin diluted in 1x PBSTC +5% BSA. The next day, the brain sections and meninges were washed three times with 1x PBSTC, and flat-mounted using ProLong Gold antifade reagent (Invitrogen). The tissues were imaged using a Zeiss LSM700 confocal microscope, and processed with ImageJ (Lu et al., 2018; Wang et al., 2019).
4.11 Treatment of experimental hematogenous E. coli meningitis
Animals were randomly divided into five experimental groups and infected with E. coli, as described above. Twenty-four hours later, animals were treated with ceftriaxone (100 mg/kg), montelukast (10 mg/kg), gefitinib (10 mg/kg), ceftriaxone plus montelukast or gefitinib, daily for 6 days, and monitored for survival. Survivors were examined for neuronal injury, astrocyte activation and memory function using Y-maze as described below.
4.12 Nissl staining
Brain sections were defatted for 10 min in a mixture of methanol/acetone (1:1, vol), and immersed in 1% nissl stain dye for 5 s and then rinsed in dH2O, followed by a series of graded alcohols, cleared in xylene and coverslipped with a mounting medium. Images were acquired on a Zeiss LSM microscope. Nissl-positive neurons with healthy morphological characteristics of nucleus were counted in the cortex region. Data were obtained from up to five surviving animals per group, six fields per slide, the average numbers of cells in each hemisphere, in five sections from each animal were analysed for quantification, and the mean was used for the statistical analysis. Graph results are expressed as the number of Nissl bodies (Del Toro et al., 2017).
4.13 Immunohistochemistry of astrocytes
The brain sections were immunostained by first blocking non-specific binding sites with 2% IgG-free bovine serum albumin and then incubating with 0.2% Triton X-100. The sections were then incubated overnight at 4°C with a primary antibody against glial fibrillary acidic protein (GFAP 1:500, Dako, Denmark), followed by fluorescence labelled secondary antibody Alexa Fluor 647 (Invitogen). The nuclei were counterstained with VectaShield DAPI (Vector Labs), and photomicrographs of cortical and hippocampal subfields were obtained on a Zeiss fluorescence microscope, and immunofluorescent GFAP were quantified in the different region of cortex and hippocampus (Iulita et al., 2018).
4.14 Assessment of memory function using Y-maze
All mice were subjected to a two-trial test separated by a 24 hr intertrial interval to assess spatial recognition memory, as previously described (Lei et al., 2012). A Y-shaped white-painted timber with three identical arms 29.5 cm long × 7.5 cm wide × 15.5 cm high, designated start arm, novel arm, and other arm, was used. Visual cues were placed on the walls of the maze. The first trial (training) was for 10 min, and the mice were allowed to explore only two arms (starting arm and other arm). For the second trial (retention) mice were placed back in the maze in the same starting arm, and allowed to explore for 5 min with free access to all three arms. Actions were recorded on video during a 5 min trial and the Ethovision video-tracking system was used for analysis. Data are expressed as the total duration of novel arm and percentage of entries in novel arm made during the 5-min second trial. Percentage of entries in novel arm is calculated: entry of novel arm/total entry (novel+ start+ other) × 100.
4.15 Statistical analysis
Data are expressed as mean ± SEM. Differences of bacterial counts in the blood, brain and kidney between different groups of mice were determined by Wilcoxon rank sum test or Student's t test. Differences of bacterial invasion in HBMEC were determined by Student's t test. Survival curves were generated using the Kaplan–Meier method, and differences were assessed by a two-sided log-rank (Mantel-Cox) test (GraphPad software, version 6.0). One-way ANOVA followed by multiple comparison of Bonferroni's post hoc test was used to determine differences of quantification of image analysis and Y-maze data. p < .05 was considered significant.
4.16 Ethics statement
The animal protocol was approved by The Johns Hopkins Animal Care and Use Committee (Animal Welfare Assurance Number: A3272-01). All efforts were made to provide the ethical treatment and minimise suffering of mice employed in this study.
ACKNOWLEDGMENTS
We thank W She for preparation of EGFR knockout HBMEC, D Pearce for preparation of HBMEC, Y Kanaoka and KF Austen for providing CysLT1 and CysLT2 knockout animals, Dr. Douglas Tilley (Temple University) for providing the floxed EGFR mice, and National Laboratory Animal Center (Taiwan) for Tek-RFP-CreERT2 mice. This work was supported by the US National Institutes of Health (NIH) grants, NS091102, AI84984, AI113273 and AI126176 to KSK.
CONFLICT OF INTERESTS
All authors declare no competing interests. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
AUTHOR CONTRIBUTIONS
Ningyu Zhu, Wei Liu, Atish Prakash, Chengxian Zhang performed the experiments, Kwang Sik Kim conceived the project and wrote the paper, and all authors edited the manuscript.