Clinical findings in individuals with duplication of genes associated with X-linked intellectual disability
Abstract
Duplication of all genes associated with X-linked intellectual disability (XLID) have been reported but the majority of the duplications include more than one XLID gene. It is exceptional for whole XLID gene duplications to cause the same phenotype as sequence variants or deletions of the same gene. Duplication of PLP1, the gene associated with Pelizaeus-Merzbacher syndrome, is the most notable duplication of this type. More commonly, duplication of XLID genes results in very different phenotypes than sequence alterations or deletions. Duplication of MECP2 is widely recognized as a duplication of this type, but a number of others exist. The phenotypes associated with gene duplications are often milder than those caused by deletions and sequence variants. Among some duplications that are clinically significant, marked skewing of X-inactivation in female carriers has been observed. This report describes the phenotypic consequences of duplication of 22 individual XLID genes, of which 10 are described for the first time.
1 INTRODUCTION
Partial or complete duplication of one or more genes are to be expected in the genomes of all individuals. These duplications may be de novo or inherited from a parent and may exist as tandem or inverted sequences. They arise because of misalignment of alleles during meiosis or mitotic divisions.1 Repeat sequences in intergenic regions of the genome predispose to misalignment during cell division and depending on the timing of the event may lead to constitutional or somatic duplications. The duplicated regions may be in tandem, inverted or even inserted a distance away from their origin.
To date, 164 X-linked genes have been associated with intellectual disability.2 Deletions and sequence alterations in these genes produce a wide spectrum of phenotypes from intellectual disability without other distinguishing manifestations (nonsyndromal XLID, IDX) to intellectual disability accompanied by characteristic craniofacial features, structural anomalies, metabolic disturbances, neuromuscular findings or behavioral abnormalities (X-linked intellectual disability (XLID) syndromes). Complete duplications of all genes associated with XLID have now been identified.3, 4 Partial duplications of these genes may interrupt their reading frames and may cause phenotypes similar to those caused by deletions or sequence variants. With few exceptions, complete XLID gene duplications result in phenotypes quite different from that caused by deletions or sequence variants. Among some clinically important duplications, marked skewing of X-inactivation in female carriers has been observed.5
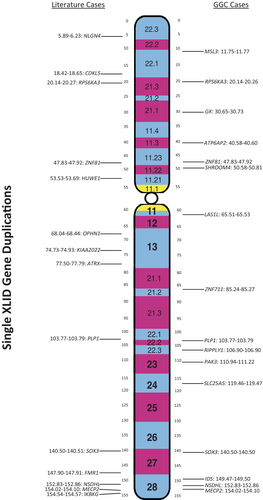
In the decade since microarray technology has become readily available for diagnostic purposes, hundreds of different segmental duplications of the X chromosome have been reported. In most instances, the duplications involve more than one gene. The literature contains reports of the duplication of 14 XLID genes without extension of the duplication into adjacent XLID genes (Figure 1, Tables 1–3). During the same period (2010–2022), 516 segmental duplications of the X chromosome were detected among the 18 664 microarray analyses conducted at the Greenwood Genetic Center (GGC). Two hundred sixteen (216) of the duplications overlapped only one XLID gene. Among those are duplications of 16 individual XLID genes, 10 of which are described herein for the first time. Individual gene duplication of RPS6KA3, ZNF81, PLP1, SOX3, NSDHL and MECP2 were found in the literature and in the GGC cohort.
| Gene | XLID syndrome | X-chromosome band | Male/female affected | Phenotype of duplication |
|---|---|---|---|---|
| OPHN1 | IDX60 and XLID-cerebellar dysgenesis | q13 | One male in literature | Severe intellectual disability, seizures, truncal hypotonia, hyperreflexia, join laxity, generalized undergrowth, and facial dysmorphism (sloped forehead, dense eyebrows, narrow nose, thin upper lip, and malformed ears). Lacks the cerebellar hypoplasia that occurs in XLID-cerebellar dysgenesis |
| ZNF711 | IDX40, IDX65, IDX97 | q21.1 | One male in GGC cohort | Normal developmental milestones but microcephaly, ADHD, seizures, inguinal hernia, balance difficulty and clumsiness in adulthood. The single case appears more severely affected than those with sequence alterations. |
| SOX3 | XLID-panhypopituitarism and XLID-growth hormone deficiency | q26 | Males and females in literature and GGC cohort | Developmental delay, intellectual disability, growth impairment, disorders of sexual development, other endocrine dysfunction and lumbosacral spina bifida, the last of which has not occurred with sequence changes or deletions of SOX3. |
| Gene | XLID syndrome | X-chromosome band | Male/female affected | Phenotype of duplication |
|---|---|---|---|---|
| GK | Glycerol kinase deficiency | p21.1 | One female in GGC cohort | Normal growth and development but ADHD, anxiety, and autoimmune manifestations. |
| ZNF81 | IDX45 | p11.23 | One male in literature and one female in GGC cohort | Developmental delay, variable growth and different craniofacial dysmorphism than described in IDX45. |
| KIAA2022 | IDX98, Cantagrel spastic paraplegia | q13 | Two brothers in literature | Duplications have similar phenotype to gene mutations with intellectual disability and autism without growth abnormalities or craniofacial dysmorphic features. |
| PLP1 | Pelizaeus-Merzbacher | q21 | Males and females in literature and GGC cohort | Same as Pelizaeus-Merzbacher. |
| Gene | XLID syndrome | X-chromosome band | Male/female affected | Phenotype of duplication |
|---|---|---|---|---|
| MSL3 | Basilicata-Akhtar | p22.2 | One male in GGC cohort | Typical early development and growth, regression resulting in ASD, poor speech development. Lacks global developmental delay, ataxia, and facial dysmorphism of Basilicata-Akhtar syndrome. |
| CDKL5 | Rett-like seizures-hypotonia | p22.1 | Males and females in literature | Developmental delay with language impairment, autism, ADHD. Lacks the normal early development followed by regression, acquired microcephaly, and seizures of Rett syndrome. |
| RPS6KA3 | Coffin-Lowry | p21.3 | Males and females in literature and GGC cohort | Mild intellectual disability, ADHD, seizures. Lacks craniofacial features typical of Coffin-Lowry syndrome. |
| ATP6AP2 | XLID-epilepsy | p11.3 | Three males in GGC cohort | Variable presentation with mild intellectual disability, large head, absence of other craniofacial anomalies, and autism mannerisms. Lacks early onset seizures of Hedera type of XLID. |
| SHROOM4 | Stocco dos Santos | p11.22 | One male in GGC cohort | Delayed development, hypotonia and autism. Lacks the craniofacial dysmorphism, skeletal anomalies, recurrent infections and severe intellectual impairment of Stocco dos Santos syndrome. |
| HUWE1 | Juberg-Marsidi-Brooks, Turner XLID-macrocephaly | p11.21 | Males in literature | Normal growth, mild-moderate intellectual disability, limited speech or dysarthria, variable facial dysmorphism with or without seizures. Lacks the prenatal and postnatal growth failure, microcephaly, blepharophimosis and contractures of Juberg-Marsidi-Brooks syndrome, and all but one lacks the macrocephaly of Turner XLID-macrocephaly syndrome. |
| LAS1L | Wilson-Turner | q11.1 | One male in GGC cohort | Developmental delay and autism. Lacks the somatic features (hypogonadism with gynecomastia, obesity and small feet) of Wilson-Turner syndrome. |
| ATRX | ATR-X | q13.3 | One male fetus in literature | Normal growth, hypertelorism, micrognathia, genital underdevelopment and undescended testes. Lacks the distinctive facial features of ATR-X syndrome. |
| RIPPLY1 | Ataxia-seizures-hearing loss | q22.3 | One male in GGC cohort | Dysmorphic craniofacial features (prominent metopic suture, sloped forehead, upslanted palpebral fissures, flat midface, short philtrum, long nose), clumsiness and possible seizures. Lacks hearing loss of sequence alterations in RIPPLY1. |
| SLC25A5 | IDX70 | q24 | One male in GGC cohort | Overgrowth, malar flatness, full lips, gynecomastia, mild ID and psychosis. Lacks the cataracts and epilepsy of sequence alteration in SLC25A5. |
| FMR1 | Fragile X | q27.3 | Two females in literature | Normal or excessive growth, developmental delay, variable facial features. Lacks the severe intellectual disability of Fragile X syndrome. |
| IDS | MPSII (Hunter) | q27 | One male in GGC cohort | Autism spectrum disorder. Lacks the somatic and biochemical findings of lysosomal storage disease. |
| NSDHL | CK | q28 | One male in literature | Normal postnatal growth, thick droopy eyelids, hypotonia, poor motor coordination, ADHD, and autism spectrum disorder. Lacks the severe ID, microcephaly, craniofacial dysmorphism, seizures, and neuronal migration disturbances of CK syndrome. |
| MECP2 | Rett | q28 | Several males in literature and one female in GGC cohort | Severe intellectual disabilities, seizures, hypotonia from birth progressing to spasticity, limited speech and ambulation, recurrent respiratory tract infections. Lacks the period of normal development followed by episodic course of neurological deterioration and loss of purposeful hand use of Rett syndrome. |
| IKBKG | Incontinentia pigmenti | q28 | One female in literature | Normal development and intelligence but abnormalities of hair growth and thin pigmentation, neuromuscular manifestations including peripheral neuropathy and seizures, and vascular malformations. Lacks the triphasic cutaneous lesions of infancy typical of Incontinentia pigmenti. |
2 SUBJECTS AND METHODS
The literature from 2010 through 2022 was surveyed for duplications of the X chromosome that included an XLID gene. Those duplications that included only a single XLID gene were selected for further evaluation and inclusion in this survey. A parallel search was made for segmental X chromosome duplications identified using microarray technologies at the GGC in the years 2010–2022. During this period, seven different microarray platforms were used at GGC, which included Genome-Wide Human SNP Array 6.0., CytoScan HD Microarray, CytoScan Dx Array, OncoScan CNV Assays, CytoScan XON Array (ThermoFisher Scientific, USA), Agilent Custom Array (Agilent Technologies, CA, USA), and OGT HRX (OGT, NY, USA). The duplications found at the GGC that included only a single XLID gene without extension into adjacent XLID genes were selected for comparison with cases from the literature and inclusion in this survey.
3 DUPLICATIONS OF XLID GENES
Individual XLID gene duplications may result in phenotypes of greater severity (Table 1), of similar severity (Table 2), and of lesser severity (Table 3) than those caused by deletions or sequence variants. Notably, the haploinsufficiency scores are well curated and are useful while interpreting deletions or sequence variants, but the triplosensitivity scores for all genes reported in this report are zero except for PLP1 (score of 3; triplosensitive), primarily due to the lack of reports with individual XLID gene duplications (Clinical Genome Resource, https://www.clinicalgenome.org/, date accessed September 19, 2023).
Individual XLID gene duplications in the literature and the GGC cohort are identified in Figure 1. Twenty two of the individual gene duplications are discussed below in order from Xpter to Xqter. Two gene duplications, a PAK3 duplication in the GGC cohort and an NLGN4 duplication from the literature, are not included because insufficient clinical information is available.6 Duplications of certain XLID-associated genes (GK, DMD, ARX, and IKBKG) and certain X chromosome regions (Xp21.33 and Xq21.33) do not appear to be associated with neurodevelopmental abnormalities although they may be associated with other somatic manifestations.7-9
3.1 MSL3 (Xp22.2)
Basilicata-Akhtar syndrome due to pathogenic variants in the MSL3 gene are characterized by hypotonia and feeding difficulties in early infancy, global developmental delay with poor or absent speech and progressive spasticity or ataxic gait, resulting in the inability to walk. Typical facial features include telecanthus, epicanthal folds, down-slanting palpebral fissures, downturned corners of the mouth, and dysplastic ears. Males and females appear equally affected.10
One male in the GGC cohort with a de novo duplication of MSL3. The birth weight at 38 weeks gestation was 2.3 kg. At age 3 years, the height was 91.8 cm (3rd centile), weight 14.3 kg (10th centile), and head circumference 50 cm (50th centile). Developmental milestones were normal until 24 months when regression occurred with loss of speech resulting in a diagnosis of autism spectrum disorder. By age 4 years, he had regained only five words (Table 3).
3.2 CDKL5 (Xp22.1)
The phenotype of males and females with CDKL5 sequence variants is characterized by early onset and intractable seizures, developmental regression, hypotonia, and postnatal slowing of head growth, a phenotype that is considered Rett syndrome-like.11
Szafranski et al.12 have provided detailed phenotypic descriptions on three unrelated males and one female with isolated whole gene duplication of CDKL5 (Table 3). The duplications ranged in size from 540 to 935 kilobases. All patients, aged 6–14 years had developmental delay with speech/language impairments. Two males had autism spectrum disorder, ADHD, and obsessive-compulsive behavior. Developmental regression, sleep difficulties, and resistance to pain were noted in individual patients. The female described had generalized overgrowth and one male had macrocephaly, tall stature, and clinical manifestations of Marfan syndrome. Facial dysmorphism was inconsistent, one male having frontal prominence, deep-set eyes and thin upper lip, and another having malar hypoplasia and facial asymmetry. A fifth patient (individual 4160 from DECIPHER) had delay of development, intellectual disability, wide nasal root, high palate, hyperopia and hypotonia.13
3.3 RPS6KA3 (Xp21.3)
Coffin-Lowry syndrome due to sequence alterations in RPS6KA3 exhibits one of the more distinctive craniofacial and musculoskeletal phenotypes in males and a milder phenotype in females.14 In their early years, affected males have prominent forehead, hypertelorism, anteverted nares, thin upper lip, high narrow palate, hypodontia and peg-shaped incisors. The face elongates and coarsens with notably large lips by adulthood. The hands are large and soft with distal tapering of the digits. Joint hyperextensibility accompanies generalized hypotonia. Growth is slow with postnatal microcephaly and short stature. Drop attacks occur in one fourth of individuals. Developmental delay becomes obvious from infancy and ultimately results in severe intellectual disability. Females have a milder phenotype characterized by short stature, coarse face, prominent lips, soft fleshy hands, and normal or mildly impaired cognitive function.
Duplications of RPS6KA3 have been identified in males and females in the literature and in the GGC cohort. They cause a phenotype with normal growth, mild to moderate impairment of cognitive function, speech impairment, attention deficit and hyperactivity (Table 3). They lack the typical craniofacial and musculoskeletal phenotype of Coffin-Lowry syndrome.15
3.4 GK (Xp21.1)
Glycerol kinase deficiency usually occurs as part of a contiguous gene deletion involving GTD, AHC, GK, DMD, and OTC.16 Alterations in GK alone may be asymptomatic or cause recurring episodes of hypothermia, lethargy, vomiting and acidemia.17
One female in the GGC cohort with duplication of GK had childhood onset of rheumatoid arthritis and IgA deficiency. Her birth weight was 3.6 kg and postnatal growth parameter was at or above the 80th centile (height 168 cm, weight 64 kg, head circumference 56.5 cm) at age 15 10/12 years. She developed ADHD and anxiety but no history of developmental delay or cognitive impairment (Table 2). The duplication was inherited from the mother and both mother and daughter had random X-inactivation (52:48 and 59:41, respectively).
3.5 ATP6AP2 (Xp11.3)
Pathogenic variants in the ATP6AP2 gene are associated with syndromic XLID with epilepsy in males.18 Seizures typically present in the first 18 months of life and may be tonic–clonic, atonic, and myoclonic. Affected males have mild intellectual disability. Three unrelated families have been reported, all with hemizygous variants affecting ATP6AP2 gene splicing.
Duplication of ATP6AP2 has been identified in three individuals at GGC. One male presented with speech delay and shyness. His birth weight at 31 weeks gestation was 1.2 kg and at 2 ½ years, the head circumference was 48.5 cm (45th centile). The craniofacial examination was normal (Table 3). The duplication was also present in unaffected brother. X-inactivation in the carrier mother was random (53:47).
The second duplication was found in a young adult man with a history of childhood developmental delay and hypotonia. His birth weight at term gestation was 3.5 kg. At age 20½ years, his height was 179 cm (70th centile), weight 113 kg (>97th centile) and head circumference 59.5 cm (>97th centile). He had large hands and feet, anxiety, ADHD, poor balance, stiff gait, and mild intellectual disability. X-inactivation in the carrier mother was random (69:31).
The third duplication was identified in a young boy with developmental delay and autistic features. His birth weight was 2.9 kg at 39 weeks gestation and his height was 90.8 cm (75th centile), weight 13.25 kg (55th centile) and head circumference 51 cm (90th centile) at age 27 months. The duplication was present in his mother.
3.6 ZNF81 (Xp11.23)
A missense alteration in ZNF81 has been reported in IDX45.19 Affected members of this six-generation pedigree had nonprogressive intellectual disability of varying severity, large hands, large ears, and normal behavior. Some affected males had short stature, high nasal bridge, full lips, high arched palate and large testes. The pathogenicity of the gene alteration has been questioned by Piton et al.20
A duplication of ZNF81 and two nonXLID genes was reported by Alesi et al.21 They described a three-year-old male with global developmental delay (walked at 27 months, few single words at 36 months) and all growth parameters less than the 3rd centile. He developed autism spectrum disorder and had delayed adaptive and cognitive skills. Additional features noted included downslanted palpebral fissures, myopia, epicanthus, everted lower eyelids, everted lower lip, micrognathia, midface hypoplasia, hypotonia, and excessive laxity of joints (Table 2).
A female with duplication of ZNF81 in the GGC cohort had developmental delay. The birth weight was 3.3 kg and at age 6 years the height was 125.4 cm (97th centile) and weight 27.4 kg (95th centile). The physical examination showed no dysmorphic features (Table 2). An older brother with the X chromosome duplication and a second duplication (17q21.31) required special education in kindergarten. The duplication was inherited from an unaffected mother.
3.7 SHROOM4 (Xp11.22)
Stocco dos Santos syndrome caused by sequence alteration in SHROOM4 (KIAA1202) is characterized by severe intellectual disability, skeletal abnormalities (short stature, kyphosis, hip dislocation), hirsutism, precocious puberty and recurrent respiratory infections. Facial dysmorphology may include heavy eyebrows, epicanthus, hypertelorism, short palpebral fissures, short philtrum, thin upper lip, and prominent nasal tip.22
One male in the GGC cohort with a maternally inherited duplication of SHROOM4 had global developmental delay (walked at 16 months, first words at 29 months). His birth weight was 2.3 kg at 35 weeks gestation and at 4½ years the height was 107.3 cm (60th centile), weight 21.4 kg (90th centile) and head circumference 53 cm (90-97th centile). He was diagnosed with autism spectrum disorder and had low muscle tone and normal craniofacial appearance. His autistic manifestations showed improvement by school age with therapies (Table 3). X-inactivation in the mother was random (60:40).
3.8 HUWE1 (Xp11.21)
Pathogenic variants in HUWE1, an E3 ubiquitin kinase gene, are associated with Juberg-Marsidi-Brooks and Turner XLID-macrocephaly syndromes.23, 24 The two disorders have little in common. Juberg-Marsidi-Brooks syndrome is characterized by severe intellectual difficulty, short stature, microcephaly, facial dysmorphism, (bifrontal narrowing, blepharophimosis, cupped ears, bulbous nose, and small mouth), contractures, seizures, poor vision, deafness and hypotonia which progresses to spasticity. In contrast, the Turner XLID-macrocephaly syndrome is characterized by mild to moderate intellectual disability, macrocephaly, downslanting palpebral fissures, elbow limitation and obesity.
Duplications of HUWE1 have been reported in 14 males in 6 families by Froyen et al.24 and one male by Orivoli et al.25 All had mild to moderate intellectual disability and were considered to have nonsyndromic XLID although several affected males had visible facial dysmorphism, gastrointestinal and genitourinary symptoms, ADHD or behavioral manifestations and abnormal encephalograms or seizures. None had the growth abnormalities or craniofacial features of Juberg-Marsidi-Brooks syndrome and only one had the macrocephaly of Turner XLID-macrocephaly syndrome (Table 3). Carrier females were not affected and X-I was markedly skewed (98:2) in the one carrier female tested.
3.9 LAS1L (Xq11.1)
The XLID disorder with global developmental delay during childhood, hypotonia, gynecomastia, hypoplastic genitalia, obesity, small feet and emotional lability has been termed Wilson-Turner syndrome.26 The causative gene LAS1L encodes a nuclear protein subunit that processes preRNAs of ribosomes.27
One male in the GGC cohort had a maternally inherited duplication of LAS1L. His birth weight at 33 weeks gestation was 3 kg and at age 4 years the height was 103 cm (50th centile), weight 15.8 kg (75th centile) and head circumference 49.5 cm (20th centile). He had a history of developmental delay and autism but did not exhibit the somatic manifestations typical of Wilson-Turner syndrome (Table 3).
3.10 OPHN1 (Xq13)
Pathogenic variants in OPHN1 are associated with nonsyndromic XLID (IDX60) and with XLID-cerebellar dysgenesis syndrome.28
Duplication of OPHN1 has been reported in a male in the literature.29 He had severe intellectual function, seizures, short stature, microcephaly, dense eyebrows, telecanthus, downslanting palpebral fissures, deep set eyes, narrow nose with broad tip, long philtrum, thin upper lip, a single lower incisor receding chin, malformed ears, joint laxity, truncal hypotonia with scoliosis, leg length asymmetry, muscle hypoplasia and hyperreflexia. There were no abnormalities of the cerebellum (Table 1). The carrier mother had marked skewing of X-inactivation.
3.11 KIAA2022 (Xq13)
Loss of function alterations in KIAA2022 (NEXMIF) are associated with intellectual disability in males and females with significant intrafamilial variability.30, 31 In some families males were severely affected but females appeared nonsymptomatic. In other females with de novo alterations in KIAA2022, intellectual disability was present, in some cases severe and accompanied by intractable seizures.
A single report of KIAA2022 duplication in brothers with intellectual disability and autism suggests that duplication of the gene may cause an identical or similar XLID disorder as sequence variants in the gene.32 In contrast to the expected increase in gene expression, KIAA2022 expression in lymphocytes and fibroblasts was reduced.
3.12 ATRX (Xq13.3)
Sequence alterations in ATRX have been found in alpha thalassemia-intellectual disability, Chudley-Lowry, Carpenter-Waziri, Holmes-Gang, XLID-hypotonic facies, XLID-arch fingerprints-hypotonia and cerebrofaciogenital syndromes. Although considerable phenotypic variability exists among individuals with sequence alterations in ATRX, all have intellectual disability and most have growth impairment, craniofacial manifestations, and genitourinary abnormalities.33
Chen et al.34 reported a fetus with a 1.5 Mb duplication of Xq13.3-q21.1. ATRX was completely duplicated as were other genes not known to be associated with XLID. Extension proximally into the coding region of MAGT1 cannot be excluded. The aborted fetus had normal growth, epicanthal folds, hypertelorism, midface hypoplasia, micrognathia, hypoplastic male genitalia and clinodactyly. The mother and maternal grandmother carried the duplication (Table 3). Gene expression and X-inactivation were not reported.
Other males with duplications of ATRX have been reported but all include other XLID genes.35, 36 The phenotype of individuals with these larger duplications includes intellectual disability, growth impairment, genital anomalies, hypotonia and minor skeletal abnormalities, but lacks the distinctive craniofacial phenotype of ATRX syndrome.
3.13 ZNF711 (Xq21.1)
Sequence alterations in ZNF711 have been identified in a number of families with nonsyndromic XLID, including IDX40, IDX65, and IDX97.37 The intellectual disability is typically mild and coexists with autism spectrum disorder in half of cases.
A single male in the GGC cohort had duplication of ZNF711. The birth weight at 34 weeks was 1.6 kg. At 9 years, the height was 133 cm (35th centile), weight 29.5 cm (50th centile) and head circumference 49.5 cm (<3rd centile). His phenotype was characterized by postnatal microcephaly, strabismus, wide mouth, inguinal hernia, undescended testes, and seizures. Early developmental milestones were normal but he had ADHD, balance difficulty and clumsiness in mid childhood (Table 1). Both parents had learning difficulties and the carrier mother's X-inactivation was random (52:48).
3.14 PLP1 (Xq21)
Sequence variants in the coding or splice site regions of PLP1 are found in less than half of families with Pelizaeus-Merzbacher syndrome. More commonly, the condition is caused by duplications of the entire gene.38 The severity of features typical of this disorder—nystagmus, initial hypotonia progressing to spastic paraplegia, ataxia, tremors, dystonia and other abnormal movements of the limbs, white matter dysmyelination, and basal ganglia deposits—does not appear to be related to the size of the duplications (Table 2).
3.15 RIPPLY1 (Xq22.3)
A frameshift alteration due to a one base deletion in exon 3 of RIPPLY1 has been reported in 2 males with severe intellectual disability with seizures, ataxia, and hearing loss.39
A single male in the GGC cohort had a maternally inherited duplication of RIPPLY1. He had delayed development with walking at 18 months and absence of speech at age 7 years. The birth weight at term was 3 kg. At age 6 9/12 years, the height was 129 cm (95th centile), weight 26.8 kg (85th centile) and head circumference 50.2 cm (10th centile). He had hypotonic face, prominent metopic suture, upslanted palpebral fissures, flat midface, short philtrum and long nose. He was clumsy and had episodes with excessive laughing followed by weakness and wandering of the eyes which were considered to possibly be seizures but no evidence of hearing loss (Table 3).
3.16 SLC25A5 (Xq24)
Deletion of SLC25A5 gene has been reported in 3 unrelated families with non-syndromic XLID in males.40 In the first family, three affected male patients were reported with moderate intellectual impairment and one potential female carrier with borderline intellectual function. In the second family, a single male was reported with borderline ID (IQ of 74), behavioral and sociability disorders at age 10. He had congenital cataract and mild facial dysmorphism including upslanting palpebral fissures and a broad forehead. The third case was a male with intellectual disability, global developmental delay, myoclonic epilepsy, and congenital cataract.
One male in the GGC cohort had a duplication of SLC25A5. The birth weight was 3.2 kg. In adult life all growth percentages were above the 97th centile (height 160 cm, weight 160 kg, and head circumference 60 cm). As a child he had speech delay and one possible seizure. In addition to global overgrowth, he had malar flatness, full lips and gynecomastia. He had mild intellectual disability, bipolar disorder, and paranoid schizophrenia (Table 3).
3.17 SOX3 (Xq26)
Duplications of SOX3 as well as pathogenic variants that reduce gene dosage have been associated with intellectual deficit, isolated growth hormone deficiency or panhypopituitarism, short stature, gynecomastia, and incomplete virilization in males. Lumbosacral spina bifida has occurred in males and females with SOX3 duplications, but has not occurred with sequence alterations.41
3.18 FMR1 (Xq27.3)
Fragile X syndrome is a predominantly male disorder characterized by large ears, prominent jaw, macro-orchidism, and intellectual disability that usually progresses to severe impairment.42 In most cases, the disorder is caused by an expansion of a CGG repeat in the 5′ UTR of FMR1. Autism cooccurs frequently.
Only two females with duplication of FMR1 without extension to other XLID genes have been reported.43 One girl had normal prenatal and postnatal growth but speech delay with first words not spoken until age 2 years. She had upslanting palpebral fissures, epicanthal folds, and a high arched palate. Temper tantrums were the only behavioral symptom. The second girl said first words by 20 months and walked by 18 months. Her height and weight were above the 95th centile. She had no craniofacial dysmorphisms but had duplication of the right ureter with hydronephrosis, two-three toe syndactyly, and a mild Chiari 1 malformation (Table 3).
The FMR1 duplications in males have all extended into adjacent XLID genes.44, 45 The phenotype includes intellectual disability, short stature, and hypogonadism (small testes, gynecomastia, obesity, scant body hair, low testosterone level, and elevated gonadotrophins).
3.19 IDS (Xq27)
Hunter syndrome (mucopolysaccharidosis II) has the typical somatic manifestations of a lysosomal storage disorder: global early overgrowth followed by failure of statural growth, coarse facial features with thickening of nostrils, lips and tongue, short neck, hepatosplenomegaly, hernias, joint limitation, thick skin with nodular lesions on arms and back, papilledema and hearing loss.46 At one end of the phenotypic spectrum, MPSIIA has severe intellectual disability. At the other extreme, MPSIIB, cognitive function is normal or only slightly disturbed.
While no duplication of IDS alone has been identified, a duplication of IDS and adjacent genes not associated with XLID was found in a male in the GGC cohort. At 2 weeks past term the birth weight was 3.9 kg. At age 7 9/12 years, the height was 122 cm (25th centile), weight 31.4 kg (75th– 90th centile) and head circumference 52.2 cm (50th centile). He had autism spectrum disorder but none of the somatic or biochemical findings of lysosomal storage disease (Table 3). The mother and one unaffected brother carried the duplication.
3.20 NSDHL (Xq28)
Severe intellectual disability, behavioral disturbances, distinctive craniofacial and musculoskeletal manifestations, and neuronal migration abnormalities, termed CK syndrome, have been associated with alterations in NSDHL, a gene that functions in the cholesterol biosynthesis pathway.47
A duplication in NSDHL has been reported in a male with low birth weight, normal postnatal growth, thick droopy eyelids, hypotonia, poor motor coordination, ADHD, and autism spectrum disorder.48 Intellect was normal. NSDHL RNA was threefold elevated and serum free fatty acids were elevated. The carrier mother's X-inactivation was random.
3.21 MECP2 (Xq28)
Rett syndrome due to deletion or sequence variants in MECP2 is characterized by a period of normal development in infancy followed by microcephaly and an episodic but unrelenting course of neurological deterioration, loss of purposeful hand use, seizures, and spasticity.49
One female in the GGC cohort had a duplication of MECP2. In adult life she had normal head circumference (55.5 cm, 75th centile), severe intellectual disability (IQ 47), seizures, fifth finger clinodactyly, gastroesophageal reflux, osteoporosis, normal muscle tone, and diminished reflexes in the lower limbs. She had a pleasant demeanor and conversational speech.
The literature contains several males with duplications of MECP2 that did not extend distally into FLNA or proximally to L1CAM.50 The phenotype of affected males appeared as severe as in affected males with larger duplications. Intellect, speech, and ambulation are severely impaired. Axial hypotonia, lower limb hyperreflexia, ataxia and recurrent infections are common and seizures occur in some patients.
3.22 IKBKG (Xq28)
Incontinentia pigmenti is one of the XLID disorders that occur primarily in females. In addition to the triphasic cutaneous manifestations (initially vesicular lesions, replaced by verrucous nodules which are then transformed into areas of irregular hyperpigmentation), the majority of cases will have ocular (microphthalmia, nystagmus, cataracts, and optic atrophy) and dental (oligodontia, abnormally shaped teeth, and delayed eruption) abnormalities. A minority, about 10%, have intellectual disability.
Duplication of the IKBKG gene and several other genes in Xq28 that do not cause XLID, was reported by van Asbeck et al.7 The female patient had normal developmental milestones and intelligence but developed numerous medical problems including joint hypermobility and dislocations, recurrent respiratory and urinary infections, easy bruising, cutaneous manifestations and absence seizures (Table 3).
4 DISCUSSION
Duplication of 12 XLID genes (MSL3, ATP6AP2, SHROOM4, LAS1L, OPHN1, KIAA2022, ATRX, ZNF711, RIPPLY1, SLC25A5, IDS, and NSDHL) have been reported/identified only in affected males, seven genes (CDKL5, PLP1, SOX3, MECP2, ZNF81, RPS6KA3, and HUWE1) in both affected males and females, and three (GK, IKBKG, and FMR1) only in affected females (Table 4). The lower incidence of XLID disorders in females is attributed to the skewed X-inactivation, reflecting selective silencing of the allele carrying the alteration.
| Gene | Genomic coordinates (Reference assembly) | Affected individual | Proband XI | Inheritance | Carrier mother's XI | Additional testing (Normal) |
|---|---|---|---|---|---|---|
| MSL3 | 11571933_11900033 (hg19)a | M | (−) | De novo | Na | FMR1, WES |
| RPS6KA3 | 19,937,032-20,732,975 (hg19)b | M | (−) | Na | Na | Subtelomeric MLPA, KT, FMR1 |
| RPS6KA3 | 20016145_20379599 (hg19)a | M | (−) | Maternal | Na | None |
| GK | 30,454,948-30,832,466 (hg18)c | F | Randomly Skewed | Maternal | Randomly Skewed | FISH, KT |
| ATP6AP2 | 40,262,370-40,374,311 (hg18)c | M | (−) | Maternal | Randomly Skewed | FMR1, FISH, KT |
| ATP6AP2 | 40,256,714-40,375,912 (hg18)c | M | (−) | Maternal | Randomly Skewed | Metabolic screen, FMR1, PW/AS testing |
| ATP6AP2 | 40,223,653-40,514,540 (hg19)b | M | (−) | Maternal | Na | FMR1 |
| ZNF81 | 47,658,045-48,046,050 (hg19)b | F | Na | Maternal | Na | None |
| SHROOM4 | 50,102,932-50,847,174 (hg18)c | M | (−) | Maternal | Randomly Skewed | FMR1, FISH, KT, Biochemical testing |
| LAS1L | 64,325,706-64,772,747 (hg19)b | M | (−) | Maternal | Na | FMR1, KT, Syndromic Autism Panel Sequencing |
| ZNF711 | 84,000,996-84,536,911 (hg19)a | M | (−) | Maternal | Randomly Skewed | FISH, KT |
| PLP1 | 102,983,229-103,305,345 (hg19)a | F | Randomly Skewed | Na | Na | KT |
| RIPPLY1 | 106,009,206-106,585,814 (hg19)a | M | (−) | Maternal | Na | Metabolic screen, FMR1, PW/AS testing |
| PAK3* | 109,744,598-110,363,085 (hg18)c | F | Randomly Skewed | Maternal | Moderately Skewed | None |
| SLC25A5 | 118,377,107-118,557,209 (hg18)c | M | (−) | Na | Na | Subtelomeric MLPA, KT, FMR1, PTEN gene sequencing |
| SOX3 | 139,137,983-139,570,778 (hg18)c | M | (−) | Maternal | Moderately Skewed | FISH, KT |
| SOX3 | 139,014,903-139,527,313 (hg18)c | F | Randomly Skewed | Na | Na | KT |
| IDS | 148,368,197-148,500,983 (hg18)d | M | (−) | Maternal | Na | Biochemical testing†, KT, FMR1, MLPA |
| NSDHL | 151981360_152112980 (hg19)a | F | Na | Na | Na | KT |
| NSDHL | 151935518_152329158 (hg19)a | F | Na | De novo | Na | KT |
| MECP2 | 153253477_153438781 (hg19)a | F | Na | Na | Na | FMR1, KT |
- Note: *Female with PAK3 duplication not described because of insufficient clinical information. †Iduronate-2-sulfatase activity slightly increased: 1081.5 nmol/4 h/mL plasma (normal 194–950 nmol/4 h/mL plasma); a: CytoScan HD Microarray; b: CytoScan Dx Assay; c: Genome-wide Human SNP 6.0 Array; d: OGT HRX.
- Abbreviations: FISH, fluorescence in situ hybridization; FMR1, FMR1 repeat analysis; KT, karyotyping; MLPA, multiplex ligation-dependent probe amplification; na, not available; PW/AS testing, Prader-Willi/Angelman syndrome methylation specific MLPA assay; WES, whole exome sequencing; (−), not applicable.
Duplications of IKBKG responsible for Incontinentia pigmenti and GK responsible for glycerol kinase deficiency have not been associated with neurodevelopmental phenotypes but may cause other phenotypes. Duplication of MECP2 with or without adjacent genes is more commonly reported than any of the other segmental duplications and has been reported in males and females.
The presence of neurobehavioral signs in a male with an XLID gene duplication and not in a brother with the same duplication (e.g., ATP6AP2 and IDS) calls into question the pathogenicity of the duplication or its penetrance and expressivity. Among the seven carrier mothers in the GGC cohort five had random X-I and two had moderately skewed X-I (Table 4). This may call into question the pathogenicity of the duplications in these cases or alternatively question the dictum that carrier mothers of pathogenic duplication will have marked skewing of X-I.5
Most reports of duplications of individual XLID genes in the literature do not include expression studies. This deficiency applies to all duplications in the GGC cohort. As most individual XLID gene duplications have been identified in only one or two cases, caution must be used in determining the phenotypic consequences until additional cases are reported.
AUTHOR CONTRIBUTIONS
Barbara R. DuPont, Alka Chaubey, and Roger E. Stevenson designed this study. Nikhil Sahajpal and Roger E. Stevenson selected the cases for inclusion. Nikhil Sahajpal, Barbara R. DuPont, Alka Chaubey, and Fatima Abidi conducted laboratory testing. Nikhil Sahajpal, Catherine Ziats, Charles E. Schwartz, and Roger E. Stevenson wrote the article; all authors reviewed and approved the article.
ACKNOWLEDGMENTS
The authors thank cytogenetics/cytogenomic technologists at the Greenwood Genetic Center for identifying duplications of XLID genes and to Anna Crockett for administrative assistance.
FUNDING INFORMATION
Partial funding was provided by the Greenwood Genetic Center Foundation and the South Carolina Department of Disabilities and Special Needs.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ETHICS STATEMENT
Ethical approval for the diagnostic and research testing used in this article was obtained from the IRB of Self Regional Healthcare, IRB number Pro00085001 “Consent for Participation in Clinical and Laboratory Studies to Determine the Causes of Disabilities and Birth Defects.” Approval for the use of deidentified clinical findings was obtained from Self Regional Healthcare, IRB number Pro00128855.
Open Research
DATA AVAILABILITY STATEMENT
The clinical and laboratory data utilized in this study are provided in the case reports.




