The third case of Marbach-Rustad progeroid syndrome caused by a de novo LEMD2 variant
Zhikun Lu and Wen Zhang have equal contribution.
Abstract
Marbach-Rustad progeroid syndrome is an extremely rare disease caused by a heterozygous variant in the LEMD2 gene. To date, only two patients and one LEMD2 pathogenic variant have been reported in Marbach-Rustad progeroid syndrome. Here we describe the third case of Marbach-Rustad progeroid syndrome worldwide, which is also the first case in China. The proband was affected with premature birth, failed to thrive, facial abnormalities, feeding difficulties, skull defects and delayed motor milestones, but had a normal intelligence and speech. Whole exome sequencing (WES) initially did not find a phenotype-causing variant when the proband was 1 year of age. The reanalysis of WES data 4 years later revealed the proband harbored a de novo heterozygous c.1436C>T(p.Ser479Phe) variant in the LEMD2 gene, which is known responsible for Marbach-Rustad progeroid syndrome. Sanger sequencing confirmed the presence of this variant in the proband and absence in his parents and two elder sisters. Our study provides accurate clinical diagnosis for the proband and adds a new patient with Marbach-Rustad progeroid syndrome. Our study suggests the LEMD2 c.1436C>T(p.Ser479Phe) variant as a hotspot. Our work also indicates reanalysis of WES data of negative cases might identify pathogenic variant and improve diagnostic efficiency.
Nuclear envelopathies is a set of rare genetic disorders with progeria, myopathy or cardiomyopathy phenotypes caused by variants in nuclear envelope protein-coding genes, including LMNA (OMIM * 150330), LMNB1 (OMIM * 150340), LMNB2 (OMIM * 150341), LBR (OMIM * 600024), BANF1 (OMIM * 603811), LEMD3 (OMIM * 166700), ZMPSTE24 (OMIM * 606480), SYNE1 (OMIM * 608441), SYNE2 (OMIM * 608442), EMD (OMIM * 300384), TOR1A (OMIM * 605204), TOR1AIP1 (OMIM * 614512), LEMD2 (OMIM * 616312), and TMEM 43 (OMIM * 612048).1-5
LEMD2 gene coding an A-type-lamin-associated inner nuclear membrane protein—LEM domain-containing protein 2 is involved in nuclear structure organization and required for cell differentiation.6, 7 It was first associated with a human disease—autosomal recessive Hutterite-type juvenile cataract, also known as Cataract 46, juvenile-onset (OMIM # 212500), in 2015.8 In 2019, this gene was implicated in a more complex syndromic phenotype—Marbach-Rustad progeroid syndrome (OMIM # 619322) with a novel autosomal dominant pattern.4
Marbach-Rustad progeroid syndrome is an extremely rare disease with only two patients and one pathogenic variant in the LEMD2 gene (NM_181336.4) reported to date (HGMD Professional 2023.2).4 Here we describe a Chinese boy with Marbach-Rustad progeroid syndrome, who is the third case worldwide and the first one in China.
The proband (II3) is the third child of the non-consanguineous healthy parents, who already had two healthy daughters without a family history (Figure 1A). He was born at 35 weeks of pregnancy and small for gestational age (Figure 1B,C)9 without birth injury or asphyxia.
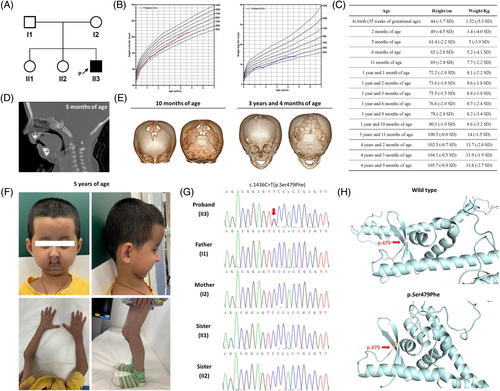
After birth, he was hospitalized because of premature birth, low birth weight and neonatal jaundice. He suffered from feeding problems due to micrognathia, retrognathia and glossoptosis (Figure 1D), and underwent bilateral mandibular distraction osteogenesis at 6 months of age. Furthermore, he displayed skull defects (Figure 1E), failure to thrive (Figure 1B,C), and delayed motor milestones as he achieved head control at 4 months of age and could sit at 9 months of age with a Gesell development quotient of 58 at 5 months of age. No abnormality was found in his biochemical and metabolic profiles and chromosomal microarray analysis.
Whole exome sequencing (WES) was performed when the proband was 1 year of age with an average depth over 100× and coverage over 99%. The proband-parents trio was analyzed with family model to screen de novo, hemizyous/homozygous and compound heterozygous variants respectively. One de novo, four hemizyous, one homozygous and two compound heterozygous variants were identified (Table S1), but none of them could explain the proband's phenotypes.
During the follow-up until 5 years of age, the proband gradually presented apparent facial abnormalities of large forehead, full cheeks, protruding eyes, ocular hypotelorism, crooked nose, small mouth, micrognathia and retrognathia, and thin habitus with little subcutaneous fat and prominent veins (Figure 1F). He enabled to walk at 1 year and 4 months of age and had a normal intelligence and speech. He started teeth eruption after 1 year of age, but only had three teeth at 3 years and 11 months of age without a chewing ability. Surprisingly, he had a catch-up growth after 2 years of age (Figure 1B,C).
His brain magnetic resonance imaging (MRI) at 2 years and 4 months of age in another hospital revealed Chiari malformation, head computed tomography (CT) at 3 years and 4 months of age showed skull defects and wormian bones (Figure 1E), and ultrasonography at 3 years and 11 months of age indicated diffuse hepatic lesions and enlarged kidneys. Moreover, his liver, renal, pancreatic and thyroid functions were impaired and showed a trend to develop diabetes (Table 1).
| Item | Value | Reference range |
|---|---|---|
| At 3 years and 11 months of age | ||
| Serum alanine aminotransferase (U/L) | 147↑ | 7–30 |
| Serum aspartate aminotransferase (U/L) | 78↑ | 14–44 |
| Serum γ-transglutaminase (U/L) | 64↑ | 5–19 |
| At 4 years and 1 month of age | ||
| Serum insulin (μIU/mL) | 122.24↑ | 3–25 |
| Serum C-peptide (μIU/mL) | 6.1↑ | 0.81–3.85 |
| Serum glucose (mmol/L) | 5.07 | 3.5–5.7 |
| HbA1c (%) | 6.4 | <6.4 |
| Serum triacylglycerol (mmol/L) | 3.85↑ | 0.23–1.7 |
| At 4 years and 3 months of age | ||
| Serum free triiodothyronine (pmol/L) | 10.69↑ | 4.23–7.21 |
| Serum free thyroxine (pmol/L) | 13.74↓ | 15.49–22.38 |
| Serum thyroid stimulating hormone (μIU/mL) | 1.538 | 0.65–7.18 |
| At 4 years and 4 months of age | ||
| Serum insulin (μIU/mL) | 134.95↑ | 3–25 |
| Serum C-peptide (μIU/mL) | 7.58↑ | 0.81–3.85 |
| Serum glucose (mmol/L) | 6.12↑ | 3.5–5.7 |
| Serum triacylglycerol (mmol/L) | 3.94↑ | 0.23–1.7 |
Based on these clinical features, a special syndrome was suspected. To further investigate the underlying molecular basis, WES data was reanalyzed after 4 years when the proband was 5 years of age. Excitingly, the de novo c.1436C>T(p.Ser479Phe) heterozygous variant in the LEMD2 gene had already been found as a pathogenic variant to cause a progeria phenotype. Sanger sequencing confirmed the de novo status of this variant with presence in the proband and absence in his unaffected parents and two elder sisters (Figure 1G). Moreover, this c.1436C>T(p.Ser479Phe) variant was predicted to affect the stability and alter the conformation of the LEMD2 protein by 3-D structure modeling (Figure 1H).
LEMD2 gene was first associated with Marbach-Rustad progeroid syndrome in two unrelated individuals from different ethnicities in 2019.4 However, to date, the patient list has not yet extended and there are still only these two patients. Our case shared similar facial appearances and physical features with the two previous patients (Table 2), supporting this gene as a cause of this disease. More interestingly, our case also shared the same LEMD2 c.1436C>T(p.Ser479Phe) variant with them, suggesting this variant as a hotspot. Thus, our patient could be diagnosed as Marbach-Rustad progeroid syndrome clinically and genetically.
| Patient | Patient 1 | Patient 2 | Patient 3 |
|---|---|---|---|
| Reference | Marbach F et al.4 | Marbach F et al.4 | Our study |
| Country | Italy | Norway | China |
| Age | 16 years | 10 years | 5 years |
| Gender | Male | Male | Male |
| Birth condition | |||
| Gestation | 36 weeks | 32 weeks | 35 weeks |
| Birth length (cm) | 44 | 40.5 | 44 |
| Birth weight (kg) | 1.91 | 1.675 | 1.52 |
| Prenatal growth retardation | + | − | + |
| Craniofacial features | − | ||
| Microcephaly | + | + | − |
| Triangular face | + | + | − |
| Prominent forehead | − | + | + |
| Full cheeks | − | + | + |
| Protruding eyes | + | + | + |
| Ocular hypotelorism | + | + | + |
| Crooked nose | + | + | + |
| Small mouth | + | + | + |
| Micrognathia | + | + | + |
| Subcutaneous tissues | |||
| Thin habitus with little subcutaneous fat | + | + | + |
| Thin skin with prominent veins | + | + | + |
| Skeletal problems | |||
| Short stature | + | − | − |
| Skull defects | − | − | + |
| Wormian bones | + | + | + |
| Delayed dentition | + | + | + |
| Crowed dentition | + | + | − |
| Hypodontia | + | + | + |
| Hypoplastic clavicles | + | + | − |
| Low bone density | + | − | NA |
| Neurological symptoms | |||
| Intellectual disability | − | − | − |
| Motor delay | − | − | + |
| Intention tremor | + | + | − |
| Abnormal head MRI | + | + | + |
- Abbreviation: NA, not available.
In this study, our initial analysis of WES in 2019 did not found the phenotype-causing variant for the proband, whereas the reanalysis 4 years later achieved. That is because LEMD2 variant-caused Marbach-Rustad progeroid syndrome was included in OMIM until 2021, though it was described in 2019. Although the de novo LEMD2 variant was found (Table S1), our initial analysis of WES based on OMIM in 2019 could not recognize it as the phenotypic cause for the proband.
In summary, we present a new patient with Marbach-Rustad progeroid syndrome, who is the third case worldwide and the first one in China. Our finding indicates the LEMD2 c.1436C>T(p.Ser479Phe) variant as a hotspot. Our experience also shows reanalysis of WES data of negative cases might improve diagnostic efficiency.
AUTHOR CONTRIBUTIONS
Li Liu and Yunting Lin designed the study. Li Liu, Wen Zhang and Xiaojian Mao enrolled the family. Yunting Lin, Zhikun Lu, Duan Li and Xiaodan Chen performed the experiments. Yunting Lin, Zhikun Lu and Wen Zhang analyzed the data. Yunting Lin wrote the paper.
ACKNOWLEDGEMENTS
We would like to thank the enrolled family for participation in this study. We thank Dr. Yongxian Shao and Meiyi Wang from Guangzhou Women and Children's Medical Center for help with 3-D structure modeling of the LEMD2 protein.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ETHICS STATEMENT
This study was approved by the Institutional Review Board of Guangzhou Women and Children's Medical Center (Guangzhou, China) (No.2015–112).
INFORMED CONSENT
Written informed consent was obtained from the guardian.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




