Genetic disorders with central nervous system white matter abnormalities: An update
Funding information: Clinical and Molecular Characterization of Leukodystrophies in Indian Children, Grant/Award Number: V.25011/379/2015-GIA/HR
Abstract
Several genetic disorders have variable degree of central nervous system white matter abnormalities. We retrieved and reviewed 422 genetic conditions with prominent and consistent involvement of white matter from the literature. We herein describe the current definitions, classification systems, clinical spectrum, neuroimaging findings, genomics, and molecular mechanisms of these conditions. Though diagnosis for most of these disorders relies mainly on genomic tests, specifically exome sequencing, we collate several clinical and neuroimaging findings still relevant in diagnosis of clinically recognizable disorders. We also review the current understanding of pathophysiology and therapeutics of these disorders.
1 INTRODUCTION
Genetic disorders with involvement of central nervous system (CNS) white matter are heterogeneous entities. Over the years, classifications based on neuropathology, imaging, genetic, and molecular mechanisms have been devised.1-3 Essentially, these disorders have been put under a single umbrella term in order to provide a diagnostic framework and the term leukodystrophies (LD) has been used interchangeably to describe most genetic white matter disorders.1, 2, 4-7 However, the rapid increase in delineation of novel phenotypes and underlying genetic mechanisms has made it challenging to accommodate these disorders into the current definitions and classification systems. The diagnostic modalities for these disorders have transformed from pathology to pattern recognition on the magnetic resonance imaging (MRI) of the brain and more recently to direct genomic testing.4 Several, but not all these disorders, share molecular pathways and pathomechanisms necessary for devising therapeutic modalities.
These disorders appear to represent a continuum ranging from isolated and primary myelin defects to those with other structural white matter components involvement and finally to those where extensive white matter involvement is secondary to metabolic defects or neuronal pathology. Acknowledging the limitations of current insight into underlying pathomechanisms, all disorders with significant and high penetrance of CNS white matter abnormalities (CNS WMAs) evident on neuroimaging have been included in this review. We attempt to devise a pragmatic approach for diagnosis of these disorders in the genomic era. We describe the current clinical spectrum, classification and its limitations, diagnostic modalities, role of genomic testing in rapid diagnosis, pathophysiology, and therapeutic modalities for these disorders.
2 DEFINITIONS AND CLASSIFICATIONS
Traditionally, all genetic disorders with CNS WMAs were referred to as LD. Recently, an attempt was made by the Global Leukodystrophy Initiative1 to define and classify white matter disorders into three categories, that is, leukoencephalopathies, genetic leukoencephalopathies (gLE), and LD based on consensus of a panel of experts in the field. Leukoencephalopathies was defined as all disorders with white matter abnormalities of the central nervous system, both acquired and genetic. Leukoencephalopathies with an underlying genetic defect were termed as gLE. The term LD was used for a subclass of gLE characterized by primary glial cell and myelin sheath pathology of variable etiology where secondary axonal pathology can emerge as the disease progresses. There were several limitations to the classification. For example, L-2 hydroxyglutaric aciduria in which there is neuropathology evidence for primary white matter involvement, was classified as genetic leukoencephalopathy and not as leukodystrophy. Cerebrotendinous xanthomatosis, classified as a true primary disorder of white matter, has involvement of gray matter structures as well on MRI along with systemic involvement.8 Several disorders for which a neuropathology evidence was lacking were designated as leukodystrophy based on the brain imaging data.
Classification of LD based on pathological changes and pathogenetic mechanisms that takes into account the primary involvement of any white matter component has also been proposed recently.2 Categories in this classification are the myelin disorders due to a primary defect in oligodendrocytes or myelin (hypomyelinating and demyelinating LD, LD with myelin vacuolization); astrocytopathies; leuko-axonopathies; microgliopathies; and leuko-vasculopathies.
Currently, the myelin-focused concept has been abandoned and all genetic disorders with involvement of any component within CNS white matter, that is, myelin, oligodendrocytes, astrocytes, microglia, axons, and blood vessels are referred to as LD.3, 6 All genetic disorders irrespective of the structural white matter component involved, the molecular process affected and the disease course, are referred to as LD. Hence, several disorders, which were designated as gLE earlier, have now been reclassified as LD.4 The exhaustive list of all disorders including metabolic, mitochondrial and those designated as leukoencephalopathies have been brought under this term. Hence, the concept of only white matter involvement, primary white matter involvement or true white matter involvement stands blurred. The experts agree that the precise meaning of this term is lost and retains the popular concept of selective, primary, predominant involvement of white matter with a progressive disease course.
Though LD is used practically for all genetic white matter disorders, this definition, which is largely based on neuropathology, does not contribute to categorizing the newly recognized phenotypes diagnosed by neuroimaging and genomic testing. The list of these disorders is bound to grow, and the diagnostic approach is likely to evolve into a combination of deep phenotyping complemented by genomic testing. Classifications based on brain imaging findings have been discussed in the respective section of the review.
3 EPIDEMIOLOGY
There is limited information on cumulative incidence and prevalence of these disorders with CNS WMAs owing to immense heterogeneity. The incidence in a pediatric cohort of genetic white matter disorders with molecular diagnosis was noted to be 1.2 in 100 000 live births9 which was comparable to the incidence of acquired white matter disorders in this age group. However, in a decade-long study of cohort of MRI diagnosed cases, the incidence of white matter disorders was estimated to be 1 in 7663 live births.10 An earlier study based on either MRI and/or biochemically confirmed cases reported the incidence of 2 per 100 000 live births.11 More robust epidemiological data is available for common and well-characterized disorders. A recent study based on genetic diagnosis by targeted and exome sequencing (ES) revealed relatively high frequency of Aicardi–Goutières syndrome, TUBB4A-related leukodystrophy, peroxisomal biogenesis disorders, POLR3-related leukodystrophy, vanishing white matter, and Pelizaeus–Merzbacher disease.12 The prevalence is noted to be 1 in 4845 to 50 000 for adrenoleukodystrophy (to cite PMID 32003821),13 1 in 40 000 to 160 000 for metachromatic leukodystrophy (MLD)14 and 1 in 2,50 000 for Krabbe disease15 across different populations. The mortality rates of Krabbe disease, Pelizaeus–Merzbacher disease, Canavan disease, Alexander disease, and MLD are reported by Barczykowski et al to be 0.089, 0.031, 0.012, 0.031 and 0.140 per 1 000 000 individuals of all ages respectively.15 The mortality rates in children below 5 years of age were noted to be three to nine folds more than those above 5 years of age.
4 ETIOLOGY
CNS WMAs are known to occur due to genetic as well as acquired causes such as autoimmune, toxins, hypoxic ischemia, infections, and several unknown factors.15 This review is focused mainly on disorders with an underlying genetic etiology. Literature search followed by manual curation revealed 422 genetic conditions with predominant CNS WMAs. The details of search methodology are provided in the supplemental data.
4.1 Monogenic disorders
Most of the well-described disorders with CNS WMAs are of monogenic etiology. A total of 406 monogenic disorders caused by pathogenic variants in 410 genes were retrieved (Table S1). One-hundred-nineteen conditions were designated as LD and 109 as gLE earlier.1, 4, 5 Thirty-one conditions have been referred to as both LD and gLE in the literature. We retrieved an additional 147 disorders with variable but consistent CNS WMAs on neuroimaging.
Subclassification of monogenic disorders based on appropriate and common principles is not achievable at present due to limited understanding of pathomechanisms involved. Based on the current knowledge, we categorize monogenic disorders affecting a known cellular or molecular process. However, these categories are not exclusive and often a disorder can be placed in more than one of these. There are very few disorders known to be caused by defects in myelin specific proteins (three disorders, three genes). The largest subgroup is that of nuclear mitochondrial disorders (91 disorders, 102 genes). Other common categories are organelle dysfunctions such as lysosomal (30 disorders, 28 genes) and peroxisomal (23 disorders, 17 genes). Defects in several enzymes involved in metabolic pathways of amino acids and organic acids (29 disorders, 32 genes), fatty acids (4 disorders, 4 genes), carbohydrate (4 disorders, 4 genes), and glycolipids (4 disorders, 4 genes) also result in marked CNS WMAs. Disorders affecting the membrane transport due to disturbed intravesicular transport (21 disorders, 20 genes) and iron and water homeostasis (39 disorders, 39 genes) are increasingly being recognized. Other prominent groups include disorders of DNA replication, transcription and their regulation (17 disorders, 17 genes), disorders of DNA repair mechanism (10 disorders, 9 genes), disorders of mRNA translation (29 disorders, 33 genes), translation modification and editing (7 disorders, 7 genes), disorders of cell–cell adhesion (4 disorders, 4 genes), cell cycle and differentiation (13 disorders, 11 genes), and apoptosis (4 disorders, 4 genes). Remaining disorders (74 disorders, 72 genes) have been listed in the miscellaneous category. All disorders of monogenic etiology and their subclassifications have been listed in the supplementary Table S1.
Twenty-two of all monogenic disorders listed above have neuropathological findings of vascular defects and are known as genetic vasculopathies.2, 4 Vascular defects involve small vessels of the brain, small veins, capillaries, small arteries, and arterioles.16 Regardless of similar pathology, the underlying cellular and processes involved are variable, mainly DNA replication and transcription regulation (six disorders), cellular growth, differentiation and apoptosis (six disorders), and one disorder each in the categories of DNA repair mechanisms and subcellular dysfunction (lysosomal).
4.2 Mitochondrial disorders
Four disorders due to variants in mitochondrial genome have CNS WMAs (Table S1).17, 18 Three of these, mitochondrial encephalopathy with lactic acidosis and stroke-like episodes, Leigh disease, and mitochondrial respiratory chain complex deficiency are caused due to defects in either protein coding (10 genes) or mitochondrial encoded transfer RNA (12 genes). The protein coding genes span subunits of all respiratory chain complexes, namely complex I (5 genes), III (2 genes), IV (2 genes), and V (1 gene), except complex II, which is entirely nuclear encoded. One disorder, Kearns–Sayre syndrome, is known to be caused by rearrangements in the mitochondrial genome.
4.3 Chromosomal abnormalities and microdeletion/microduplication syndromes
CNS WMAs are known to occur in very few chromosomal abnormalities and microdeletion/microduplications syndromes consistently. The chromosomal causes include tetrasomy 12p,19, 20 ring chromosome 18,21, 22 and 49, XXXXY syndrome.23, 24 Chromosome 18q deletion syndrome (MIM#601808) is the most common and is consistently associated with WMAs.25, 26 Other rare microdeletion/duplication syndromes with WMAs include 6p25 microdeletion,27-29 3p21.31 deletion,30-32 14q12-q13.1 triplication,33 5q14.3 deletion,34 11q14.3 deletion,35, 36 11q24 deletion,37 17p13.3 deletion,38-41 and 22q11.2q13 duplication.42 Supplementary Table S2 provides the clinical and radiological findings associated with these disorders.
5 DIAGNOSIS
The clinical heterogeneity and ever-increasing number of disorders with CNS WMAs pose a significant challenge in clinical diagnosis. Though genomic testing is increasingly being applied as a first line diagnostic test, a combination of inheritance pattern, age of onset, characteristic neurological or non-neurological clinical findings and MRI brain pattern is helpful in accomplishing a clinical diagnosis and more often a set of differential diagnosis for these disorders.
5.1 Inheritance pattern
Most disorders with CNS WMAs follow an autosomal recessive inheritance pattern (322 conditions). Sixty-four disorders are inherited exclusively in an autosomal dominant pattern and four disorders can be inherited both as autosomal recessive and dominant. Sixteen disorders show X-linked inheritance patterns. Four disorders follow mitochondrial inheritance. Hence, pedigrees with autosomal dominant or X-linked patterns provide a good handle for clinical diagnosis. The sporadic cases must be carefully evaluated to rule out the acquired conditions. The inheritance patterns of all disorders are provided in supplementary Table S1.
5.2 Age of onset
The age of onset of most disorders is in the pediatric age group. However, several disorders have onset ranging from pediatric to adulthood (Table S3). Very few disorders with CNS WMAs are known to be exclusively adult onset conditions and are listed in Table 1.43 These disorders would need dissection from the late onset forms of other disorders and acquired conditions.
| S. No | Condition | MIM# | Gene |
|---|---|---|---|
| 1 | Hereditary diffuse leukoencephalopathy with spheroids | 221820 | CSF1R |
| 2 | Autosomal dominant adult onset demyelinating leukodystrophy | 169500 | LMNB1 |
| 3 | Adult polyglucosan body disease | 263570 | GBE1 |
| 4 | Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy | 125310 | NOTCH3 |
| 5 | Cerebral autosomal recessive cerebral arteriopathy with subcortical infarcts and leukoencephalopathy | 600142 | HTRA1 |
| 6 | Cathepsin A-related arteriopathy with strokes and leukoencephalopathy | – | CTSA |
| 7 | Cerebral leukodystrophy with retinal vasculopathy | 192315 | TREX1 |
| 8 | Small vessel disease with ocular abnormalities | 175180 | COL4A1 |
| 9 | Gordon Holmes syndrome | 212840 | RNF216 |
| 10 | Polycystic lipomembranous osteodysplasia with sclerosing leukoencephalopathy 1 | 221770 | TYROBP |
| 11 | Polycystic lipomembranous osteodysplasia with sclerosing leukoencephalopathy 2 | 618193 | TREM2 |
5.3 Clinical features
Several but not all disorders with CNS WMAs are progressive disorders.6 Those with a progressive course present with regression of milestones after a period of normalcy and the others as delayed development. Often hypotonia is the presenting feature that progresses to hypertonia and spasticity, ataxia, nystagmus, swallowing and speech difficulties later in the course.5 Those disorders, which begin with motor manifestations alone usually, manifest cognitive and behavioral changes as the disease advances. The neurological findings alone are seldom useful for establishing a clinical diagnosis owing to the immense heterogeneity of these disorders. However, extra-neurological features involving endocrine, ophthalmologic, auditory, musculoskeletal, skin, gastrointestinal, and cardiovascular systems in presence of significant white matter involvement often aids clinical diagnosis (Table 2).
| Clinical feature | Disorders |
|---|---|
| Macrocephaly | Alexander disease, Canavan disease, megalencephalic leukoencephalopathy with subcortical cysts, 1,2-hydroxy glutaric aciduria, GM2 gangliosidosis |
| Coarse facies | Sialic acid storage disease, fucosidosis, multiple sulfatase deficiency, mucopolysaccharidosis |
| Progeroid appearance | Cockayne syndrome |
| Enamel hypoplasia and other enamel defects | Oculodentodigital dysplasia, peroxisomal disorders |
| Oligodontia, hypodontia, delayed eruption, altered sequence of eruption, abnormal color/shape | POLR3 related disorders (not universal and highly variable) Oculodentodigital dysplasia |
| Propensity for cavities | Cockayne syndrome |
| Cataract | At birth Hypomyelination with congenital cataract, childhood ataxia with central nervous system hypomyelination (only connatal cases), Peroxisomal disorders Childhood onset Cerebrotendinous xanthomatosis, POLR3 related disorders |
| Cherry red spot | Sialidosis, GM2 gangliosidosis, metachromatic leukodystrophy (some cases) |
| Glaucoma | Aicardi–Goutières syndrome, oculodentodigital dysplasia |
| Optic atrophy | Metachromatic leukodystrophy, Canavan disease Childhood ataxia with central nervous system hypomyelination, cerebrotendinous xanthomatosis, peroxisomal disorders (+/−), POLR3 related disorders (+/−), hypomyelinating leukodystrophies, mitochondrial disorders, oculodentodigital dysplasia |
| Retinitis pigmentosa | Refsum disease (adolescent and adult onset), peroxisomal disorders |
| Vascular retinal defects | Cerebroretinal microangiopathy with calcifications and cysts (coats plus syndrome) |
| Angiokeratoma corporis diffusum | Fucosidosis |
| Ichthyosis | Congenital Sjogren–Larsson syndrome, Ichthyotic keratoderma, spasticity, hypomyelination, and dysmorphic facies Childhood onset Multiple sulfatase deficiency, sialic acid storage disorder, Peroxisome biogenesis disorders including neonatal Zelleweger syndrome adrenoleukodystrophy and infantile Refsum disease Adulthood onset Refsum disease |
| Hyperpigmentation | X-Adrenoleukodystrophy, mitochondrial neurogastrointestinal encephalopathy |
| Xanthomas | Cerebrotendinous xanthomatosis |
| Photosensitivity | Cockayne syndrome, Tay syndrome |
| Adrenal insufficiency | X-linked adrenoleukodystrophy, peroxisome biogenesis disorders |
| Hypothyroidism | POLR3 related disorders, Aicardi–Goutières syndrome, cerebrotendinous xanthomatosis, peroxisomal biogenesis disorders |
| Hypogonadotropic hypogonadism | POLR3 related disorders |
| Growth hormone deficiency | POLR3 related disorders, Aicardi–Goutières syndrome |
| Ovarian dysgenesis (Premature ovarian failure) | Ovarioleukodystrophy, AARS2-related leukoencephalopathy Peroxisome biogenesis disorders |
| Hepatosplenomegaly | Multiple sulfatase deficiency, galactosialidosis, sialic acid storage disorders |
| Hepatic dysfunction | Peroxisomal disorders, Aicardi–Goutières syndrome, mitochondriopathies Fucosidosis, sialic acid storage disorders |
| Chondrodysplasia punctata | Peroxisomal disorders |
| Dysostosis multiplex | Multiple sulfatase deficiency, Sialidosis |
Acquired disorders may present with acute or subacute onset following an episode of infection, toxicity, or hypoxia. They may have monophasic illness of acute onset followed by partial or complete recovery as in acute disseminated encephalomyelitis (ADEM)44 or a chronic illness with recurrent episodes of relapsing signs and symptoms as seen in multiple sclerosis.45, 46 Often, they may mimic disorders of genetic etiology in clinical presentation, especially mitochondrial disorders precipitated by an intercurrent illness.5
5.4 Neuroimaging
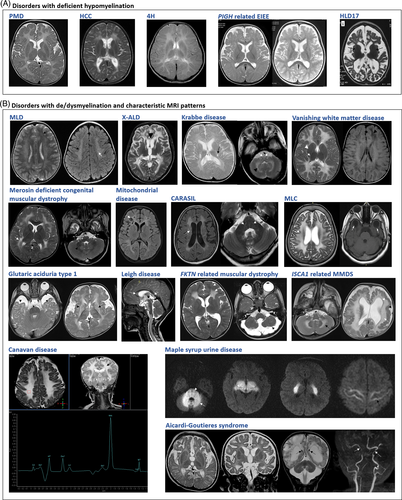
MRI has a vital role in the diagnosis of disorders with CNS WMAs. The minimum requirements for a standard MRI investigation are T1-weighted, T2-weighted and fluid-attenuated inversion-recovery (FLAIR) images. The radiological diagnostic algorithm devised by van der Knaap et al remains a useful aid for clinical diagnosis of common and recognizable disorders.3 The pattern recognition on neuroimaging involves differentiation into hypomyelination or other white matter pathologies, the confluency and predominant area of localization of WMAs and certain specific MRI characteristics. In addition, other magnetic resonance sequences including contrast enhanced MRI, susceptibility weighted imaging and diffusion-weighted sequences are useful diagnostic tools for disorders with inflammatory component, calcifications and/or vascular lesions.5 MR spectroscopy serves as a sensitive method for diagnoses of metabolic and mitochondrial disorders among these disorders (Table 3).
| Confluency and area of predominance | ||
|---|---|---|
| Diffuse and symmetric | Area of predominance | Disorders |
| Subcortical | Glutaric aciduria (Figure 1(B)) Canavan disease (Figure 1(B)) Urea cycle defects |
|
| Frontal lobe | Alexander disease Metachromatic leukodystrophy (Figure 1(B)) Neuroaxonal leukodystrophy with axonal spheroids |
|
| Periventricular | Metachromatic leukodystrophy (Figure 1(B)) Krabbe disease Leukoencephalopathy with brainstem and spinal cord involvement and lactate elevation Periventricular leukomalacia (Figure S1) |
|
| Occipital | Krabbe disease (Figure 1(B)) X-linked adrenoleukodystrophy Peroxisomal disorders |
|
| Diffuse cerebral | Vanishing white matter disease (Figure 1(B)) Megalencephalic leukoencephalopathy with subcortical cysts (Figure 1(B)) Merosin deficient congenital muscular dystrophy (Figure 1(B)) Mitochondrial disorders Most leukodystrophies at the advanced stages HIV encephalopathy (Figure S1) Toxic leukoencephalopathy (Figure S1) |
|
| Cerebellar | Leukoencephalopathy with brainstem and spinal cord involvement and lactate elevation Leigh disease (Figure 1(B)) Alexander disease Maple syrup urine disease (Figure 1(B)) Adult onset autosomal dominant leukodystrophy |
|
| Brainstem | Leigh disease (Figure 1(B)) Wilson disease Alexander disease Krabbe disease (Figure 1(B)) Leukoencephalopathy with brainstem and spinal cord involvement and lactate elevation |
|
| Spinal Cord | Alexander disease Mitochondrial disorders (Figure 1(B)) Leukoencephalopathy with brainstem and spinal cord involvement and lactate elevation |
|
| Focal and asymmetric | Genetic vasculopathies | Brain small vessel disease Proliferative vasculopathy and hydranencephaly–hydrocephaly syndrome Microangiopathy and leukoencephalopathy, pontine, autosomal dominant Aicardi-Goutières syndrome (Figure 1(B)) Cerebral amyloid angiopathy Leukoencephalopathy with calcifications and cysts Cerebral AD arteriopathy with subcortical infarcts and leukoencephalopathy Cerebral AR arteriopathy with subcortical infarcts and leukoencephalopathy (Figure 1(B)) RNASET2-related leukodystrophy NGLY1 related congenital disorder of deglycosylation Cathepsin A-related arteriopathy with strokes and leukodystrophy |
| Acquired disorders with white matter abnormalities | Optic neuritis Transverse myelitis Acute disseminated encephalomyelitis (Figure S1) Multiple sclerosis (Figure S1) |
|
| Special MRI characteristics | ||
| Cystic changes | Megalencephalic leukoencephalopathy with subcortical cysts (Figure 1(B)) Mitochondrial disorders (Figure 1(B)) Vanishing white matter disease (Figure 1(B)) Glutaric aciduria (basal ganglia) |
|
| Calcifications | Aicardi–Goutieres syndrome (Figure 1(B)) Galactosialidosis Brain small vessel disease with or without ocular anomalies Labrune's syndrome or leukoencephalopathy with calcifications and cysts Cerebro retinal microangiopathy with calcifications and cysts (coats plus syndrome) Pseudo-TORCH syndrome 1 AARS2-related disease KARS2-related disease Adult onset leukoencephalopathy with axonal spheroids and pigmented glia COL4A1-related disorders Retinal vasculopathy with cerebral leukoencephalopathy Cerebral amyloid angiopathy Primary familial brain calcification Pseudohypoparathyroidism Leukoencephalopathy with calcifications and cysts |
|
| Magnetic resonance spectroscopy | Elevated lactate | Mitochondrial disorders (Figure 1(B)) Some metabolic disorders (mild elevation) |
| Altered metabolites | Ribose 5-phosphate isomerase deficiency (Elevated levels of arabitol and ribitol) Canavan disease (elevated N-acetylaspartic acid) (Figure 1(B)) |
|
Deficient myelination, either permanent hypomyelination or delayed myelination (Figure 1(A)), is seen as less marked T2-weighted hyperintensities and T1-weighted hypointensities, isointensities or mild hyperintensities of white matter relative to gray matter.3 Seventy-six disorders with deficient CNS myelination were retrieved from the literature (Supplementary Table 4). Of these, 46 conditions are reported with hypomyelination and 27 with delayed myelination consistently. Hypomyelination and delayed myelination have been used interchangeably in three conditions. The number of disorders with deficient myelination, particularly permanent hypomyelination is limited. Hence, hypomyelination on MRI in conjunction with other specific findings is a good clinical handle for diagnosis of these disorders. Two major group of disorders with deficient myelination are hypomyelinating LD and early infantile onset epileptic encephalopathies (EIEE). Nineteen disorders with permanent hypomyelination have been cataloged as hypomyelinating leukodystrophies (HLDs, PS312080) in OMIM. WMAs, mainly delayed myelination, has been observed in 35 of the 84 (EIEE) reported till date (OMIM PS308350). This could be attributed to seizure activity arresting the normal process of myelination, which progresses and usually normalizes after seizure control.47 Clinically identifiable disorders with hypomyelination are listed in Table 4 and other disorders with deficient myelination are provided in Table S4.
| Disease | Gene | Clinical clues | Neuroimaging findings |
|---|---|---|---|
Leukodystrophy, hypomyelinating, 1 (Pelizaeus–Merzbacher disease) |
PLP1 | Developmental delay, hypotonia, rotatory nystagmus | Hypomyelination (Figure 1(A)) Thinning of corpus callosum |
Leukodystrophy, hypomyelinating, 2 (Pelizaeus–Merzbacher like disease 1) |
GJC2 | Developmental delay, hypotonia, rotatory nystagmus | Hypomyelination Involvement of the corticospinal tracts with abnormal T2-weighted signal extending into the brain stem resulting in extensive brain stem involvement not seen in Pelizaeus–Merzbacher disease Cerebral atrophy at advanced stages |
Leukodystrophy, hypomyelinating 5 (Hypomyelination with congenital cataract) |
FAM126A | Sudden motor regression Cataract |
Hypomyelination (Figure 1(A)) Cerebral atrophy at advanced stages |
| Pol III related leukodystrophiesLeukodystrophy, hypomyelinating, 8, with or without oligodontia and/or hypogonadotropic hypogonadism | POLR3B | Hypodontia Hypogonadotropic hypogonadism |
Hypomyelination (Figure 1(A)) Thinning of corpus callosum |
| Leukodystrophy, hypomyelinating, 7, with or without oligodontia and/or hypogonadotropic hypogonadism | POLR3A | Hypodontia Hypogonadotropic hypogonadism |
Hypomyelination (Figure 1(A)) Thinning of corpus callosum |
| Ichthyosis, acanthosis nigricans, hypomyelination, spastic paraplegia, high frequency deafness and optic atrophy | ELOVL1 | Ichthyosis, acanthosis nigricans, deafness |
Hypomyelination |
| Hypomyelinating neuropathy, congenital, 3 | CNTNAP1 | Arthrogryposis multiplex congenita | Hypomyelination Thin corpus callosum Cerebellar atrophy Pontine atrophy |
| Hypermethioninemia with deficiency of S-adenosylhomocysteine hydrolase | AHCY | Facial dysmorphism Abnormal hair and teeth Myocardiopathy Hypermethioninemia |
Severely delayed myelination |
De/dysmyelination presents with T2-weighted hyperintensities and T1-weighted hypointensities of affected white matter relative to the gray matter.3 Confluent, bilateral and symmetric signal abnormalities are predominantly observed in genetic disorders while focal asymmetric involvement of white matter may be indicative of a nongenetic cause. However, there are several exceptions to this phenomenon. Specific pattern recognition of WMAs including predominant areas of white matter dysmyelination in MRI remains vital for diagnosis of several common disorders like Krabbe disease, metachromatic leukoencephalopathy, megalencephalic leukoencephalopathy (Figure 1(B)), Leigh disease, and so on (Figure 1(B)). Seldom, it also aids in diagnosis of rare disorders with very characteristic and unique combination of brain imaging findings such as multiple mitochondrial dysfunction syndrome 5 (MIM# 617613) (Figure 1(B)) with extensive diffuse cerebral WMAs, ventriculomegaly, pachygyria, and cerebellar atrophy.48-50 Characteristic neuroimaging findings for common disorders is provided in Table 3 and Figure 1. Of the 147 disorders not categorized as LD/gLE earlier, 24 (21 with dysmyelination, two with hypomyelination along with dysmyelination, and one with hypomyelination) present with confluent and recognizable pattern of WMAs (Table S5).
Acquired white matter disorders are more likely to have multifocal asymmetric white matter abnormalities.3 However, this imaging appearance may also be noted in several genetic disorders such as neuroaxonal leukodystrophy with spheroids, CADASIL, vascular leukoencephalopathies (Table 3) and some disorders of mitochondrial etiology (Figure 1(B)). Conversely, some acquired causes such as hypoxic ischemia, periventricular leukomalacia, HIV encephalopathy, and toxic leukoencephalopathies lead to symmetrical and confluent white matter abnormalities thus mimicking genetic disorders. Large and ill-defined lesions, which involve gray matter structures as well suggest the possibility of acquired demyelinating disorders.3 MRI may reveal a single or multiple lesions in both white matter (periventricular and subcortical) and gray matter (basal ganglia, thalamus, cortex) in individuals affected with ADEM.44, 51 Multiple sclerosis is diagnosed by neuroimaging evidence of two or more brain and/or spinal cord lesions disseminated by space and time (McDonald criteria, 2005).45, 46 Neuroimaging findings of common acquired white matter disorders are provided in Figure S1.
6 GENETIC TESTING
ES has emerged as a highly efficient diagnostic modality for genetic disorders with CNS WMAs as most of these disorders are monogenic and predominantly recessive. The earlier diagnostic rate of 50% has now increased to more than 70–80% with the use of ES.6, 7 The widespread availability of ES relative to several other specialized tests has also led to a decrease in the discrepancy in patients receiving a definitive diagnosis worldwide. In the past decade, approximately 72 novel gene-disease associations for these disorders have been identified with the application of ES.
However, ES has limitations in terms of diagnosis of genomic variants beyond exonic single nucleotide variants and small indels owing to technical difficulties.52 Variants in regions with repetitive sequence, CNVs, intronic, and variants in regulatory regions cannot be identified by ES. The most common genomic variants for disorders like Pelizaeus–Merzbacher disease and Krabbe disease are large deletions/duplications of PLP1 and GALC respectively, which may not be detected in ES, thus emphasizing the role of clinical diagnosis in the era of genomics. A good clinical diagnosis or a set of narrow differential diagnoses is more likely to facilitate definitive molecular diagnosis and overcome the uncertainties of broad-spectrum genomic tests like exome and genome sequencing.
Literature on the utility of gene panels for diagnosis of these disorders is very limited. Yield of a custom panel was noted to be 13.3% in a cohort of individuals with adult onset LD.53 In an Argentinian cohort of individuals with genetic disorder with WMAs, a virtual panel analysis from exome data rendered a diagnostic yield of 46.1%.54 Though whole genome sequencing (WGS) outweighs the diagnostic yield of ES, there is limited data on use of this technique as well for investigation of these disorders.55, 48 A recent study reported trio genome sequencing in 41 families who were undiagnosed with trio ES.56 This resulted in a diagnosis in 14 (34%) additional families. Further decrease in sequencing cost and increase in the ease of data analysis is likely to result in WGS as a test of choice for these disorders.
7 BIOCHEMICAL TESTS
The role of biochemical testing for genetic disorders including those with CNS WMAs is getting redefined in the era of broad-spectrum genomic testing. In scenarios with a diagnostic MRI pattern, biochemical analysis aids in validating the clinical diagnosis (Table 5). This can be followed by often inexpensive targeted genetic testing. Also, easily available biochemical tests like enzyme assays may often be used to resolve the variants of uncertain significance, a major concern with the broad-spectrum genomic tests. In these cases, biochemical testing adds to the evidence of pathogenicity while interpreting the observed variants.
| Testing parameter | Disorders |
|---|---|
| Creatine phosphokinase (serum) | Muscular dystrophies Merosin deficient congenital muscular dystrophy Dystroglycanopathies |
| Lactate, pyruvate (plasma, cerebrospinal fluid) | Mitochondrial disorders |
| Glycosaminoglycans (urine) | Metachromatic leukodystrophy Multiple sulfatase deficiency |
| Organic acids (urine) | L-2-hydroxyglutarate N-acetylaspartic acid for Canavan disease Metabolic disorders of Krebs cycle Other mitochondrial disorders |
| Amino acids (urine) | Aminoacidopathies Maple syrup urine disease Homocystinuria Hyperornithinemia–hyperammonemia–homocitrullinemia syndrome Pyruvate dehydrogenase complex deficiency |
| Very long chain fatty acids (plasma) | Peroxisomal disorders X-linked adrenoleukodystrophy |
| Lysosomal enzymes (leukocytes/fibroblasts) | Krabbe (galactosyl cerebrosidase) Metachromatic leukodystrophy (arylsulfatase A) Multiple sulfatase deficiency (arylsulfatase A, B, C, D) GM1 gangliosidosis (beta galactosidase) GM2 gangliosidosis (hexosaminidase A and B) Sialidosis (neuraminidase) Galactosialidosis (neuraminidase and beta galactosidase) |
| 1,4-alpha-glucan-branching enzyme activity | Adult polyglucosan body disease |
| Cholestanol | Cerebrotendinous xanthomatosis |
| Fatty aldehyde dehydrogenase enzyme | Sjogren–Larsson syndrome |
8 PATHOPHYSIOLOGY
Genetic disorders with CNS WMAs result from molecular defects, which lead to abnormalities in myelin production and maintenance or myelin destruction.2 The myelin sheath is a modified plasma membrane of the oligodendrocytes and is wrapped around neuronal axons. It consists of lipids (70–85%) and proteins (15–30%).57 The production of myelin in developmental stages and its maintenance through adult life requires tightly regulated cues within the oligodendrocytes, glial cells and the neuronal axons.58 Most of the oligodendrocytic organelles including nucleus, rough endoplasmic reticulum, Golgi apparatus, microtubules, lysosomes, and peroxisomes play critical and unique roles in formation and maintenance of CNS white matter.
There are three main mechanisms of white matter abnormalities based on cellular pathology. First, is a primary defect in oligodendrocytic activity of myelin production and maintenance. The illustrative example for defect in myelin production is duplication variants in PLP1, which cause misfolding of myelin followed by mislocalization. This leads to accumulation of PLP1 in the late endosome resulting in oligodendrocytic death. Second, is a defect on astrocytic regulation of myelination process, as seen in Alexander disease, where mutant glial fibrillary acidic protein accumulation activates multiple stress pathways inside the astrocytes. An astrocytic defect may also result in an ionic imbalance and fluid accumulation in myelin (vacuolation) as observed in several mitochondrial disorders and megalencephalic leukoencephalopathy with subcortical cysts. Third, there is no formation or there is degeneration of previously formed neuronal axons. Variants in neuronal axon specific genes may lead to improper formation of axons (FAM126A related hypomyelinating leukodystrophy) or degeneration of previously formed neuronal axons (GM1 gangliosidosis).
In addition to this, WMAs can occur as a consequence of genetic defects which lead to vascular pathology.2 The classical example is cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy, caused by defects in NOTCH3. Variants and deletions of NOTCH3 result in accumulation of the aberrant protein, which ultimately causes a reduced cerebral blood flow in brain white matter leading to white matter abnormalities.59 Several external factors like metabolic, mitochondrial, and nongenetic factors (toxins, hypoxic insults) can also lead to WMAs. Defects in several genes, which are not yet known to have a direct role in oligodendrocyte or glial cell function are increasingly being identified to cause these disorders.
9 TREATMENT
A substantial insight into the pathomechanisms has enabled advances in specific therapeutic strategies for these disorders. The timing of initiating therapy is of utmost importance as these therapies are effective only if initiated before or immediately after the onset of symptoms. Hence, an early diagnosis is warranted for optimal management and outcome for these disorders. However, supportive care remains the mainstay of treatment for most and involves management of spasticity, adequate nutrition including supplementation of vitamin D and calcium, treatment of neuropathic pain, epilepsy, drugs for sialorrhea and insomnia, and monitoring for orthopedic complications like hip dislocation and scoliosis. One of the most common symptoms, spasticity, can be managed effectively by oral spasmolytic drugs, intramuscular botulinum toxin, intrathecal baclofen,60 or selective dorsal rhizotomy.61
The effective and curative therapies developed till date for these disorders include hematopoietic stem cell transplant and ex vivo gene therapy. Other forms of therapies like antisense oligonucleotides, targeted drug therapy, enzyme replacement therapy and stem cell-based therapy need further evidence before they are proved efficacious in the clinic. Table 6 summarizes the currently recommended therapeutic measures and Table S6 lists the emerging treatment modalities for common and selected disorders.4, 62-95
| S. No | Disorder | Therapy | References | |
|---|---|---|---|---|
| Recommended therapies | ||||
| 1 | ALD | Asymptomatic males | Lorenzo oil and dietary restriction of very long chain fatty acids | Moser et al., 2005; Sassa et al., 2014 |
| Males with adrenal insufficiency | Oral corticosteroid replacement | Raymond, Moser, & Fatemi, 1993 | ||
| Cerebral ALD | HSCT effectively arrests progression of demyelination if initiated early in course of disease | Shapiro et al., 2000; Peters et al., 2004; Miller et al., 2011 | ||
| Adrenomyeloneuropathy | Corticosteroid replacement for adrenocortical insufficiency Supportive treatment |
Raymond, Moser, & Fatemi, 1993 | ||
| 2 | MLD | Early onset forms | Cholecystectomy for preventing complications like gall bladder dysfunction, polyps and carcinoma Variable outcomes and several complications in patients treated with allogenic HSCT |
Peters et al., 2004 |
| Juvenile or adult forms | Allogenic HSCT halts demyelination in minimally symptomatic Individuals |
Peters et al 2004; Weinberg 2005 | ||
| 3 | Krabbe disease | Infantile onset | HSCT prevents progression of disease in presymptomatic individuals | Escolar et al., 2005 |
| Late onset | HSCT may be beneficial | Krivit et al 1999; Krivit 2004 | ||
| 4 | Aicardi–Goutieres syndrome | Systemic corticosteroids are effective for treatment of autoimmune manifestations but not for neurologic symptoms | Chahwan and Chahwan 2012 | |
| 5 | Vanishing white matter disease | Preventive care to avoid trauma and infections Treatment of ovarian failure |
van der Knaap et al., 2019 | |
| 6 | Canavan disease | Oral lithium citrate showed improvement in myelination in six patients but minimal clinically effectivity in tolerable lithium dosage | Assadi et al.,2010; Pleasure et al., 2020 | |
| 7 | Cerebrotendinous xanthomatosis | Oral chenodeoxycholic acid Cholic acid |
Berginer et al 1984; Mandia et a., 2019 | |
| 8 | Adult onset polyglucosan body disease | Triheptanoin, a seven-carbon triglyceride has been found to be beneficial in slowing the clinical course | Roe et al 2010; (NCT00947960) | |
| 9 | Hypomyelination with brain stem and spinal cord abnormalities and leg spasticity | Steroids may be beneficial in patients with subacute disease onset | Wolf et al., 2014 | |
| 10 | Pol-III related leukodystrophies | Supplemental hormonal therapies including growth hormone, thyroid hormone and hormone replacement therapy for hypogonadism | Bernard and Vanderver, 1993 | |
- Abbreviations: HSCT, Hematopoietic stem cell transplantation; MLD, Metachromatic leukodystrophy; X-ALD, X-linked adrenoleukodystrophy.
10 CONCLUSION
The last few decades have added several insights for disorders with CNS WMAs. A combination of clinical, radiographic, biochemical, and genomic expertise has helped us to overcome the challenges of a definitive diagnosis in disorders with CNS WMAs. The improvement in understanding of etiopathogenesis in future is likely to result in sustained advancement in therapeutics for several of these disorders.
ACKNOWLEDGMENTS
We thank Department of Health Research, Ministry of Health and Family Welfare, Government of India for funding the project titled “Clinical and Molecular Characterization of Leukodystrophies in Indian Children” (V.25011/379/2015-GIA/HR) which led to our understanding of disorders with CNS WMAs and assisted in this review.
CONFLICT OF INTEREST
The authors declare no conflict of interest
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as it is a review of pre-existing information.




