X-linked intellectual disability: Phenotypic expression in carrier females
Abstract
To better understand the landscape of female phenotypic expression in X-linked intellectual disability (XLID), we surveyed the literature for female carriers of XLID gene alterations (n = 1098) and combined this with experience evaluating XLID kindreds at the Greenwood Genetic Center (n = 341) and at the University of Adelaide (n = 157). One-hundred forty-four XLID genes were grouped into nine categories based on the level of female phenotypic expression, ranging from no expression to female only expression. For each gene, the clinical presentation, gene expression in blood, X-inactivation (XI) pattern, biological pathway involved, and whether the gene escapes XI were noted. Among the XLID conditions, 88 (61.1%) exhibited female cognitive phenotypic expression only, while 56 (38.9%) had no female phenotypic expression (n = 45), phenotype expression with normal cognition in females (n = 8), or unknown status for female phenotypic expression (n = 3). In twenty-four (16.6%) XLID genes, XI was consistently skewed in female carriers, in 54 (37.5%) XI showed variable skewing, and in 33 (22.9%) XI was consistently random. The XI pattern was unknown in 33 (22.9%) XLID conditions. Therefore, there is evidence of a female carrier phenotype in the majority of XLID conditions although how exactly XI patterns influence the female phenotype in XLID conditions remains unclear.
1 INTRODUCTION
The classical concept of X-linked inheritance holds that pathogenic variants in genes on the X chromosome produce a phenotype only or primarily in the hemizygous males, while heterozygous carrier females are protected by their normal X-chromosome, in accordance with the Lyon hypothesis. However, exceptions to this rule have been noted since the first descriptions of males with X-linked intellectual disability (XLID).1-5 In the first report of the fragile X syndrome [MIM: 300806] in 1943 by Martin and Bell, the authors noted that “… the milder form of deficiency, exhibited by females … would remind the reader, however, that occasionally genes appear to be incompletely recessive, in that they may manifest to a less severe degree in heterozygotes …“2 Moreover, while it has become clear that some female carriers of XLID conditions show variable cognitive dysfunction similar to male patients, the mechanisms driving this variability in females are still unclear.6
Protection from phenotypic expression in carrier females of XLID conditions has been largely attributed to skewed inactivation of the X chromosome and compensation by the normal allele, with evidence for this coming from studies showing more frequent skewing of X-inactivation (XI) in female carriers of XLID syndromes as compared to non-carrier females.6, 7 X-inactivation is established in the blastocyst stage of embryonic development and is responsible for random transcriptional downregulation of one of the two X-chromosomes in somatic cells.8, 9 This process is mediated by a variety of epigenetic mechanisms including methylation, XIST RNA-mediated gene silencing, and chromatin modification and once established is maintained in all subsequent daughter cells.10 Although primarily considered a random process, XI can be skewed in normal females, with inactivation favoring either the maternally or paternally inherited X-chromosome.9 Additionally, pathogenic XI skewing in females does occur, primarily if the X-inactivating complex is disrupted, for instance with pathogenic variants in XIST, or secondarily as with post-inactivation cell selection due to variants or large structural rearrangements on the X-chromosome that affects cell proliferation.11, 12
The process of XI is now known to be more complex than initially thought, with evidence suggesting that patterns of XI are heterogeneous among healthy non-carrier females, varying over time and in different tissues, even within individuals.13, 14 Further complicating this process is the increasing number of X-linked genes that are now known to have some expression from the allele on the inactive X-chromosome, termed escape genes.15 Although expression of these genes from the inactivated X-chromosome can be as little as 10% of that from the active allele, several groups have suggested that even this small amount of added expression from the inactivated X-chromosome may be responsible for the phenotypic expression observed in some female carriers of XLID conditions, for example, those caused by mutations in genes such as DDX3X [MIM: 300160] and SMC1A [MIM: 300040].6, 16, 17
In an effort to better characterize the prevalence and nature of female phenotypic expression in XLID, we searched the literature for evidence of phenotypic expression in female carriers of XLID conditions and combined it with the experience of many years of XI testing at the Greenwood Genetic Center and at the University of Adelaide. We found that the majority of XLID conditions do have female phenotypes and that subphenotypes become apparent based on pattern of phenotypic expression in females—from conditions with only minimal or no phenotypic expression to those with a phenotype only in females. Furthermore, grouping XLID syndromes based on the nature of the phenotype in females offers insight into the factors that may be driving female phenotypes in XLID conditions.
2 MATERIALS AND METHODS
One hundred forty-four XLID-associated genes were identified using published data and the most recent XLID update.18 For each gene, clinical presentation, gene expression in blood, phenotype of carrier females, results of XI studies, functional pathway, and if the gene escapes XI were noted. Gene expression in blood was determined using UniGene (https://www.ncbi.nlm.nih.gov/unigene), and gene escape from XI was determined using published data.19, 20 Skewed XI was defined as inactivation of the same X-chromosome in greater than 90% of cells, which is often considered the cutoff for biological and clinical relevance; all other XI results were considered random.6, 21 The XI pattern was determined for each XLID gene by noting the number of cases with skewed XI. The XI pattern was classified as skewed if greater than 90% of cases had skewed XI and similarly classified as random XI if greater than 90% of cases had random XI. All other XLID genes with XI information were classified as having a variable XI pattern. It was also documented if no XI information could be found for a particular gene (Tables S1-S10).
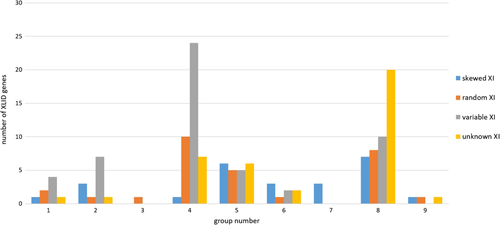
Female carriers of XLID gene alterations (n = 1596) were identified through the literature (n = 1098), the Greenwood Genetic Center (n = 341) and the University of Adelaide (n = 157). Based on the findings of phenotypic expression patterns in carrier females, each XLID gene was assigned to one of nine groups: predominantly female phenotypic expression presumed with male lethality (group 1), female phenotypic expression equivalent or greater than males (group 2), female phenotypic expression with normal males (group 3), cognitive deficits in females that are milder than in males (group 4), rare female phenotypic expression (group 5), non-cognitive female phenotypes (group 6), female phenotypes only in X:autosome translocations (group 7), no female phenotype (group 8), and unknown status of phenotypic expression in females (group 9). The presence of skewed XI was noted for genes in each group (Table 1, Figure 1).
| Group | Female phenotypic expression | Number of genes (% total) | Skewed XI (%) | Random XI (%) | Variable XI (%) | No XI information (%) | Escape gene (%) | No expression in blood (%) |
|---|---|---|---|---|---|---|---|---|
| 1 | Predominantly female phenotypic expression and male lethality | 8 (5.6) | 1 (12.5) | 2 (25.0) | 4 (50.0) | 1 (12.5) | 2 (25.0) | 1 (12.5) |
| 2 | Female phenotypic expression equivalent or greater than males | 13 (9.0) | 3 (23.1) | 1 (7.7) | 8 (61.5) | 1 (7.7) | 5 (38.5) | 3 (23.1) |
| 3 | Female phenotypic expression and normal males | 1 (0.69) | 0 | 1 (100.0) | 0 | 0 | 1 (100.0) | 1 (100) |
| 4 | Cognitive defects in females, milder than males | 41 (28.5) | 2 (4.9) | 10 (24.4) | 23 (56.1) | 6 (14.6) | 4 (9.8) | 9 (22.0) |
| 5 | Rare female phenotypic expression | 22 (15.2) | 5 (22.7) | 6 (27.3) | 6 (27.3) | 5 (22.7) | 0 | 5 (22.7) |
| 6 | Non-cognitive phenotypic expression in females | 8 (5.6) | 2 (25.0) | 1 (12.5) | 3 (37.5) | 2 (25.0) | 0 | 1 (12.5) |
| 7 | Female phenotypes in X-autosome translocations only | 3 (2.1) | 3 (100.0) | 0 | 0 | 0 | 0 | 2 (6.7) |
| 8 | No female phenotypic expression | 45 (31.3) | 7 (15.5) | 11 (24.4) | 10 (22.2) | 17 (37.8) | 4 (8) | 12 (26.7) |
| 9 | Unknown status in females | 3 (2.1) | 1 (33.3) | 1 (33.3) | 0 | 1 (33.3) | 0 | 1 (33.3) |
| Total | 144 (100.0) | 24 (16.6) | 33 (22.9) | 54 (37.5) | 33 (22.9) | 16 (11.1) | 35 (24.3) |
- Note: Percentage listed is percentage of total number within group, unless indicated otherwise.
- Abbreviation: XI, X-inactivation.
Kindreds from the Greenwood Genetic Center and from the University of Adelaide were reviewed for similar characteristics (Table S10). Genes with different XI patterns in the Greenwood cohort, the University of Adelaide cohort and the literature cohort were noted, and final XI pattern was determined by considering all cases together. All patients examined at the Greenwood Genetic Center and at the University of Adelaide were enrolled in a study protocol approved by the Self Regional Health Care or the Women's and Children's Health Network Human Research Ethics Committee in Adelaide (REC786/7/2020), respectively. All patients evaluated at both institutions provided appropriate informed consent.
3 RESULTS
Forty-five (31.3%) of the 144 XLID genes had no evidence of female phenotypic expression in female carriers, and in three (2.1%) XLID genes, the status of female phenotypic expression was unknown (Table 1). Female phenotypic expression with associated cognitive impairment was seen in 88 (61.1%) XLID genes, while in eight (5.6%) genes female phenotypic expression was present but cognitive function was normal. Twenty-four (16.6%) XLID genes had skewed XI reported in over 90% of cases reviewed (Table 2). Fifty-four (37.5%) had variable XI, with cases of both random and non-random XI. Thirty-three (22.9%) XLID genes had predominantly random XI (Table 3), while the XI pattern was unknown in 33 (22.9%). The XI pattern was consistent between the literature, the Greenwood Genetic Center, and the University of Adelaide cohorts in 78.5% (113/144, Table S10a) of the XLID genes studied. Thirty-five (24.3%) of the XLID genes studied are not expressed in white blood cells (Table 4), and sixteen (11.1%) of the XLID genes show incomplete XI (Table 5).
| HDAC6 | HDAC8 | RLIM | FTSJ1 |
|---|---|---|---|
| DKC1 | SMC1A/SMC1L1 | RNF113A | HCFC1 |
| ACSL4 | SRPX2 | KLF8 | OGT |
| KLHL15 | MTM1 | ZDHHC15 | RAB40AL |
| HPRT | SLC25A5 | ZMYM3 | SHROOM4 |
| IDS | CUL4B | FANCB | UBE2A |
| ALG13 | PDHA1 | CNKSR2 | FAM50A | ZDHHC9 |
|---|---|---|---|---|
| MECP2 | PCDH19 | NDUFA1 | RBM10 | NXF5 |
| GDI1 | AP1S2 | PLP1 | SLC16A2 | KIAA2022 |
| FGD1 | CLCN4 | THOC2 | SYP | SLC6A8 |
| OTC | CLIC2 | NDP | ZNF711 | SLC9A7 |
| SLC9A6 | GPC3 | ATP6AP2 | ZFP92 | RAB39B |
| NLGN3 | CCDC22 |
| MECP2 | DLG3 | SRPX2 | PCDH19 | CLCN4 |
|---|---|---|---|---|
| DCX | GRIA3 | IL1RAPL1 | KIAA2022 | MID1 |
| NHS | OCRL1 | SYN1 | ARX | DMD |
| FRMPD4 | NLGN4 | PTCHD1 | NDP | KLF8 |
| ZDHHC15 | AGTR2 | MID2 | NLGN3 | SLC16A2 |
| SLC9A7 | SOX3 | USP27X | ZNF711 | ZDHHC9 |
| ZNF81 | EFHC2 | HS6ST2 | MAOA | IQSEC2 |
| NEMO | OFD1 | DDX3X | MSL3 | SMC1A/SMC1L1 |
|---|---|---|---|---|
| USP9X | PCDH19 | AP1S2 | IQSEC2 | KDM5C |
| KDM6A | SSR4 | EIF2S3 | HS6ST2 | MAOA |
| TAF1 |
Group 1 was comprised of eight XLID genes associated with syndromes with predominantly female phenotypic expression and presumed male lethality (Table S1). Several of these genes were associated with widely recognized syndromes: Rett syndrome [MIM: 312350] (MECP2 [MIM: 300005]), incontinentia pigmenti [MIM: 308300] (IKBKG [MIM: 300248]), oral-facial-digital syndrome 1 [MIM: 311200] (OFD1 [MIM: 300170]) and Goltz syndrome [MIM: 305600] (PORCN [MIM: 300651]). Two genes within this group, IKBKG and OFD1, escape X-inactivation. Chassaing-Lacombe chrondrodysplasia [MIM: 300863] (HDAC6 [MIM: 300272]) was the only syndrome in this group that had consistently skewed X-inactivation. MIDAS [MIM: 3098010] (HCCS [MIM: 300056]), incontinentia pigmenti (IP, IKBKG), oral-facial-digital 1 (OFD1) and Goltz syndrome (PORCN) syndromes occasionally had skewed XI (variable XI). Congenital disorder of glycosylation 1s [MIM: 300884] (CDG1s, ALG13 [MIM: 300776]) and Rett Syndrome (MECP2) consistently had random XI; however, MECP2 also does not have evidence of gene expression in blood. Clinical presentations of the syndromes within this group varied widely. However, most were associated with seizures; only Goltz syndrome and Chassaing-Lacombe chondrodysplasia were not. Four genes were associated with microcephaly, either congenital in the case of incontinentia pigmenti and Goltz or acquired in Bain type XLID [MIM: 300986] and in Rett syndrome. Three syndromes (MIDAS, IP, and Goltz) had notable cutaneous manifestations. Cognitive function within this group varied and was reported to be normal in up to 50% or more cases of IP, Goltz and OFD1.
Group 2 was comprised of 13 XLID genes associated with syndromes with equivalent male and female expression (Table S2). In X-linked Cornelia de Lange [MIM: 300882] (HDAC8 [MIM: 300269] and SMC1A [MIM: 300040]) and XLID-rolandic seizures [MIM: 300643] (SRPX2 [MIM: 300642]), XI was skewed, and in another eight XLID genes, the XI pattern was variable. Rett-like seizures-hypotonia [MIM: 300672] (CDKL5 [MIM: 300203]) showed predominantly random XI. Five genes within this group escape XI (DDX3X, IQSEC2 [MIM:300532], MSL3 [MIM:300609], SMC1A, USP9X [MIM:300072]), and DLG3 [MIM: 300189], IQSEC2 and SRPX2 do not have evidence of gene expression in blood. As in group 1, the clinical presentation of syndromes within this group varied considerably, as did the functional pathways of the associated genes. In most of the syndromes within this group, cognitive delay was a major feature of the clinical presentation. The notable exception was in periventricular heterotopia, which is typically associated with normal cognitive function in heterozygote females. Seizures, hypotonia, facial dysmorphism and brain abnormalities were common presentations within this group of syndromes. In contrast, cutaneous symptoms were rare and only seen in IDX102 (DDX3X) and IDX99 (USP9X).
Group 3 was only associated with one XLID gene, PCDH19 [MIM: 300460], which causes epilepsy-intellectual disability in females, a syndrome with phenotypic expression in females and no transmitting male phenotype (Table S3). The PCDH19 gene encodes a protocadherin protein involved in cell adhesion. This gene has been found to be subject of XI across 27 human tissues studied.20 Female carriers develop neurologic manifestations in infancy with the onset of seizures. Neurologic regression and cognitive impairment of variable severity is also characteristic. In this syndrome, the XI pattern as tested on blood and primary skin fibroblasts was predominantly random.
Group 4 was comprised of 41 XLID genes associated with syndromes that had cognitive defects in females milder than males (Table S4). In this group, dyskeratosis congenital [MIM: 305000] (DKC1 [MIM: 300126]) and XLID-hypogonadism-tremor [MIM: 300354] (CUL4B [MIM: 300304]) showed consistently marked skewing of XI. Twenty-three (58.5%) genes in this group had variable skewing of XI. Ten (24.4%) XLID genes had predominantly random XI, and in six (14.6%), the XI pattern was unknown. Four genes in this group escape XI, and nine genes do not have gene expression in blood. Seizures and abnormal muscle tone were relatively common symptoms, reported in over half of the syndromes within this group. Additionally, the majority (80%, 33/41) of the syndromes within this group had significant non-neurologic findings, with associated skeletal and cardiac anomalies predominating. The genes within this group are involved in a variety of different cellular and molecular pathways, although over half (21/41) of the genes function in processes related to neuronal and synaptic function, as well as transcriptional regulation.
Group 5 was comprised of 22 XLID genes associated with syndromes with rare female phenotypic expression (Table S5). Five (22.7%) genes within this group had consistent skewing of XI, and six (27.3%) had variable skewing of XI. In six (27.3%) genes, the XI pattern was consistently random, and in five (22.7%), the XI pattern was unknown. None of the genes in this group escapes XI; however, five are not expressed in blood. Facial dysmorphism and brain abnormalities were present in 12 (54.5%) associated conditions, while growth deficiencies were uncommon and only present in four: ARX-associated XLID syndromes [MIM: 300419], ATRX-associated XLID syndromes [MIM: 301040], IDX 30 and 47 [MIM: 300558] (PAK3 [MIM: 300142]), and IDX 12 and 35 [MIM: 300957] (THOC2 [MIM: 300395]. Cutaneous findings were also rare and only present in Hunter syndrome [MIM: 309900] (IDS [MIM:300823]).
Group 6 was comprised of eight XLID genes associated with XLID syndromes with non-cognitive phenotypes in females (Table S6). Trichiodystrophy 5 [MIM: 300953] (RNF113A [MIM: 300951]) and TOKAS (Tonne-Kalscheuer [MIM: 300878], RLIM [MIM: 300379]) had consistent skewing of XI. GPKOW-associated XLID [MIM: 301003], Schimke syndrome [MIM: 312840] (BCAP31 [MIM: 300398]), and IFAP (ichthyosis follicularis, atrichia, and photophobia [MIM: 309205]; MBTPS2 [MIM: 300294]) had variable skewing of XI. Only Norrie syndrome [MIM: 310600] (NDP [MIM: 300658]) had consistently random XI. X-inactivation status was unknown in MEND (male EBP disorder with neurologic defects [MIM: 300960], EBP [MIM: 300205]) and Mircsof-Langouet [MIM: 300967] (NONO [MIM: 300084]) syndromes. None of the genes within this group escapes XI, and only NDP is not expressed in blood. All associated syndromes had evidence of multi-organ system involvement, and all except IFAP (MBTPS2) were associated with growth deficiency. Additionally, structural brain abnormalities were present in all conditions within this group, with the exception of Norrie syndrome (NDP).
Group 7 contained three genes that were associated with XLID syndromes with female phenotypes in X:autosome translocations only (KLF8-associated XLID [MIM: 300286], ZMYM3-associated XLID [MIM: 300061], and IDX91 [MIM: 300577] (ZDHHC15 [MIM: 300576]). All three conditions showed skewed XI (Table S7). Growth deficiency, abnormal head size and brain abnormalities were not part of the phenotype in any of the conditions within this group, while all three syndromes had associated minor skeletal anomalies as part of the disease presentation. None of the genes within this group escapes XI, and both KLF8 and ZDHHC15 are not expressed in blood.
Group 8 contained 45 XLID genes associated with syndromes with no phenotypic expression in female carriers, making this group the largest of the nine categories (Table S8). Seven (15.5%) XLID genes had consistent skewing of XI, and 10 (22.2%) XLID had variable skewing of XI. Eleven (24.4%) XLID genes had random XI, and no XI information was available for 17 (37.8%) genes within this group. Escape from XI has been reported in EIF2S3 [MIM: 300161] (MEHMO syndrome [MIM: 300148]), HS6ST2 [MIM: 300545], MAOA [MIM: 309850] (monoamine oxidase A deficiency [MIM: 300615]), and TAF1 [MIM: 313650] (XLID-associated craniofacialcaudal syndrome [MIM: 300966]), and 12 genes in this group are not expressed in blood. This group included a number of syndromic and non-syndromic XLID conditions encompassing a wide range of clinical presentations. However, some of the more common clinical findings included seizures (present in 24/45 syndromes), facial dysmorphism (present in 30/45 syndromes), and abnormalities in multiple organ systems (present in 28/45 syndromes).
Group 9 was comprised of three genes (KLHL15 [MIM: 300980], NXF5 [MIM: 300319], EFHC2 [MIM: 608815]) associated with syndromes where the female phenotypic expression was unknown (Table S9). IDX103 [MIM: 300982] (KLHL15) had consistent skewing of XI, and NXF5-associated XLID had consistent random XI, while the XI status was unknown in IDX74 (EFHC2). None of the genes within this group escapes XI, and only EFHC2 is not expressed in blood. All associated syndromes had speech delay as part of the male phenotype. Additionally, minor skeletal anomalies and facial dysmorphism were part of the male phenotype in IDX103 (KLHL15) and NXF5-associated XLID. IDX103 (KLHL15) also had associated polymicrogyria and ventricular enlargement as part of the disease pathology.
4 DISCUSSION
XLID syndromes have classically been considered recessive conditions, with rare phenotypic expression in female carriers secondary to protection offered by the unaffected allele on the normal X chromosome, in accordance with the Lyon hypothesis.6 However, the findings presented here suggest that describing XLID conditions as predominantly recessive may not be accurate for all XLID conditions. Moreover, it shows that female phenotypic expression of XLID variants exists on a continuum, ranging from those following a dominant inheritance pattern in conditions with a severe phenotype in females to truly recessive conditions with no phenotype in carrier females.
Evidence showing a higher frequency of skewing in carriers of XLID conditions supports a role for skewing of XI in determining the presence of a phenotype in carrier females. However, with recent studies providing evidence for variable patterns of XI with age and tissue type within individuals, it is clear that the relationship between XI and phenotypic expression patterns in female carriers of XLID conditions is more complex than initially thought.13, 14 The results presented here provide further support for this observation and show that categorizing XLID genes by female phenotype does not clearly identify shared patterns of XI in carrier females. In this study, the majority of XLID genes had variable patterns of XI regardless of female phenotype. Additionally, only 16.7% (16/96) of XLID genes with associated female phenotypic expression and 17.7% (8/45) of XLID genes with no female phenotype or an unknown status of female phenotypic expression had skewing of XI. While these numbers are higher than the reported prevalence of skewing in normal non-carrier females (3.6%), it does not appear that skewed XI favoring expression of the variant allele in affected females or favoring expression of the normal allele in non-affected carriers explains female phenotypic expression patterns in the majority of XLID syndromes.22 However, XLID genes with female phenotypes only in X:autosome translocations is a notable exception to the above observations, and in these cases specifically, skewing of XI is a well-documented phenomenon believed to occur in order to ensure the correct gene dosage of the autosomal segment.23 This study supports these findings with all cases of X:autosome translocations reported here having skewed XI.
Several groups have shown that in some families with XLID conditions, skewing of XI seems to influence the severity of the phenotype present in carrier females, and in a few families presented here, this was also shown to be the case (Table S10).24, 25 For instance, in one family described with MASA syndrome (caused by variants in L1CAM [MIM: 308140]), marked skewing of XI was only observed in symptomatic carrier females, while random XI was only observed in the non-affected female carrier in the kindred, suggesting that in this particular family, skewing of XI may be driving the phenotype of female carriers.26 In cases where female carriers show preferential expression of the variant allele, follow-up studies to determine if additional pathologic variants or structural rearrangements are present on the normal allele, which may explain preferential inactivation of the normal X-chromosome and expression of the chromosome with the variant, may be warranted. In most families, presence or absence of phenotype did not correlate with the level of X-inactivation. However, several groups have shown that XI can be a heritable trait that is influenced by one or more X-linked alleles; it is therefore possible that in some families, the genetic background/modifiers rather than the presence of an XLID variant have a greater influence on the observed skewing pattern.27-29
As several groups have emphasized, incomplete inactivation of genes on the inactive X-chromosome should be considered when interpreting XI studies and in this study 11% (16/144) of XLID genes were found to escape inactivation, which is similar to the percentage of all X-linked genes currently predicted to escape inactivation (15%).19, 30 Evidence suggests that expression of individual genes from the inactive X-chromosome likely represents a continuum from complete silencing to full expression. Escape genes are considered to be those with expression from the inactive X-chromosome of at least 10% of that from the active X-chromosome.30 However, in this study, four escape genes (EIF2S3, HS6ST2, MAOA, and TAF1) did not show phenotypic expression in carrier females, and although some degree of gene expression from the variant allele would be expected, perhaps in these cases gene expression from the inactive X-chromosome is insufficient to result in clinical symptoms.16, 20, 31 Further work should clarify the relative expression level of the inactive X-chromosome in these four escape genes with no female phenotype, as it is plausible that the threshold of clinically relevant gene expression is higher than 10% of that from the active X-chromosome. Additionally, several X-linked genes are thought to have variable escape—showing tissue-specific differential gene expression from the inactive X-chromosome—and so tissue-specific gene escape patterns may also be contributing to these obseverations.32
X-inactivation studies are typically performed on leukocytes because of ready availability of these cells, and in this survey, only XI studies performed on blood were included in analysis.32 Importantly, however, as studies have shown XI patterns vary among different tissue types, expression from the gene of interest in blood should also be considered when interpreting XI patterns as the results are only relevant if the gene of interest is expressed in blood.14, 33 Thirty-five of the 144 XLID genes (24.3%) studied do not have gene expression in blood, suggesting that for these genes, the results of XI studies performed using blood samples should be interpreted with caution. The presence of skewed XI co-occurring with a variant in an XLID gene that is not expressed in blood may indicate the existence of a modifier of XI elsewhere on the genome or the presence of mutations in another XLID gene. Notably, among the genes not expressed in blood was MECP2, a gene in which the XI pattern has been well studied in blood.34, 35 While some early studies of individual families suggested skewed XI may influence the phenotype of female carriers with variants in MECP2, more recent studies have not shown this to be the case.32 The results presented here suggest that conflicting XI studies using blood samples may be explained in part by the absence of MECP2 expression in blood, and so in Rett syndrome specifically, work attempting to incorporate XI studies in mouse or post-mortem human brain tissue would be of significant interest and perhaps help clarify the role of XI in the female phenotype.32
Notably, the XI status was unknown in 33 (22.9%) XLID conditions studied. The majority of these conditions (22/33) are associated with XLID genes that have rare or no female phenotypic expression—highlighting the bias in XI studies toward syndromes with a known female phenotype. However, many of the XI studies in the literature were performed using the human androgen receptor assay, which relies on differential parental expression of short tandem repeats at the androgen receptor (AR) site, by definition requiring a prior knowledge of the parental genotype, which in many cases is not available.36 As RNA-sequencing (RNA-seq), which uses computational techniques to phase alleles instead of knowledge of parental genotype, is becoming more commonly used to determine XI status, expect this technique may provide more insight into the XI status of many of the XLID conditions with unknown XI patterns in female carriers.37 Additionally, the validity of 25 of the 144 genes included in our survey as XLID-associated genes has been questioned by Piton et al.38 Ten of the genes have been removed from the list of questioned genes, leaving 15 (ZNF41 [MIM: 314995, ZNF674 [MIM: 300573], MAGT1 [MIM: 300715], ZCCHC12 [MIM: 300701], RPL10 [MIM: 312173], CLIC2 [MIM: 300138], ZNF81 [MIM: 314998], KLF8, IGBP1 [MIM: 300139], ZMYM3, ZDHHC15 [MIM: 300576], SRPX2, NXF5, AGTR2 [MIM: 300034], and ARHGEF6 [MIM: 300267]) in need of replication or evidence of pathogenicity.
In summary, this survey finds that, in contrast to a commonly held view, cognitive impairment occurs in the majority (61.1%) of female carriers of pathogenic XLID gene variants, even though usually at a lesser level of severity than in males, given the partial compensatory action of the wild-type X. Second, and again contrary to expectation, the presence of cognitive impairment in carrier females does not consistently correlate with XI, as measured in blood. Significantly skewed XI was found in only a minority of carrier females and in all cases of X:autosome translocations. However, in these latter cases, inactivation of the normal X is obligatory to protect against inactivation of the autosomal segments involved in the translocation. Third, the carrier female phenotype, when present, does not necessarily match that of the affected males in the family, and occasionally, it does not include cognitive impairment. Finally, no correlation was found between carrier female phenotypic expression and the functional pathway of the disease-causing gene.
Recognition of phenotypic expression in the majority of females heterozygous for XLID gene variants has significance in the clinical setting, bearing on recurrence risks and disease severity in the different sexes. With the increased use of whole exome sequencing, mutations in XLID genes are being detected in singleton females. These cases may be informative when compared to female carriers in large pedigrees but are subject to the bias that most cases studied in this fashion are likely to have a phenotype. The provisional and tentative conclusion is that female phenotypic expression in XLID disorders is multifactorial in etiology, with the genetic background in any given family possibly playing a significant modulating role. Future work incorporating newer techniques such as RNA-seq may allow for a better understanding of how skewing of XI, the functional pathway of the gene, and the variant type may act to influence phenotypic expression in carrier females.
ACKNOWLEDGMENTS
This study was made possible by support from the South Carolina Department of Disabilities and Special Needs. This manuscript is dedicated to the memory of Ethan Francis Schwartz, 1996 to 1998.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.