New SMARCE1 variant in a patient with features overlapping with oculoauriculofrontonasal syndrome
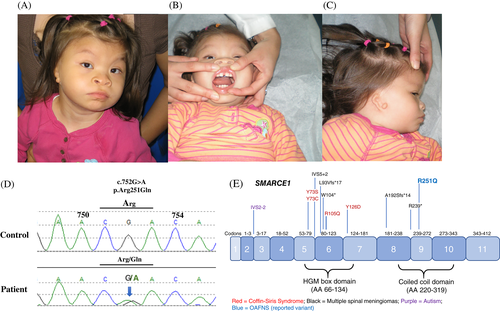
We report a Hispanic female with significant delay in intellectual and motor development, microcephaly, hypotonia, deafness, congenital heart defects including a patent ductus arteriosus and a bicuspid aortic valve, and craniofacial features overlapping with oculoauriculofrontonasal syndrome (OAFNS) including hypertelorism, mandibular hypoplasia, broad bifid nose, depressed nasal bridge, widely spaced maxillary central incisors, left microtia (Grade I), right microtia/atresia (Grade III), right ear skin tag, right iris coloboma, and macrostomia (Figure 1A-C). The proband was born to non-consanguineous healthy parents after uneventful pregnancy without complications including maternal diabetes, referred to us at age 3 years. She had long slender fingers and toes with normal fingernails and toenails. Brain MRI studies at 1 year of age showed hypogenesis of the splenium of the corpus callosum. Differential diagnoses included OAFNS based on her craniofacial dysmorphic features although severe intellectual disability was atypical for OAFNS. Single-nucleotide polymorphism (SNP) microarray study was normal. Whole exome sequencing was performed at age 10 years in comparison with her parents and a healthy younger brother. A novel de novo SMARCE1 missense alteration c.752G > A, p.Arg251Gln (ENST00000348513 NM_003079), was identified and confirmed by Sanger sequencing in the patient (Figure 1D). Variant analysis was conducted based on annotation from multiple annotation sources including ClinVar, dbSNP, dbNSFP, Broad ExAC, HGMD, MedGen, NCBI, OMIM, UCSC Genome Browser, Alamut, locus specific databases, literature searches, and other molecular biological principles.
OAFNS is a rare disorder of unknown etiology with features of both oculoauriculovertebral spectrum (OAVS) and frontonasal dysplasia (FND). It is thought to be a result of the abnormal development of structures in the midface and in the first and second branchial arches. OAVS is a first- and second-branchial arch syndrome of unknown etiology characterized by epibulbar dermoids, microtia, preauricular tags, and hemifacial microsomia. FND is due to the defects in the frontonasal process characterized by ocular hypertelorism, anterior cranium bifidum occultum, broad nasal root, and bifid nose, and is known to be caused by mutations in the Aristaless-like homeobox genes including ALX4. Homozygous mutations in ALX4 are known to cause FND2. ALX4 interacts with LEF1, which encodes a transcription factor that interacts with one of the SWI/SNF-related genes, that is, SMARCA4, via CTNNB1.1 Inactivation of ARID1A, a component of the SWI/SNF complex, in neural crest cells is known to cause craniofacial defects in mice.2
SMARCE1 encodes the protein which is a part of the SWI/SNF complex. Gain-of-function mutations or dominant-negative effects and loss-of-function mutations in SMARCE1 have been reported in four patients with Coffin-Siris syndrome 5 (CSS5)3 and in patients with familial spinal meningiomas,4 respectively (Figure 1E). All of the mutations in CSS5 are in the highly mobility group domain of SMARCE1. Mutations reported in familial spinal meningiomas distribute throughout the gene including the coiled-coil domain, where the identified variant located in the proband. The heterozygous missense variant in the coiled-coil domain may act as a dominant-negative or a gain-of-function mutation. In fact, there is no evidence that the identified variant has any relation to the phenotype, there are a few common findings described in patients with CSS5, that is, abnormal corpus callosum, flat nasal bridge, thin upper lip, prenatal and postnatal growth delay, and intellectual disability5. Interactions between ALX4 and the SWI/SNF complex may suggest the involvement of the SMARCE1 variant in the observed craniofacial findings. Further studies with more cases are necessary to evaluate if SMARCE1 is responsible for the patient's distinctive clinical phenotype overlapping with OAFNS. Approval of the Institutional Review Board was not required for this single case report. Written informed consent for publication was obtained.
ACKNOWLEDGEMENT
We thank the family for allowing us to publish this case report.





