Age-related alterations in immune responses to West Nile virus infection
Summary
West Nile virus (WNV) is the most important causative agent of viral encephalitis worldwide and an important public health concern in the United States due to its high prevalence, severe disease, and the absence of effective treatments. Infection with WNV is mainly asymptomatic, but some individuals develop severe, possibly fatal, neurological disease. Individual host factors play a role in susceptibility to WNV infection, including genetic polymorphisms in key anti-viral immune genes, but age is the most well-defined risk factor for susceptibility to severe disease. Ageing is associated with distinct changes in immune cells and a decline in immune function leading to increased susceptibility to infection and reduced responses to vaccination. WNV is detected by pathogen recognition receptors including Toll-like receptors (TLRs), which show reduced expression and function in ageing. Neutrophils, monocyte/macrophages and dendritic cells, which first recognize and respond to infection, show age-related impairment of many functions relevant to anti-viral responses. Natural killer cells control many viral infections and show age-related changes in phenotype and functional responses. A role for the regulatory receptors Mertk and Axl in blood–brain barrier permeability and in facilitating viral uptake through phospholipid binding may be relevant for susceptibility to WNV, and age-related up-regulation of Axl has been noted previously in human dendritic cells. Understanding the specific immune parameters and mechanisms that influence susceptibility to symptomatic WNV may lead to a better understanding of increased susceptibility in elderly individuals and identify potential avenues for therapeutic approaches: an especially relevant goal, as the world's populating is ageing.
OTHER ARTICLES PUBLISHED IN THIS REVIEW SERIES
Immunosenescence: the importance of considering age in health and disease. Clinical and Experimental Immunology 2017, 187: 1–3.
The convergence of senescence and nutrient sensing during lymphocyte ageing. Clinical and Experimental Immunology 2017, 187: 4–5.
Immune senescence: significance of the stromal microenvironment. Clinical and Experimental Immunology 2017, 187: 6–15.
Innate immune responses in the ageing lung. Clinical and Experimental Immunology 2017, 187: 16–25.
Intracellular signalling pathways: targets to reverse immunosenescence. Clinical and Experimental Immunology 2017, 187: 35–43.
Ageing and inflammation in patients with HIV infection. Clinical and Experimental Immunology 2017, 187: 44–52.
Considerations for successful cancer immunotherapy in aged hosts. Clinical and Experimental Immunology 2017, 187: 53–63.
Ageing and obesity similarly impair antibody responses. Clinical and Experimental Immunology 2017, 187: 64–70.
The life cycle of a T cell after vaccination – where does immune ageing strike? Clinical and Experimental Immunology 2017, 187: 71–81.
Herpes zoster and the search for an effective vaccine. Clinical and Experimental Immunology 2017, 187: 82–92.
Adult vaccination against tetanus and diphtheria: the European perspective. Clinical and Experimental Immunology 2017, 187: 93–99.
Emergence of West Nile virus infection
West Nile virus (WNV) is a mosquito-borne, enveloped, positive-strand RNA virus belonging to the family Flaviviridae. This virus family is particularly well known for yellow fever, dengue and the recently emerging Zika virus 1. Identified originally in Uganda more than 70 years ago, WNV was first identified in the United States in New York City in 1999 and has now spread across North America, South America and the Caribbean. More than 43 000 cases have been reported in the United States, including 1884 fatalities 2, 3, and the cumulative incidence of WNV infection is estimated to reach 3 million people 4. WNV is the most important causative agent of viral encephalitis worldwide and has become an important public health concern in the United States due to its high prevalence, severe disease in humans and the absence of effective treatments or vaccines 1, 3, 5, 6.
Notably, despite documented presence of WNV in Europe, no cases were noted from 1962 to 1985, and WNV was not considered a public health concern there 6. Europe has seen a rise in the number of cases since 2008, with > 2500 cases in the European Union (EU) (n = 788) and neighbouring countries (n = 1786) between 2011 and 2015, approximately 30% of which were in the Russian Federation 7, 8. WNV is now endemic in northern Italy 9. None the less, the case numbers are considerably lower than in the United States, and indeed no cases were detected, despite surveillance programmes, in the United Kingdom, Germany and Switzerland 6, 10. Lower incidence in Europe may be due in part to the lower density of competent Culex and Aedes species mosquito vectors, in contrast to the abundant presence of these mosquitoes across tropical and subtropical regions 11-13. Furthermore, as birds are required for the viral life cycle 1, bird population management is a factor in local levels of infection and levels of disease in birds can be used to monitor endemic infection 11, 14. The worldwide variation in incidence and severity of WNV infection highlights the many factors that contribute to infection, including climate, vector prevalence, bird populations, viral strain virulence and exposure history 6, 15.
Ageing is a risk for severe West Nile virus infection
The majority of WNV infections are asymptomatic (∼80%); however, some infected patients develop mild symptoms of West Nile fever (∼20%) and a small subset (<1%) develop severe neuroinvasive disease, which may include meningitis, encephalitis, acute flaccid paralysis and death 3. Resistance to infection is multi-factorial but, notably, age is the most well-defined risk factor – an approximately 20-fold increased risk at age > 60 – for susceptibility to neurological involvement and retinopathy associated with severe disease 7, 15-19. In this study, our understanding is reviewed of risk factors for severe infection with WNV and the effects of ageing on elements of immune responses relevant to those factors are highlighted. Understanding the specific immune parameters and mechanisms that influence susceptibility to symptomatic WNV may lead to a better understanding of increased susceptibility in elderly individuals and could be used to identify potential avenues for therapeutic approaches, an especially relevant goal as the world's populating is ageing 20.
Host factors are a risk for severe West Nile virus infection
Beyond risk factors related to exposure to infected mosquitoes, individual host factors have been shown to play a role in susceptibility to WNV infection (Fig. 1a). In particular, increased risk for severe infection has been identified with a history of immunosuppression, cardiovascular disease, chronic renal disease and hepatitis C virus infection 7, 21-23. Genomic studies of WNV cohorts have identified markers associated with host susceptibility to severe WNV infection, including certain human leucocyte antigen (HLA) types and single nucleotide polymorphisms (SNPs) in immune response genes, e.g. interferon (IFN) response pathway elements oligoadenylate synthetase (OAS), IFN regulatory factor 3 (IRF3), myxovirus resistance 1 (MX)-1, the chemokine C-C chemokine receptor type 5 (CCR5) and a replication factor and a sodium channel were associated with severity of infection 5, 24-30. Recent exome sequencing also identified HLA alleles, as well as ion channels, and immune regulation and IFN responses 31. In addition, studies show that a moderate, not exuberant, early response of type I IFN is crucial to control WNV without severe symptoms 32. Thus it is notable that the IFN-inducible transcription factor E74-like ETS transcription factor 4 (ELF4), which up-regulates and amplifies production of IFN, is elevated in subjects with a history of severe infection with WNV 33, 34. Further, other cytokines are also noted to be elevated in patients with neuroinvasive WNV infection, including elevated numbers of WNV-specific T lymphocytes which produced both IFN-γ and interleukin (IL)-4 35, and a heightened IFN-γ production in response to WNV in natural killer (NK) cells from symptomatic WNV subjects (Yao Y, Strauss-Albee DM, Zhou JQ, Malawista A, Garcia MN, Murray KO, Blish CA, Montgomery RR., unpublished article). WNV patients homozygous for high-expression alleles of the proinflammatory cytokine macrophage migration inhibitory factor (MIF) were > 20-fold more likely to have WNV encephalitis, consistent with a murine model showing that MIF is an important determinant of WNV neuropathogenesis 36, 37.
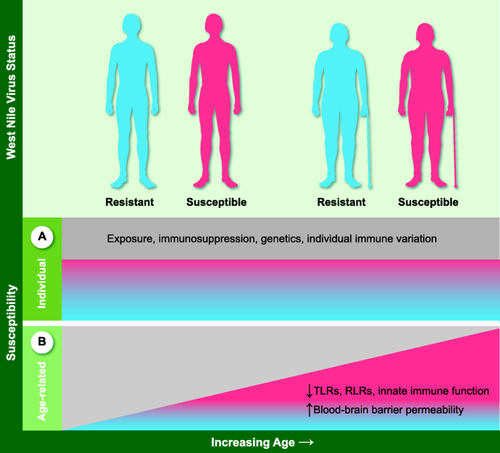
Determinants of susceptibility to West Nile Virus (WNV). Resistance and susceptibility to WNV infection result from exposure history and immune status including immunosuppression, cardiovascular disease, chronic renal disease and hepatitis C virus infection 7, 21-23. Individual susceptibility also derives from host factors such as single nucleotide polymorphisms (SNPs) in immune response genes 5, 24-30, and control of cytokine responses to infection 32-37 (a). Increased susceptibility to WNV in the elderly encompasses these determinants as well as impairments of immunity in ageing 38, 39 (b).
Effects of ageing on susceptibility to WNV: pathogen recognition and innate immune responses
Ageing is a critical risk factor for severe infection with WNV 7, 15-19. Our understanding of the basis for this increased susceptibility remains incomplete and is being investigated actively (Fig. 1b). Ageing is associated with distinct changes in immune cell populations and a progressive decline in immune function leading to increased susceptibility to infection 38, 39. Age-related changes in immunity are relevant at each step of WNV infection, from recognition of the virus to dissemination and immune responses (Fig. 2). WNV is detected by pathogen recognition receptors including Toll-like receptors (TLRs), which show reduced expression and functional efficiency in older donors 39. This is particularly relevant in the skin (Fig. 2a) for innate immune cells of the myeloid lineage, monocyte/macrophages and dendritic cells (DCs), which employ TLRs to recognize pathogens 40. In addition, TLR-7 has been shown to mediate immune cell homing to WNV-infected cells 41. Many functions of macrophages are known to be diminished with ageing, such as chemotaxis, phagocytosis, intracellular killing, production of reactive oxygen species and cytokines, expression of major histocompatibility complex (MHC) class II and co-stimulatory molecules. These changes are mediated largely by reductions in signalling mediators such as p38 mitogen-activated protein kinase (MAPK), nuclear factor kappa B (NF-κB), myeloid differentiation primary response gene 88 (MyD88) and the phosphorylation capacity of signal transducer and activator of transcription (STAT)-1α 42, 43. In WNV studies, primary human macrophages showed age-dependent impairment in TLR-3-mediated anti-WNV responses, leading to an early and sustained elevation of cytokines 44. While cytokine responses are essential to initiate immune responses and viral control, elevated levels of some cytokines have been shown to be detrimental in WNV infection 32-37. Thus, age-related over-exuberant cytokine production that disrupts the balance may be expected to be detrimental to anti-WNV responses.
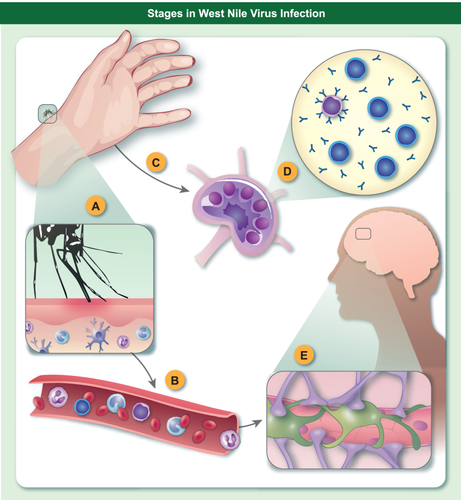
Schematic of key interfaces of West Nile Virus (WNV) susceptibility. Barriers to WNV infection by mosquito vector occur at numerous levels in the host from (a) skin surveillance by innate immune cells which recognize WNV and initiate innate immune responses; (b) blood-borne or (c) lymphatic dissemination; (d) lymph nodes for priming of adaptive T and B lymphocyte responses; and (e) the blood–brain barrier which protects the brain from infection. Each barrier shows reduced efficiency in ageing that contributes to increased susceptibility to WNV.
DCs also demonstrate an age-related decline in chemotaxis, endocytosis, a global reduction in expression of expression of TLR-1, -3, -5, -7 and -8, production of IL-12 and antigen presentation, leading to impaired activation of naive T cells 39. Paradoxically, DCs from elderly individuals also produce a higher basal level of cytokines [e.g. tumour necrosis factor (TNF)-α, IL-6 and IL-2], which can contribute to inflammation. Induction of co-stimulatory markers (CD80, CD86), TLR-7 and WNV-induced type I IFN were significantly lower in dendritic cells (DCs) from older human donors compared to younger donors 45, and older mice showed impaired TLR-7 signalling following WNV infection 46. Such critical deficits in DC signalling pathways would be expected to contribute to the increased susceptibility to WNV infections in elderly people.
Retinoic acid inducible protein 1 (RIG-I)-like receptors (RLRs) are important in the recognition of WNV 47, 48 and have been shown recently in monocytes from older donors to have impaired signalling leading to low induction of IFN and reduced resistance to influenza virus infection 49. RIG-I activation is regulated by acetylation mediated by histone deactylase 6 50. Notably, histone deactylation contributes to ageing-related muscle atrophy in a mouse model 51 and may be relevant to RIG-I activation and the age-related heightened immune state of ‘inflammageing’ 52. Potential effects of ageing on the viral DNA sensor cGAS, which also plays a role in WNV responses 53, remain to be determined.
Neutrophils, which are the first cells to respond to infection, show impairment with ageing of many functions relevant to anti-viral responses, including chemotaxis, phagocytosis, superoxide production, neutrophil extracellular traps (NET) formation, TLR expression and apoptosis 54-57. Although, in the early stages of infection, neutrophils serve as a reservoir for WNV replication and dissemination (Fig. 2b,c), they play a role later in WNV clearance 58 and have been noted to play a similar dual role in related arbovirus infections 59. Thus age-related reduction in functional activity probably contributes to the increased susceptibility to WNV infection in older subjects.
Tissue-specific IFN signalling pathways and expression of IFN-induced genes are critical in restricting WNV infection 60, 61. IFNs induce multiple response genes with anti-viral activity, such as IFN-response gene (ifit)2 and ifit3 62, 63, and detailed studies have identified IFN-β promoter stimulator 1 (IPS-1), working through RIG-I, to induce IFN in myeloid cells 64. In addition, IFNs are critical for the recruitment and activation of NK cells 65. NK cells have been associated with the control of many viral infections including HIV-1 and WNV 66-70. Recent studies have used mass cytometry (CyTOF) to highlight the NK cell repertoire. Notably, increases in diversity of the NK repertoire following infection with either HIV or WNV lead to terminal differentiation and reduced degranulation and an increased risk of viral acquisition 70. In ageing, NK cells show increased levels of a mature phenotype (CD57+) and receptor repertoire co-ordinated with higher cytokine production, reduced expression of activating receptors DNAX accessory molecule 1 (DNAM-1) and NKp30 and NKp46, as well as impaired cytotoxicity and decreased production of granzyme A 71-73. These age-related changes effect NK function and may contribute to reduced efficiency of anti-WNV responses.
Although studied in less depth, γδ T cells produce inflammatory cytokines (IL-17, IL-10 and TGF-β) in mouse models of WNV infections and deficient mice [T cell receptor (TCR)δ–/–] show increased susceptibility to WNV 74. The frequency and absolute number of γδ T cells are reduced in ageing, which may contribute to reduced anti-viral efficiency in WNV infection 75, 76.
Effects of ageing on susceptibility to WNV: adaptive immune responses
In addition to the reduction in innate recognition and signalling in ageing, age-related alterations of adaptive immunity compound these reduced efficiencies and play a critical role in increased susceptibility to WNV in elderly people (Fig. 2d). The reduction in naive T and B cells and dysregulated signal transduction and cytokine production, probably unrelated to chronic herpesvirus infection 77, 78, lead to decreased expansion and function of antigen-specific T and B cells 79. However, production of antibodies to WNV antigens did not differ with disease severity, suggesting that antibody levels do not correlate with susceptibility 80. Both CD4 and CD8 T cells 81, 82 are involved in resistance to WNV, as shown by elevated levels of T cell immunoglobulin and mucin domain-3 (Tim-3)+ (inhibitory) T cells in WNV infection 83. Regulatory T cells (Tregs) control acute infection 84 and were elevated in patients with a history of severe disease 34. Although human CD8 T cell responses to WNV are maintained despite ageing phenotypes 85, 86, several studies have shown age-related dysregulation of responses to WNV in T cells from older mice 46, 87, including an age-dependent decrease in trafficking of T cells in lymph nodes compounded by deficient cytokine production 88. Moreover, aged mice show lower levels of primary and memory T and B cell responses induced by vaccination with West Nile encephalitis vaccine, and repeated in-vivo restimulation is needed to generate protective cellular and humoral immunity in older animals 89. It is interesting to speculate whether reduced response to vaccination in ageing may be beneficial overall in infection with WNV, although not for other pathogens, by tempering cytokine production and inflammatory damage. While recent observations suggest that caloric restriction or treatment with rapamycin may be beneficial for longevity, these treatments reduced the function of T cells in infection with WNV with an adverse outcome 90.
Factors contributing to neurological involvement
Permeability of the blood–brain barrier, which is enhanced by cytokine responses, has been shown to be critical to susceptibility of neuroinvasive WNV infection (Fig. 2e) 91. The blood–brain barrier is a specialized physiological and functional barrier that separates the nervous system from the circulatory system and is essential to maintain a carefully regulated homeostasis within the brain. The blood–brain barrier has a well-documented functional decline with ageing leading to increased leakage of soluble factors and immune cell infiltration 92. Elements that decrease the integrity of the blood–brain barrier contribute to entry of WNV into the brain and susceptibility to severe infection with WNV 15. These include cytokines, adhesion molecules, proteases and infected leucocytes 37, 41, 93-97. TNF-α may contribute to severe disease by promoting blood–brain barrier permeability 93, and recent studies have identified activity of IFN-λ at epithelial cell barriers as a critical factor in restricting viral invasion of the brain 98, 99. During infection with WNV, CD8+ T cells expand dramatically and migrate to the site of CNS infection in response to neuronally derived CXCL10 100-102. Leucocytes crossing the blood–brain barrier may enhance disease by trafficking virus into the brain or increasing inflammation, or alternatively protect from severe disease, by promoting monocyte accumulation, which is critical to anti-viral activity in the brain or CD8 T cell control of virus 103, 104.
Effects of ageing on these pathways are not defined fully; however, for control of virus in the brain, IL-1β has been shown to be critically important in resistance to WNV, and animals lacking IL-1β or inflammasome signalling had elevated viral load and reduced CD8 cell immunity in the central nervous system (CNS) 105. In the brain, IL-1β and IL-1R1 signalling restrict WNV 105, 106, as does ifit2 62. Notably, chief among the differences revealed from a systems immunology profile of a stratified cohort of subjects with a history of asymptomatic or severe infection with WNV was decreased IL-1β production by macrophages and decreased CXCL10 expression from myeloid dendritic cells in response to WNV infection 34. Relevant to these observations is that IL-1β is also a key factor in neural inflammation in autoimmune disease 38. The IFN-IL-1β pathway is closely connected, with myeloid cells exhibiting suppression of IL-1β transcription upon IFN receptor engagement, perhaps mediated by the IRF8/IRF1 transcription regulation 107, 108. While effects of ageing on these pathways are not defined, these observations may be key to protection from severe disease.
Immune regulation in susceptibility to WNV
Several microRNAs, non-coding short RNAs involved in post-transcriptional regulation of gene expression, including micro-RNA (miR)-196a, -202-3p, -449c and -125a-3p, have been shown to be expressed differentially following WNV infection, suggesting their potential role in WNV resistance and pathogenesis 109, 110. Notably, numerous miRNAs are altered with ageing, which may be relevant to deficits noted in age-related immune responses 111, 112. Specific mechanisms have yet to be elucidated for miRNA regulation in age-related WNV susceptibility.
The TAM receptors [TYRO3 protein tyrosine kinase (Tyro3), AXL receptor tyrosine kinase (Axl) and MER proto-oncogene, tyrosine kinase (Mertk)] are a family of homologous receptor-tyrosine kinases expressed in monocytes and macrophages that suppress TLRs and lead to downstream pathways to control excess stimulation and restore homeostatic balance 113-115. Mice deficient for the homeostatic regulatory receptors, Mertk and Axl were more susceptible to WNV due to elevations in blood–brain barrier permeability 116. Mertk and type I IFN mediate tightening of tight junctions and reduce blood–brain barrier permeability 116. Recently, another role for these receptors has been recognized, related to identification of a family of receptors including TAMs and CD300a that facilitate viral uptake mediated by phosphatidyl serine, which binds virus and bridges binding to Mertk 117-119. Age-related up-regulation of one TAM receptor, Axl, has been noted previously in human DCs from older donors 45 and might contribute to increased viral uptake.
Conclusions
WNV gained public attention following its rapid spread throughout the continental United States and neighbouring countries. Thanks to active investigation, we have a good understanding of the ecology of WNV reservoirs and epidemiology of cases. However, unlike other recent dramatic viral epidemics, such as Ebola and Zika viruses, WNV is a particular concern for elderly people. Development of treatments is ongoing, with recent testing in vitro of Parkinson's drugs showing effective reduction of viral reproduction 120; development of some small molecule therapeutics that induce anti-viral gene transcription relevant against broad viral targets 121; and use of a synthetic TLR-4 agonist to increase efficacy of a WNV envelope-protein based vaccine candidate 122. However, no novel therapeutics are yet available for WNV treatment or prevention. With ongoing research efforts, our understanding of the role of cytokines and inflammation in age-related susceptibility to WNV infection is growing. An increased proinflammatory milieu prevails in ageing, and immunotherapeutics now provide specific pharmacological blockade of IL-1β activity in inflammatory diseases. Agents such as anakinra have an excellent safety record and multiple routes of administration, and resveratrol shows a strong inhibitory effect on proinflammatory marker secretion 123, 124. However, IL-1β plays an important role in controlling WNV in the neurological WNV infection, so tissue-specific balance is essential to guide development of targeted therapeutic approaches.
The need for greater understanding and treatments is compelling. A large portion of the world has a habitat suitable for Aedes mosquitoes and our population is ageing. Thus the range of the main WNV vector mosquito leads to potential exposure of > 2 billion people 13. Heightened attention to preventive measures and precautions is essential, particularly for older people, as well as intensified effort for discovery of targeted immune therapeutics.
Acknowledgements
This work is supported in part by awards from the NIH (N01-HHSN272201100019C and U19 AI 089992). The author thanks many colleagues for helpful discussions, Anna Malawista for valuable assistance, and regrets the omission of many excellent papers due to limitations of space.
Disclosure
The author has no disclosure to declare.