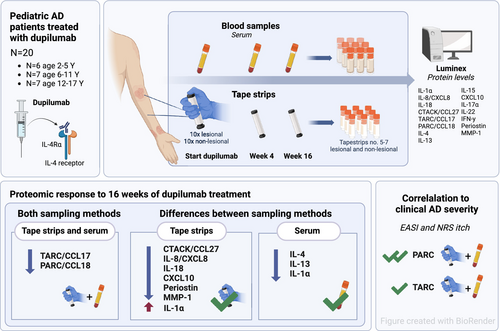
Complementary Analysis of Local and Systemic Effects of Dupilumab in Paediatric AD Using Tape Strips and Serum
Marjolein S. de Bruin-Weller and Marlies de Graaf contributed equally.
Funding: Patients included in this manuscript participated in the BioDay registry sponsored by Sanofi, Regeneron Pharmaceuticals, Lilly and Company, Pfizer, and LEO Pharma. Data collection, analysis, and publication of this article were not funded.
ABSTRACT
Objective
This study investigates local and systemic immune-related proteins in tape strips and serum of paediatric atopic dermatitis (AD) patients treated with dupilumab, and explores their correlation with clinical severity.
Methods
Twenty paediatric AD patients (< 18 years) starting dupilumab treatment were included. Serum samples and tape strips from lesional and non-lesional skin were collected at baseline, 4 and 16 weeks of treatment. Fifteen pre-specified proteins were measured at each visit by Luminex multiplex immunoassay. Clinical severity outcome measures included the Eczema Area and Severity Index (EASI) and Numeric Rating Scale (NRS) itch. Statistical analyses included Wilcoxon signed-rank tests and Spearman correlations.
Results
Along with clinical improvement, 16 weeks of dupilumab treatment resulted in a rapid and significant reduction in the disease-associated mediators PARC/CCL18 and TARC/CCL17 in both tape-stripped skin and serum. While the cytokine and chemokine profiles differed between the sampling methods, both effectively captured immunological changes associated with dupilumab treatment. Tape strips demonstrated significant reductions in innate pro-inflammatory cytokines (IL-8/CXCL8, IL-18), the T cell-recruiting chemokine CTACK/CCL27, the Type 1 immune mediator CXCL10, and tissue repair and remodelling proteins (periostin, MMP-1) in response to treatment, but were less sensitive in detecting T cell-derived cytokines (IL-4, IL-13). In both skin and serum, several proteins were significantly correlated with AD severity, as measured by EASI and NRS itch, with PARC/CCL18 emerging as the strongest correlated protein.
Conclusion
Our findings provide insight into the distinct local and systemic proteomic changes in response to dupilumab treatment in paediatric AD patients. These findings underscore the complementary roles of tape strips and serum in profiling immune and epidermal barrier proteins, highlighting the utility of minimally invasive tape stripping for monitoring proteomic responses to targeted therapies in paediatric AD.
Graphical Abstract
Abbreviations
-
- AD
-
- Atopic Dermatitis
-
- ADQ
-
- Atopic Dermatitis Quickscore
-
- CTACK
-
- Cutaneous T-cell-attracting Chemokine
-
- CXCL
-
- C-X-C motif chemokine ligand
-
- EASI
-
- Eczema Area and Severity Index
-
- IFN
-
- Interferon
-
- IGA
-
- Investigator's Global Assessment
-
- IL
-
- Interleukin
-
- IQR
-
- Interquartile Ranges
-
- MMP
-
- Matrix Metalloproteinase
-
- NRS
-
- Numerical Rating Score
-
- PARC
-
- Pulmonary and Activation Regulated Chemokine
-
- SCORAD
-
- SCORing Atopic Dermatitis
-
- TARC
-
- Thymus- and Activation-Regulated Chemokine
-
- Th
-
- T-helper
Summary
- Dupilumab significantly reduces immune-related proteins in tape-stripped skin and serum from paediatric atopic dermatitis patients.
- Skin tape strips and serum serve complementary roles in profiling immune and epidermal barrier proteins.
- Several proteins in skin and serum correlated with AD severity, with PARC/CCL18 showing the strongest correlation.
1 Introduction
Atopic dermatitis (AD) is a common chronic inflammatory skin disease affecting up to 20% of children worldwide [1]. Its pathogenesis is complex and highly heterogeneous, characterised by a predominant T helper (Th)2-driven inflammation, variable activation of Th22, Th17, and Th1 pathways, and a dysfunctional epidermal barrier [1-3]. The understanding of the underlying local and systemic mechanisms of AD, derived from molecular studies in skin biopsies and serum, has advanced in recent years. This has led to the development of targeted therapies [4].
Dupilumab, a monoclonal antibody blocking interleukin (IL)-4 and IL-13 receptor binding, was the first targeted treatment approved for (moderate to) severe AD, and is currently available for patients aged ≥ 6 months [5-7]. Molecular studies have been valuable in elucidating the modulatory effects of dupilumab on systemic AD-related proteins in paediatric AD patients [5-11]. However, its modulatory effects on local skin proteins in this paediatric population remain poorly characterised. This is primarily due to the limited data available and the challenges associated with skin biopsies, which are invasive, painful, and potentially scarring [12-14].
Tape stripping, a minimally invasive technique that samples the stratum corneum and upper stratum granulosum, is emerging as an alternative to skin biopsies for determining local proteomic signatures [15-17]. Recent studies have shown that tape strips effectively profile key immune and epidermal barrier dysregulations in skin inflammation, including AD and psoriasis, in both lesional and non-lesional skin [13, 17-26]. Moreover, tape strips have proven useful for tracking molecular changes in adult AD patients and a single paediatric cohort (n = 14) treated with dupilumab [14, 18, 20]. While previous studies have primarily focused on direct comparisons between local tape strips and skin biopsies, studies comparing tape strips with serum are limited, particularly in evaluating treatment response [16, 17, 24, 25, 27, 28]. However, such comparisons may provide crucial insights into distinct local and systemic proteomic signatures and changes, and may enhance our understanding of the proteomic changes in response to targeted treatment.
Therefore, this study investigated local and systemic immune-related proteins in tape strips and serum of paediatric atopic dermatitis (AD) patients treated with dupilumab, and explored their correlation with clinical severity.
2 Methods
2.1 Study Design
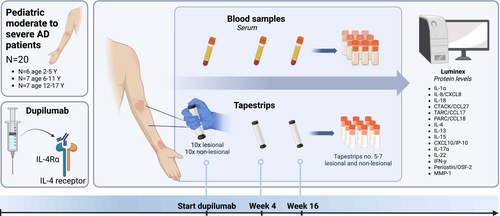
Twenty paediatric patients (aged < 18 years) with moderate-to-severe AD, eligible for systemic treatment and starting dupilumab treatment, were recruited from the Department of Dermatology at the Wilhelmina Children's Hospital (University Medical Center Utrecht), The Netherlands, between October 2022 and November 2023 (Figure 1). Exclusion criteria were the use of oral corticosteroids, small molecule inhibitors, or cyclosporine A within 1 week, methotrexate within 4 weeks, and biologics within 10 weeks prior to the start of treatment. All patients participated in the BioDay registry, a prospective, multicenter registry of patients treated with new systemic treatments for AD in daily practice. Written informed consent was obtained from all patients (and/or their parents). This study did not fall under the scope of the Medical Research Involving Human Subjects Act (METC 18/249 and 23/014) and has been performed according to the declaration of Helsinki.
2.2 Treatment
Dupilumab was administered subcutaneously at a dose of 200 or 300 mg every 2 or 4 weeks, including a loading dose of 400 or 600 mg at baseline or 200 or 300 mg on day 15 of treatment, depending on age and weight (label use) [5-8]. Background therapy with topical corticosteroids and calcineurin inhibitors was allowed during the observation period.
2.3 Clinical Assessment
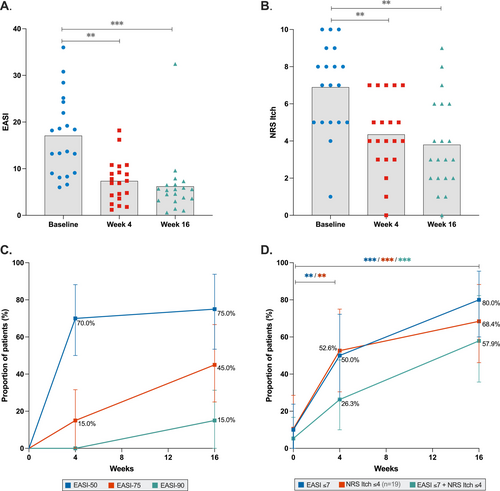
Patients visited the outpatient clinic at the start (baseline) and after 4 and 16 weeks of treatment. Demographics were collected at baseline. Clinical severity outcome measures, including the Eczema Area and Severity Index (EASI), Investigator's Global Assessment (IGA) score, and patient-reported Numeric Rating Scale (NRS) of average itch (NRS itch) and pain (NRS pain) over the past week (0–10 scale), were assessed at baseline and during follow-up. Outcome measures were further stratified according to EASI responses, reported as the proportion of patients achieving ≥ 50%, ≥ 75%, or ≥ 90% improvement in EASI score (EASI-50, EASI-75, and EASI-90, respectively) and absolute cutoff scores indicating disease control (EASI ≤ 7, NRS itch ≤ 4) [8, 29].
2.4 Blood and Tissue Sampling
Blood serum samples and ten consecutive tape strips from both lesional and non-lesional skin were collected at each visit. Adhesive D-Squame tape strips (3.8 cm2, D-squame, CuDerm, Dallas TX, USA) were applied with a standardised pressure (225 g cm2) using a D-Squame pressure instrument (D500, CuDerm, Dallas, TX, USA). Lesional tape strips were preferably taken from the elbow flexure, followed by the knee flexure. If these areas were unaffected, a site with representative lesional skin was selected. Non-lesional tape strips were preferably collected from the same anatomic region as lesional tape strips. To avoid bias in sampling, lesional and non-lesional tape strips were collected from the same site at each visit. After collection, tape strips and blood serum samples were stored frozen at −80°C until analysis.
For tape strip analysis, the 5th, 6th, and 7th tape strips were pooled for subsequent protein extraction, based on findings from our pilot study (unpublished data) and previous studies [22, 25, 30]. Tape strips were eluted overnight on an IKA Roller 10 using a 1 mL elution buffer containing PBS (0.5%), Tween 20 (1%), BSA, and one cOmplete protease inhibitor tablet per 25 mL. Elution buffer was removed and debris centrifuged the next day. The supernatant was subsequently yielded and added to a microwell plate for analysis. Supernatant samples were corrected for elution buffer background, and values above or below the assay detection limits were assigned values equal to the lower limit divided by two or the upper limit multiplied by two (Table S1).
2.5 Protein Analyses
Luminex multiplex immunoassay was used to quantify the levels of 15 pre-specified proteins in serum and tape strips collected from lesional and non-lesional skin [31]. All protein measurements were performed at the ISO9001:2008 certified multiplex core facility of the University Medical Center Utrecht, The Netherlands. Protein selection was based on findings from previous studies in both adult and paediatric AD [2, 8, 9, 17, 20, 22, 25, 28]. These included innate pro-inflammatory cytokines (IL-1α, IL-8/C-X-C motif chemokine ligand 8 [CXCL8], IL-18), Type 2 immune mediators (thymus- and activation-regulated chemokine [TARC/CCL17], pulmonary and activation-regulated chemokine [PARC/CCL18], cutaneous T-cell-attracting chemokine [CTACK/CCL27], IL-4, IL-13), Type 1 immune mediators (interferon-gamma [IFN-γ], CXCL10/interferon gamma-induced protein 10), T cell stimulating cytokine IL-15, Th17-associated cytokine IL-17α, Th22-associated cytokine IL-22, and tissue repair and remodelling proteins (periostin/osteoblast-specific factor 2, matrix metalloproteinase [MMP]-1).
2.6 Statistical Analyses
Unsupervised hierarchical clustering was performed to visualise differentially expressed proteins in serum, lesional, and non-lesional skin at baseline. Box and whisker plots were used to visualise the distribution of protein levels. Wilcoxon's signed-rank tests were applied for paired comparisons (e.g., baseline versus week 16). Sub-analyses were performed to evaluate potential differences in lesional skin protein levels between patients with and without topical corticosteroid use. Patients were stratified into age groups (2–5, 6–11, and 12–17 years) to assess age-related differences in protein expression. Radar plots were performed to illustrate protein levels by age group at baseline. Spearman correlation coefficients were used to determine associations between protein levels and disease severity, as measured by EASI and NRS itch, using data from all time points. p-values of < 0.05 were considered statistically significant. Data were analysed and figures were created using SPSS Statistics (version 29.0.1.0, IBM Statistics for Windows), RStudio (version 2024.04.1, PBC, Boston, USA), and GraphPad Prism (version 10.2.2, GraphPad Software, La Jolla, USA).
3 Results
3.1 Study Population and Clinical Improvement During Dupilumab Treatment
Twenty paediatric patients were included in the study (median age 10.0 years, interquartile range (IQR) 4.3–13.5 years; 70.0% female). Patient characteristics are shown in Table 1. Patients were evenly distributed across the age groups: 2–5 years (n = 6), 6–11 years (n = 7), and 12–17 years (n = 7). None of the patients reported a history of other inflammatory or autoimmune diseases (e.g., psoriasis, rheumatoid arthritis). The majority of tape strips were collected from the upper extremities (Table S2). Non-lesional tape strips were collected at least 4 cm from the lesional site, except in one patient where the distance was 2 cm. Most patients applied concomitant topical corticosteroids prior to sampling: 95.0% at baseline, 85.0% at week 4, and 75.0% at week 16 (median: 1.0 day prior; range 0–7 days) (Table S3). None of the patients applied concomitant topical calcineurin inhibitors to the sampling site.
| Characteristic | N = 20 |
|---|---|
| Sex (female), n (%) | 14 (70.0) |
| Age (years), median (IQR) | 10.0 (4.3–13.5) |
| 2–5 years, n (%) | 6 (30.0) |
| 6–11 years, n (%) | 7 (35.0) |
| 12–17 years, n (%) | 7 (35.0) |
| Age of AD onset (years), median (IQR) | 0.0 (0.0–0.0) |
| Atopic comorbidity, ≥ 1, n (%) | 17 (85.0) |
| Asthmaa | 13 (65.0) |
| Allergic rhinitisa | 16 (80.0) |
| Allergic conjunctivitisb | 11 (55.0) |
| Food allergyc | 6 (30.0) |
| First-degree relatives with atopic diseases,d ≥ 1, n (%) | 19 (95.0) |
| Atopic dermatitis, n (%) | 15 (75.0) |
| Allergic rhinitis, n (%) | 12 (60.0) |
| Asthma, n (%) | 10 (50.0) |
| Food allergy, n (%) | 2 (10.0) |
| Previous use of immunosuppressants, n (%) | 15 (75.0) |
| Oral corticosteroids | 4 (20.0) |
| Cyclosporine A | 10 (50.0) |
| Methotrexate | 1 (5.0) |
| EASI score, median (IQR) | 16.0 (9.0–23.7) |
| IGA score, n (%) | |
| Mild | 3 (15.0) |
| Moderate | 12 (60.0) |
| Severe | 5 (25.0) |
| Very severe | 0 (0.0) |
| NRS itch, median (IQR) | 7.0 (5.0–9.0) |
| Missing, n (%) | 1 (5.0) |
| NRS pain, median (IQR) | 6.0 (1.0–8.0) |
| Total IgE levels (kU/L), median (IQR) | 2335.0 (223.5–4777.5) |
| Missing, n (%) | 4 (20.0) |
| Eosinophil levels (×109/L), median (IQR) | 0.6 (0.4–1.0) |
| Eosinophilia (≥ 0.5 × 109/L), n (%) | 11 (55.0) |
- Abbreviations: AD, atopic dermatitis; EASI, Eczema Area and Severity Index; IGA, Investigator Global Assessment Scale; IgE, immunoglobulin E; IQR, Interquartile range; NRS, Numeric Rating Scale.
- a Diagnosed by pulmonologist.
- b Patient reported.
- c Confirmed by elevated (≥ 0.35 kU/L) food specific IgE levels and corresponding symptoms.
- d Self-reported.
Patients demonstrated significant clinical improvement during dupilumab treatment, with a median percentage improvement in EASI of 64.3% and NRS itch score of 37.5% at week 4, increasing to 67.0% and 46.4%, respectively, at week 16 (all p < 0.001) (Figure 2). At week 16, 80.0% of patients achieved EASI ≤ 7, 68.4% achieved NRS itch ≤ 4, and 57.9% met both criteria.
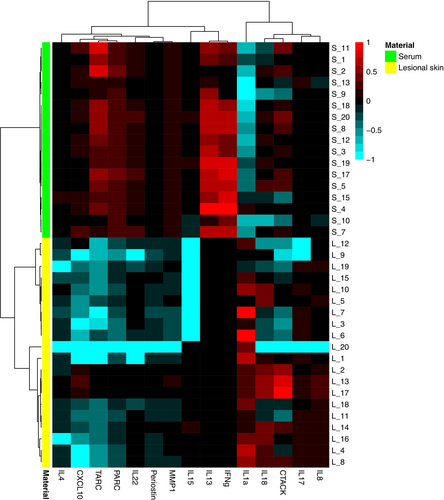
3.2 Tape Strips and Serum Capture Distinct Protein Expressions at the Start of Treatment
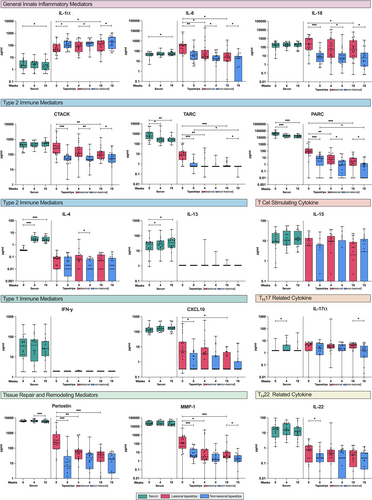
Differentially expressed proteins in tape-stripped lesional skin and serum at baseline are depicted in an unsupervised hierarchical clustering heatmap (Figure 3). Relatively high expression of innate pro-inflammatory cytokines (IL-1α, IL-18) was observed in tape strips of lesional skin, while T cell-derived cytokines were either undetectable (IL-13, IFN-γ) or poorly expressed (IL-4, IL-15, IL-22). In contrast, relatively high levels of T cell-derived cytokines (IL-13, IFN-γ) and Type 2 immune mediators TARC and PARC were observed in serum (Figure 3). Protein expression in non-lesional skin was generally lower than in lesional skin, except for IL-1α (Figure S1). Despite small sample sizes, consistent protein expression was observed across all age groups at baseline (Figure S2).
3.3 Proteomic Changes in Tape Strips and Serum During Dupilumab Treatment
In both lesional skin and serum, the disease-associated Type 2 immune mediators TARC and PARC significantly decreased after 4 and 16 weeks of dupilumab treatment, and significantly decreased in non-lesional skin after 16 weeks of treatment. However, distinct proteomic changes were observed in the skin and serum during treatment. In lesional skin, T cell-recruiting chemokine CTACK, innate pro-inflammatory cytokines (IL-8, IL-18), tissue repair and remodelling proteins (periostin, MMP-1), and Type 1 immune mediator CXCL10 significantly decreased after 16 weeks of treatment, while innate pro-inflammatory cytokine IL-1α significantly increased (Figure 4). Significant reductions in IL-8, periostin, and MMP-1 levels were observed after 4 weeks of treatment. In serum, IL-4 and IL-13 levels significantly increased after 4 and 16 weeks of treatment.
3.4 Tape Strip and Serum Proteins Correlate With Disease Severity
Several proteins were significantly correlated with AD severity as measured by EASI and NRS itch (Tables S4 and S5). In lesional skin, the strongest correlations with EASI and NRS itch were observed for Type 2 immune mediators PARC (r = 0.62 and r = 0.54, respectively), CTACK (r = 0.57 and r = 0.46, respectively), and TARC (r = 0.56 and r = 0.52, respectively). Tissue repair and remodelling proteins MMP-1 (r = 0.56 and r = 0.36, respectively) and periostin (r = 0.55 and r = 0.43, respectively), and innate pro-inflammatory cytokine IL-8 (r = 0.55 and r = 0.41, respectively) and IL-18 (r = 0.45 and r = 0.43, respectively) also showed significant correlations (Figure 5A, Figure S3A). Proteins in non-lesional skin demonstrated correlations with disease severity that were generally comparable to those in lesional skin, though less robust (Figure 5B, Figure S3B).
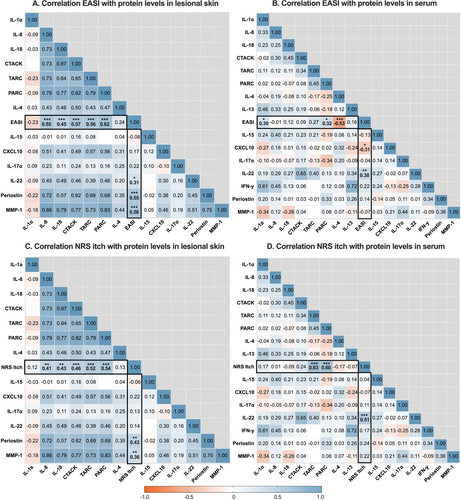
In serum, EASI and NRS itch were significantly correlated with Th22-related cytokine IL-22 (r = 0.38 and r = 0.51, respectively) and PARC (r = 0.32 and r = 0.60, respectively) (Figure 5C, Figure S3C). In addition, NRS itch was correlated with TARC (r = 0.63), whereas TARC was not significantly correlated with EASI. EASI was inversely correlated with Th2-related cytokine IL-4 (r = −0.53), which could be interpreted as IL-4 inhibition by dupilumab rather than being associated with EASI.
3.5 Monitoring Predictive Biomarkers in a Single Case of Atopic Dermatitis Flare
During the observation period, one patient experienced an acute AD flare at 16 weeks of treatment (EASI increased from 7.8 at week 4 to 32.5 at week 16). At weeks 4 and 16, skin and serum protein levels of innate (IL-8, IL-18) and Type 2 immune mediators (TARC, PARC, CTACK) increased in this patient, whereas they decreased in all other patients in whom AD severity decreased (Figure S4). Notably, in both lesional skin and serum, levels of CTACK and IL-18 were elevated in this patient before the flare (week 4) and further increased at week 16.
4 Discussion
This study explores the local and systemic immune signatures of paediatric AD patients treated with dupilumab by analysing tape strips and serum samples. By analysing both local and systemic proteins, we captured a partially overlapping yet distinct immune response characterised by robust Type 2-mediated inflammation. Tape strips capture reductions in innate pro-inflammatory cytokines (IL-8, IL-18) and tissue remodelling proteins (periostin, MMP-1) in response to treatment, reflecting improvements in local inflammation and barrier repair. In contrast, serum is more sensitive in detecting T cell-derived cytokines (IL-4, IL-13), reflecting systemic immune modulation. Additionally, the Th2-related chemokine PARC emerged as the strongest protein associated with clinical severity in both skin and serum.
Consistent with our findings at the start of dupilumab treatment, Andersson et al. and McAleer et al. observed elevated levels of innate mediators (IL-18, IL-8, IL-1β) and T cell-recruiting chemokines (TARC, CTACK) in tape-stripped lesional skin of paediatric AD patients, compared to non-lesional skin and healthy controls [23, 25]. In line, Del Duca et al. observed upregulation of innate pro-inflammatory cytokines (IL-8, IL-1β) in adult AD patients. In contrast to our findings, upregulation of IL-13 was observed in lesional tape-stripped skin compared to skin biopsies, possibly due to the higher sensitivity of transcriptomic analysis used [24]. Similar to our findings, Andersson et al. and McAleer et al. demonstrated that levels of T cell-derived cytokines (e.g., IL-4, IL-5, IL-13, IL-15, IL-31) were low or undetectable in tape-stripped skin, but were highly expressed in skin biopsies and plasma of paediatric AD patients [23, 25]. Similarly, Del Duca et al. showed that dermal T-cell cytokines IL-22 and IFN-γ were uniquely upregulated in skin biopsies compared to tape strips in adult AD patients [24]. Accordingly, a consistent pattern emerges wherein tape strips effectively capture local innate and barrier-related proteins but demonstrate limited sensitivity for detecting deeper, T cell-derived cytokines.
Our results demonstrate that tape strips accurately capture a rapid suppression of Type 2 immune mediators TARC and PARC, in both lesional and non-lesional skin, along with innate pro-inflammatory cytokines IL-8 and IL-18, and Th1-related chemokine CXCL10 in lesional skin, aligning with findings in adults [18, 20]. Although not assessed in studies of adult patients treated with dupilumab, we additionally observed a significant suppression of T cell-recruiting chemokine CTACK during treatment in lesional skin. IL-4 and IL-13 promote dendritic cell maturation and activation, which correlate with AD severity and drive Th2 skewing [32, 33]. Our findings suggest that dupilumab, by blocking the IL-4/IL-13 pathway, significantly reduces mediators associated with dendritic cell activity in lesional skin, including TARC, PARC, IL-18, and CXCL10. Although CTACK is not directly associated with dendritic cells, it is induced by pro-inflammatory cytokines [34]. Consequently, by reducing pro-inflammatory proteins, dupilumab may indirectly reduce CTACK levels. Furthermore, CTACK plays a critical role in recruiting CCR10+ T cells to the epidermis, which subsequently promotes the expression of pro-inflammatory proteins that interact with dendritic and other cells [35, 36]. Thus, a reduction of CTACK may in turn suppress the associated pro-inflammatory cascade.
Beyond its effects in the skin, dupilumab treatment resulted in a rapid decrease in serum TARC and PARC levels, supporting previous paediatric studies evaluating its impact on serum proteins [8-11]. While TARC and PARC levels significantly decreased during treatment in both skin and serum, reductions in CTACK were observed only in lesional skin. The lack of detectable changes in serum CTACK levels may reflect the spatial variability of protein expression within the skin. Tape strips primarily sample the stratum corneum, where CTACK is expressed by epidermal keratinocytes [36]. Conversely, TARC and PARC are predominantly produced in deeper dermal layers, which may explain their presence in both skin and serum [37, 38]. These findings highlight the complementary roles of tape strips and serum in capturing treatment-induced proteomic changes in AD.
Notably, we observed a significant increase in IL-1α levels in lesional tape-stripped skin during treatment, trending towards levels observed in non-lesional skin. Consistent with previous studies, IL-1α expression is generally lower in lesional tape-stripped skin compared to non-lesional or healthy skin, hypothesised to result from skin barrier damage [22, 25, 39]. As IL-1α is crucial for local inflammatory responses that maintain skin integrity (i.e., inducing wound repair) and support barrier function, its increasing levels in lesional skin may reflect barrier repair and remodelling processes induced by dupilumab treatment [40].
Dupilumab treatment also significantly increased serum IL-4 and IL-13 levels, likely due to its blocking effect on IL-4Rα, which prevents the binding of IL-4 and IL-13, resulting in elevated unbound circulating levels [8]. The greater increase in serum IL-4 relative to IL-13 suggests that IL-4Rα is more frequently bound by IL-4 in untreated patients. This is supported by findings suggesting that IL-13 predominantly drives peripheral inflammation, as it is more abundant in the skin, while IL-4, which is low or undetectable in the skin, primarily exerts central effects [41-43].
We identified proteins correlating with clinical severity, measured by EASI and NRS itch, with lesional tape strips showing the strongest correlations. Notably, PARC emerged as the strongest correlated protein. Similarly, previous studies have reported positive correlations between skin and serum PARC levels and clinical severity [44]. In some studies, PARC even showed a stronger correlation with clinical severity than TARC, which showed a significant but less pronounced correlation in our study [45]. Guttman et al. reported a positive correlation between PARC in tape strips and the Pruritus Atopic Dermatitis Quickscore, but not with EASI or Scoring Atopic Dermatitis (SCORAD), whereas TARC showed no correlation [26]. Esaki et al. and Ungar et al. reported a positive correlation between PARC in skin biopsies and SCORAD, which was not observed for TARC [28, 46]. Khattri et al. found a slightly stronger correlation between PARC in skin biopsies and SCORAD compared to TARC [47]. Although evidence for the generalisability of PARC as an AD biomarker remains limited due to the small number of studies, these findings suggest its potential and warrant further investigation [45].
Other proteins correlating with clinical severity in lesional and non-lesional skin included innate pro-inflammatory cytokines (IL-8, IL-18), T cell-recruiting chemokine CTACK, and tissue repair and remodelling proteins (periostin, MMP-1). These results align with prior studies investigating tape-stripped skin from paediatric AD patients, demonstrating positive correlations between TARC, CTACK, IL-8, and IL-18 and clinical severity (EASI, SCORAD) [23, 25]. In serum, IL-22 and TARC were notable correlated proteins with NRS itch, consistent with the findings of Kishi et al., demonstrating a positive correlation between IL-22 and TARC levels and Visual Analogue Scale Itch in adult patients. Kishi et al., along with other studies, also observed a positive correlation of these proteins with EASI and/or SCORAD [45, 48-51]. However, while serum TARC strongly correlated with NRS itch in our study, its correlation with EASI was weak and non-significant, possibly due to the small sample size.
With the development of novel targeted treatments for paediatric AD, proteomic profiling is increasingly important for enhancing patient and treatment selection, treatment monitoring, monitoring side effects, and tapering strategies [15, 52, 53]. It may also help predict treatment response and AD flares. In our study, one patient experienced an AD flare after 16 weeks of dupilumab treatment. Interestingly, levels of IL-18 and CTACK were already elevated at week 4 in lesional tape-stripped skin. These proteins, along with IL-8 and PARC, further increased up to the AD flare (week 16). These findings demonstrate the clinical relevance of these proteins and support the potential of tape strips for predicting treatment response and AD flares. However, given the single-patient observation, these findings require cautious interpretation and validation in larger cohorts before clinical implementation.
This study has several limitations, including the relatively small sample size. Furthermore, the real-world nature of our BioDay registry did not allow inclusion of a healthy control group. However, by comparing lesional and non-lesional skin, and pre- and post-treatment samples within the same patient, each patient effectively served as their own control. The real-world clinical setting allowed patients to use concomitant topical therapies, which possibly skewed the immunologic response. Nevertheless, sub-analyses revealed no significant differences in protein levels at week 16 between patients using topical corticosteroids and those who did not (Table S6). Moreover, patients were followed for 16 weeks, while longer follow-up is needed to assess the long-term persistence of dupilumab-induced proteomic changes. Additionally, we eluted tape strips in a buffer containing serum albumin to reduce skin protein loss via adsorption onto plastic surfaces, limiting comparison to other studies that normalise eluted proteins to the total amount of proteins. Lastly, a limited panel of proteins was used, thereby potentially excluding other relevant proteins.
In conclusion, our findings provide insight into the distinct local and systemic proteomic changes in response to dupilumab treatment in paediatric AD patients. These findings underscore the complementary roles of tape strips and serum in profiling immune and epidermal barrier proteins, highlighting the utility of minimally invasive tape stripping for monitoring proteomic responses to targeted therapies. Moreover, several proteins measured in both tape-stripped skin and serum correlated with clinical severity, suggesting their potential utility as biomarkers for disease and treatment response monitoring.
Author Contributions
Lisa P. van der Rijst: conceptualization; investigation; methodology; writing – original draft preparation; visualization; writing – review & editing; formal analysis; data curation. Edward F. Knol:conceptualization; methodology; investigation; writing – review & editing; formal analysis. Nicolaas P.A. Zuithoff: methodology; formal analysis; writing – review & editing. Constance F. den Hartog Jager: investigation; formal analysis; data curation. Femke van Wijk: conceptualization; methodology; project administration; resources; writing – review & editing; supervision. Marjolein S. de Bruin-Weller: conceptualization; funding acquisition; methodology; project administration; resources; writing – review & editing; supervision. Marlies de Graaf: conceptualization; writing – original draft preparation; methodology; project administration; resources; writing - review & editing; supervision.
Acknowledgements
We would like to acknowledge Mariska van Dijk for the Luminex analysis and all the patients and their parents for participating in the BioDay registry.
Ethics Statement
All included patients participated in the Dutch BioDay registry and (parents) provided written informed consent (Medical Ethics Committee, University Medical Center Utrecht; approval 18/239). In addition, all included patients (and/or parents) provided written informed consent for the collection of blood and tape strip samples during the observation period (Medical Ethics Committee, University Medical Center Utrecht; approval 23/014).
Conflicts of Interest
Lisa P. van der Rijst has been a speaker for AbbVie and Novartis; fees were paid directly to the institution. Femke van Wijk has previously served as a speaker and/or consultant for Janssen, Johnson & Johnson, and Takeda and has received grants from Regeneron Pharmaceuticals, LEO Pharma, Sanofi, BMS, Galapagos, and Takeda. Edward F. Knol has previously served as a speaker and consultant for Sanofi, Thermo Fisher Scientific, Astra Zeneca, and GSK. Nicolaas P.A. Zuithoff was involved in studies financed by Eli Lilly; fees were paid directly to the institution. Constance F. den Hartog Jager reports no conflict. Marjolein S. de Bruin-Weller has been a consultant, advisory board member, and/or speaker for AbbVie, Almirall, Amgen, Aslan, Eli Lilly, Galderma, Janssen, LEO Pharma, Pfizer, Regeneron Pharmaceuticals, and Sanofi; fees were paid directly to the institution. Marlies de Graaf has been a consultant, advisory board member and/or speaker for AbbVie, Almirall, Eli Lilly, ALK, Janssen, LEO Pharma, Novartis, Pfizer, Regeneron Pharmaceuticals and Sanofi; fees were paid directly to the institution.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.