Synthesis and Antiproliferative Activity of Silybin Conjugates with Salinomycin and Monensin
Abstract
Aiming at development of multitarget drugs for the anticancer treatment, new silybin (SIL) conjugates with salinomycin (SAL) and monensin (MON) were synthesized, in mild esterification conditions, and their antiproliferative activity was studied. The conjugates obtained exhibit anticancer activity against HepG2, LoVo and LoVo/DX cancer cell lines. Moreover, MON-SIL conjugate exhibits higher anticancer potential and better selectivity than the corresponding SAL-SIL conjugate.
A combination of two or more covalently bonded pharmacophores into a single molecule is an effective tool to design novel, highly active entities, for example in the fight against cancer 1, 2. Additionally, bioconjugation is an inventive current strategy used in medicinal chemistry, because it is generally believed that bioconjugates could have superior efficacy compared with the single target drugs as well as minimize the unwanted side-effects and allow for a synergic action 3, 4.
Our previous efforts towards the synthesis of a variety of bioconjugates of polyether ionophores such as salinomycin and monensin (Scheme 1) with 5-fluoro-2′-deoxyuridine (floxuridine) 5 and several Cinchona alkaloid derivatives 6 led to the development of efficient anticancer agents. We envisaged that the polyether ionophore subunits within the single molecule of bioconjugate could enhance the cytotoxic effects and improve the selectivity.
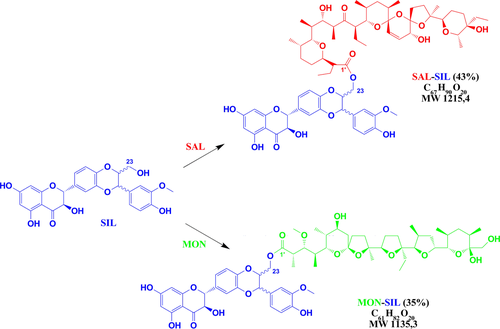
In this study, we report the synthesis of two novel silybin conjugates with monensin and salinomycin having an ester linkage (Scheme 2) and evaluation of their antiproliferative activity.
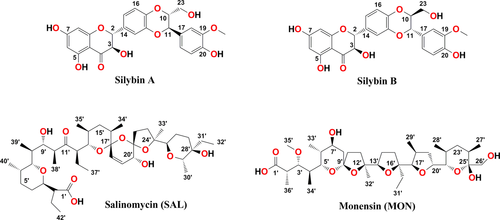
Silybin (SIL) is a major flavonolignan component of silymarin isolated from seeds of the milk thistle (Silybum marianum). Natural SIL is a mixture of two diastereoisomers – silybin A and silybin B 7. Besides hepatoprotective effects, SIL has been lately reported as an anticancer, hypocholesterolemic, antioxidant, free radical scavenger, chemo- as well as dermatoprotective agent 8-13.
Up to now, several conjugates of SIL were obtained. It was demonstrated that glyco-conjugates of SIL are more water soluble than unmodified SIL and exhibit very interesting antioxidant as well as neuroprotective activities 14. Furthermore, the conjugates of SIL with fatty acids show antioxidant and anti-influenza virus activities 15.
Natural polyether antibiotics, such as salinomycin (SAL) and monensin (MON), were objects of great interest because of their antibacterial, antifungal, antiparasitic as well as antiviral activities 16-18. The polyether skeleton of these compounds is able to form complexes with metal cations and transport them across lipid cell membranes, which leads to destruction of the natural cation balance across the membranes. Finally, it causes the apoptosis process as well as cell death 18-20.
Recently, it was found that polyether ionophores might also be important chemotherapeutic agents for the treatment of cancer, especially against the proliferation of various cancer cells, including those that display multidrug resistance (MDR) and against cancer stem cells (CSCs) 21-23.
The screening of 16 000 natural and commercially available chemical compounds performed in 2009 by Gupta and co-workers 24 has shown that SAL is the best agent against breast CSCs with 100-fold stronger activity than the well-known anticancer agent – paclitaxel. Since that time, extensive research work has been undertaken to explain the unusual anticancer properties of this compound, because CSCs play crucial role in tumour progression, chemoresistance as well as recurrence 25.
SAL was able to induce apoptosis by multiple mechanisms in many human cancer cells resistant to cell death, but not in normal cells. SAL showed strong inhibition of proliferation, migration and invasion. It has been demonstrated that SAL is not only able to kill CSCs, but also regular tumour and highly indolent tumour cells displaying resistance to cytotoxic drugs and radiation 26-30. SAL is also currently used in clinical tests, which clearly show that this compound is able to induce partial clinical regression of heavily pretreated and therapy-resistant cancers 27.
On the other hand, MON significantly inhibits the proliferation of human colon, lymphoma and myeloma cancer cell lines 31-33. Additionally, potential in vitro cytotoxicity of MON towards immunotoxins and its beneficial role in overcoming MDR cancer cells have been proved 34. It has been also shown that, from 4910 tested compounds, MON selectively inhibits prostate cancer cell growth at nanomolar concentrations 35.
The last inspiration for our studies was the recent discovery that the cytotoxicity against human hepatoma (HepG2) and other cells has decreased considerably, in relation to the combination of SAL or MON with SIL and their co-action 36. The results obtained are of interest according to recent findings of applicability of SAL and MON for human therapy. Hence, there is a great challenge that exists in this area, towards the identification of new conjugates of SIL with MON and SAL comprising of pharmacophores of known antitumour agents for enhancing the selectivity as well as anticancer activity.
Herein we report the synthesis of MON-SIL and SAL-SIL conjugates fused with ester bond and evaluation of their antiproliferative in vitro potential using human liver cancer cell line (HepG2), human colon adenocarcinoma cell line (LoVo) and doxorubicin-resistant subline (LoVo/DX) as well as normal murine embryonic fibroblast cell line (Balb 3T3).
Methods and Materials
SAL was prepared conveniently by isolation of its sodium salt from commercially available veterinary premix – SACOX following acidic extraction using the procedure described previously 37, 38, whereas MON and SIL were purchased from Sigma-Aldrich, Hamburg, Germany.
General procedure for the synthesis of silybin conjugates with monensin and salinomycin
To a mixture of SAL (500 mg, 0.66 mmol) in tetrahydrofuran (15 mL), the following compounds were added: DCC (206 mg, 1.0 mmol), PPy (50 mg, 0.33 mmol), p-TSA (28.5 mg, 0.15 mmol) and SIL (795 mg, 1.65 mmol). The mixture was first stirred at a temperature below 0 °C for 6 h and then for further 18 h at room temperature. The solvent was subsequently evaporated under reduced pressure to dryness. The residue was suspended in hexane and filtered off. The filtrate was evaporated under reduced pressure, and the residue was purified chromatographically on silica gel (Fluka type 60) to give silybin conjugate with salinomycin (SAL-SIL, 43% yield) as a colourless oil showing a tendency to form the glass state. The same procedure was used to obtain the silybin conjugate with monensin (MON-SIL, 35% yield). The exemplary FT-IR, NMR and ESI MS spectra of conjugates are included in the Figures S1–S7.
Antiproliferative activity
Cells
Three human cancer cell lines and one normal cell line were used: human hepatocellular carcinoma HepG2, human colon adenocarcinoma cell lines sensitive (LoVo) and resistant to doxorubicin (LoVo/dx) as well as also normal murine embryonic fibroblast cell line (BALB/3T3). The BALB/3T3 and HepG2 cell lines were purchased from the American Type Culture Collection (ATCC, Rockville, MD, USA), LoVo and LoVo/DX courtesy of Prof. E. Borowski (Technical University of Gdansk, Gdansk, Poland). All the cell lines are maintained at the Institute of Immunology and Experimental Therapy (IIET), Wroclaw, Poland.
Human colon adenocarcinoma cell lines were cultured in a mixture of OptiMEM and RPMI 1640 (1:1) medium (IIET) supplemented with 5% foetal bovine serum (Thermo Fisher Scientific, Waltham, MA, USA), 2 mm l-glutamine, 1 mm sodium pyruvate (Sigma-Aldrich) and 10 μg/100 mL doxorubicin for LoVo/dx (Sigma-Aldrich). Human hepatocyte carcinoma HepG2 cells and murine embryonic fibroblast cells were cultured in Dulbecco medium (Gibco, Darmstadt, Germany) supplemented with 10% foetal bovine serum (Thermo Fisher Scientific) and 2 mm l-glutamine (Sigma-Aldrich). All culture media contained antibiotics: 100 U/mL penicillin (Sigma-Aldrich) and 100 μg/mL streptomycin (Polfa-Tarchomin, Warsaw, Poland). All cell lines were cultured during entire experiment in humid atmosphere at 37 °C and 5% CO2.
The antiproliferative assay in vitro
Twenty-four hours before adding the tested compounds, all cell lines were seeded in 96-well plates (Sarstedt, Germany) in appropriate media with 104 cells per well. All cell lines were exposed to each tested agent at four different concentrations from the range 100 to 0.1 μg/mL for 72 h. The cells were also exposed to the reference drugs: cisplatin (Accord, London, UK) used as internal laboratory control and doxorubicin hydrochloride CRS (EDQM) used as reference drug for monitoring multidrug resistance of LoVo/DX cells. Additionally, all cell lines were exposed to ethanol or DMSO (solvents used for tested compounds, Sigma-Aldrich) at concentrations corresponding to those present in the tested agent dilutions. Ethanol and DMSO in such concentrations revealed no cytotoxicity.
After 72 h of incubation with the compounds tested, the cells were fixed in situ by gently adding of 50 μL per well of cold 50% trichloroacetic acid TCA (Avantor Performance Materials, Gliwice, Poland) and were incubated at 4 °C for 1 h. Then, the wells were washed four times with water and air-dried. Next, 50 μL of 0.2% solution of sulforhodamine B (Sigma-Aldrich) in 1% acetic acid (Avantor Performance Materials) was added to each well and plates were incubated at room temperature for 0.5 h. After incubation time, unbound dye was removed by washing plates four times with 1% acetic acid, whereas the stain bound to cells was solubilized with 150 μL of 10 mm Tris base (Sigma-Aldrich). Absorbance of each solution was read at a Synergy H4 photometer (BioTek Instruments, Winooski, VT, USA) at 540 nm wavelength.
Results are presented as mean IC50 (concentration of the tested compound that inhibits cell proliferation by 50%) ± standard deviation. IC50 values were calculated in Cheburator 1.0.2, Dmitry Nevozhay software for each experiment 39. Compounds at each concentration were tested in triplicates in a single experiment, and each experiment was repeated at least three times independently.
Results and Discussion
Chemistry
The selective esterification of the primary alcoholic group of SIL has been performed previously by the reaction between SIL and respective acyl chlorides in the presence of a Lewis acid. This method cannot be used for the conjugation of SIL with polyether ionophores, because SAL and MON contain in their structures several potentially reactive hydroxyl groups and they are very sensitive to acidic conditions as well as heating. Therefore, mild reaction conditions for the SAL and MON esterification were chosen.
Previously, we have developed a valuable method for the synthesis of ester derivatives of SAL 40 and MON 41, and also we used it here for the conjugation with SIL. This method based on the reaction between SAL and MON with SIL in the presence of N,N′-dicyclohexylcarbodiimide (DCC), 4-pyrrolidinopyridine (PPy) and p-toluenesulfonic acid monohydrate (p-TSA), which led to the selective esterification of the C(23)-OH group of SIL and give two diastereoisomeric MON-SIL and SAL-SIL conjugates with 35% and 43% yield, respectively.
The synthesized conjugates were fully characterized by 1H and 13C NMR, ESI MS and FT-IR techniques. The 1H and 13C NMR signals were assigned using two-dimensional (COSY, HETCOR and HMBC) spectra. A set of the representative spectra of the conjugates obtained are included in the Figures S1–S7.
In the 13C NMR spectra of MON-SIL and SAL-SIL, the most characteristic signal of C(1′) atom of the ester group was observed at 173.4 and 172.0 ppm, while the signal of C(1′) atom of the carboxyl group of MON and SAL was at 177.4 and 177.8 ppm, respectively. The characteristic 13C signal of the –OCH2– group in the ester substituent in MON-SIL and SAL-SIL was observed at 61.8 and 61.5 ppm, respectively. Very similar position of the respective 13C NMR signals has been observed in the spectra of previously obtained C(23)-O-acetyl silybin derivatives 15.
In the 1H NMR spectra of MON-SIL, the characteristic signals of the –OCH2- protons from the ester substituent were observed as two separate signals at 3.52 ppm (dd, J = 12.4, 4.2 Hz) and 3.76 ppm (dd, J = 11.7, 2.3 Hz). For SAL-SIL, these signals were also observed at 3.55 ppm (dd, J = 12.5, 4.1 Hz) and 3.79 ppm (dd, J = 10.1, 2.1 Hz).
In Figure S4, the FT-IR spectrum of MON and SIL is compared with that of the MON-SIL conjugate. In the spectrum of MON-SIL, the band assigned to the ν(C=O) stretching vibrations of the carboxylic group, which is present in the spectrum of MON at 1706 cm−1, vanishes. Instead of this band in the spectrum of MON-SIL, a new band assigned to the ν(C=O) stretching vibrations of the ester group at 1763 cm−1 is present indicating the esterification reaction. On the other hand, in the FT-IR spectrum of SAL-SIL conjugate (Figure S5) the ν(C=O) stretching vibrations of the ester group are also observed at 1763 cm−1. The band with the maximum at about 1714 cm−1 is a result of the ν(C=O) vibrations of the ketone group present in SAL moiety.
Antiproliferative activity
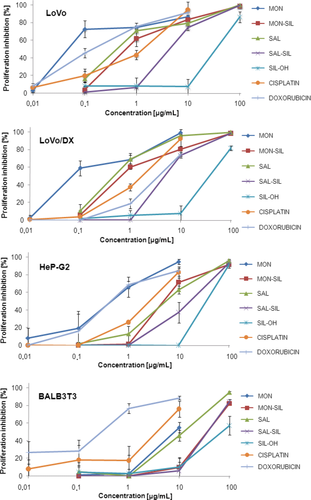
The MON-SIL and SAL-SIL conjugates obtained and their precursors (SAL, MON and SIL) as well as two reference anticancer drugs – doxorubicin and cisplatin, were evaluated for their in vitro antiproliferative effect on three cancer cell lines (LoVo, LoVo/DX and HepG2) and one normal murine embryonic fibroblast cell line (BALB/3T3) to estimate the toxicity of the studied compounds, following the previously published procedures 42, 40. The IC50 ± SD values of the tested compounds are collected in Table 1 and Figure 1.
| Compound | Cancer cells | Normal cells | ||
|---|---|---|---|---|
| LoVo | LoVo/DX | HepG2 | BALB/3T3 | |
| SAL | 0.61 ± 0.36 | 0.52 ± 0.17 | 12.44 ± 6.34 | 35.18 ± 6.86 |
| SAL-SIL | 3.63 ± 0.27 | 3.93 ± 0.04 | 14.01 ± 5.39 | 30.10 ± 5.00 |
| SIL | 72.32 ± 12.59 | 76.97 ± 6.20 | 73.78 ± 3.49 | 121.08 ± 13.67 |
| MON | 0.06 ± 0.03 | 0.07 ± 0.03 | 0.76 ± 0.04 | 6.54 ± 1.09 |
| MON-SIL | 0.59 ± 0.18 | 0.57 ± 0.04 | 4.43 ± 0.83 | 31.58 ± 5.65 |
| Doxorubicin | 0.28 ± 0.11 | 6.73 ± 0.81 | 0.77 ± 0.22 | 0.53 ± 0.20 |
| Cisplatin | 4.40 ± 0.87 | 5.67 ± 0.50 | 8.93 ± 1.37 | 12.43 ± 5.90 |
- The IC50 value is defined as the concentration of a compound that corresponds to a 50% growth inhibition. Human liver cancer cell line (HepG2); human colon adenocarcinoma cell line (LoVo) and its doxorubicin-resistant subline (LoVo/DX); normal murine embryonic fibroblast cell line (BALB/3T3). Data are expressed as the mean ± SD.
Human colon adenocarcinoma cell line (LoVo) and its doxorubicin-resistant subline (LoVo/DX), a pair of cell lines displaying various levels of drug resistance, were used for evaluation of the activity of the compounds studied against the cells with MDR phenotype. Comparison between the cancer cell lines and the corresponding normal BALB/3T3 cell line was made to define the in vitro selectivity indexes as a measure of the therapeutic potential (Table 2 and Figure 2). After the IC50 values were calculated for each tested cancer cell line for all compounds studied, using the published software 39, the resistance indexes (RI) for a given agent were calculated as the ratio of the IC50 value for the resistant cell line to the IC50 value for the sensitive one (Table 2 and Figure 2). The cells can be classified to one of three categories depending on the RI value: for RI is in the range of 0–2, the cells are drug sensitive; for RI in the range of 2–10, the cells are moderately drug resistant; and for RI higher than 10, the cells are markedly drug resistant 43.
| Compound | LoVo | LoVo/DX | HepG2 | |
|---|---|---|---|---|
| SI | SI | RI | SI | |
| SAL | 57.67 | 67.65 | 0.85 | 2.83 |
| SAL-SIL | 8.29 | 7.66 | 1.08 | 2.15 |
| SIL | 1.67 | 1.57 | 1.06 | 1.64 |
| MON | 109.00 | 93.43 | 1.17 | 8.61 |
| MON-SIL | 53.53 | 55.40 | 0.97 | 7.13 |
| Doxorubicin | 1.89 | 0.08 | 24.04 | 0.69 |
| Cisplatin | 2.83 | 2.19 | 1.29 | 1.39 |
- The RI (resistance index) indicates how many times a resistant subline is chemoresistant relative to its parental cell line The RI was calculated for each compounds using formula: RI = IC50 for LoVoDX/IC50 for LoVo cell line. When RI is 0–2, the cells are sensitive to tested compound; RI of the range 2–10 means that the cell shows moderate sensitivity to a drug; RI above 10 indicates strong drug resistance.
- The SI (selectivity index) was calculated for each compounds using formula: SI = IC50 for normal cell line (BALB/3T3)/IC50 for respective cancerous cell line. A beneficial SI > 1.0 indicates a drug with efficacy against tumour cells greater than toxicity against normal cells.

As shown in Table 1, unmodified MON was highly active against LoVo and LoVo/DX cell lines with IC50 values at low submicromolar concentrations (0.06 and 0.07 μm, respectively). The antiproliferative activity of SAL against these lines is also high, but about ten times less potent in comparison with that of MON. Both ionophores have significantly lower activity against HepG2 cell line (IC50 = 0.76 μm and IC50 = 12.44 μm for MON and SAL, respectively). It is important to note that these ionophores exhibit low toxicity against normal murine embryonic fibroblast cell line (BALB/3T3). Thus, the SI values calculated for unmodified MON and SAL are impressive, especially when compared with the SI values of the currently used anticancer drugs, such as cisplatin and doxorubicin (Table 2 and Figure 2).
The selectivity index (SI), an important pharmaceutical parameter that facilitates the estimation of possible future clinical development, was calculated as the ratio of IC50 value for normal cell line (BALB/3T3) to IC50 value for a respective cancerous cell line. Higher values of SI indicate greater anticancer specificity, and a compound displaying SI higher than 3 is considered as highly selective. The calculated SI values for MON and SAL for human colon adenocarcinoma cell lines (LoVo and LoVo/DX) indicate that these compounds can be treated as the potential anticancer drugs (Figure 2 and Table 2).
Contrary, SIL displays rather low cytotoxicity against all tested cancer cells (IC50 in range from 72.32 to 76.97 μm). Therefore, it was interesting to check the anticancer activity exhibited by the conjugates of highly active polyether ionophores and relatively low active SIL. Our studies showed that the newly synthesized conjugates exerted antiproliferative activity at micromolar concentrations (IC50 from 0.57 to 14.01 μm) against the tested human cancer cell lines and, simultaneously, relatively low toxicity against normal murine embryonic fibroblast cell line (IC50 > 30.00 μm), wherein the more active conjugate was MON-SIL. It is worth noting that both MON-SIL and SAL-SIL conjugates are more active than cisplatin against LoVo and LoVo/DX cancer lines and less active than doxorubicin against HepG2. Only MON has comparable activity as doxorubicin against HepG2 cell line.
According to the data given in Table 2, both obtained conjugates were very active against the cell lines expressing drug-resistant phenotype (RI is near 1.00), while for doxorubicin this factor is almost 24.00. In addition, the RI values for MON-SIL and SAL-SIL conjugates are better (lower) than for MON and cisplatin, and comparable to those of SAL and SIL. Furthermore, MON-SIL conjugate broke the drug resistance of LoVo/DX cancer cells (RI = 0.97).
The selectivity index (SI) values are better (higher) for MON-SIL than those observed for SAL-SIL. The in vitro SI for MON-SIL is in the range from 7.13 to 55.40, indicating high selective cytotoxic activity and low toxicity against the non-tumour cells. These results demonstrated that the presence of the MON component in the conjugate moiety was critical for the selectivity and cytotoxicity in human cancer cells. On the other hand, both conjugates are much more potent than unmodified SIL in inhibition of cell proliferation and promotion of death of cancer cells (Table 2).
Conclusion
All discussed biological properties of the conjugates obtained from SIL and polyether ionophores indicate that the chemical modification of SIL can change the anticancer activity of substrates giving weaker (SAL-SIL) or stronger (MON-SIL) anticancer drug candidates. The inspection of toxicity (selectivity) of the conjugates, expressed as their SI values, revealed that MON conjugate was less toxic (higher SI values) as compared to the SAL analogue. Generally, it was found that parent polyether ionophores as well as their conjugates appeared to be more selective against cancer cells than cisplatin and doxorubicin.
No hydrolysis of the conjugates was observed under all experimental conditions applied in our study, but for the understanding of biological properties, the in vivo hydrolysis of the conjugates by cellular carboxylesterases and the role of conjugates obtained as untypical prodrugs should be also discussed here. Carboxylesterases, which are among the best characterized prodrug hydrolysing enzymes involved in the activation of several therapeutic ester prodrugs, are able to cleavage ester bonds in several prodrugs 44. They catalyse the conversion of a carboxylic ester to an alcohol and a carboxylic acid. Carboxylesterases are widely distributed throughout the body, abounded in the liver, kidney, testis, lung and plasma, both in normal and tumour tissues 45. These enzymes are present both in microsomes and in cytosol.
We suppose that the ester bond presents in both conjugates should be rather stable in circulating system, but upon internalization into the cancer cells the conjugates can be readily cleaved by carboxylesterases. The hydrolysis of the conjugates in the living cells and tissues, such as the liver, can give two starting compounds of different biological properties. On the one hand, there are polyether ionophores (MON and SAL) as representatives of highly anticancer agents, which are active against the proliferation of various cancer cells, including those that display MDR and against CSCs. On the other hand, the product of the cellular hydrolysis is SIL, which is a well-known chemoprotective, antioxidant and free radical scavenger agent.
In conclusion, the conjugates obtained in the esterification reaction between well-known hepatoprotective natural compound silybin (SIL) and two natural polyether ionophores monensin (MON) and salinomycin (SAL) displayed interesting antiproliferative activity in various human cancer cells, including MDR cells at low micromolar concentrations. This anticancer activity is also connected with high selectivity indexes (SI) and low toxicity against normal cells, and the results obtained provide an excellent starting point for further drug discovery optimization.
Acknowledgments
Financial support from the budget funds for science in years 2013-2015 – grant ‘Iuventus Plus’ of the Polish Ministry of Science and Higher Education – No. IP2012013272, is gratefully acknowledged. Michał Antoszczak wishes to thank the Polish National Science Centre (NCN) for a doctoral scholarship ‘ETIUDA’ (No. 2014/12/T/ST5/00710).




