Roles of Basic Amino Acid Residues in the Activity of μ-Conotoxin GIIIA and GIIIB, Peptide Blockers of Muscle Sodium Channels
Abstract
To study in detail the roles of basic amino acid residues in the activity of μ-conotoxin GIIIA (μ-GIIIA) and GIIIB (μ-GIIIB), specific blockers of muscle sodium channels, seven analogs of μ-GIIIA, and two analogs of μ-GIIIB were synthesized. μ-GIIIA analogs were synthesized by replacing systematically the three Arg residues (Arg1, Arg13, and Arg19) with one, two, and three Lys residues. μ-GIIIB analogs were synthesized by replacing simultaneously all four Lys residues (Lys9, Lys11, Lys16, and Lys19) with Arg residues and further replacement of acidic Asp residues with neutral Ala residues. Circular dichroism spectra of the synthesized analogs suggested that the replacement did not affect the three dimensional structure. The inhibitory effects on the twitch contractions of the rat diaphragm showed that the side chain guanidino group of Arg13 of μ-GIIIA was important for the activity, whereas that of Arg19 had little role for biological activity. Although [Arg9,11,16,19]μ-GIIIB showed higher activity than native μ-GIIIB, highly basic [Ala2,12, Arg9,11,16,19]μ-GIIIB showed lower activity, suggesting that there was an appropriate molecular basicity for the maximum activity.
Abbreviations
-
- Boc
-
- t-butoxycarbonyl
-
- Bzl
-
- benzyl
-
- CD
-
- circular dichroism
-
- 2-Cl-Z
-
- 2-chloro benzyloxycarbonyl
-
- DIPCDI
-
- diisopropylcarbodiimide
-
- FAB-MS
-
- fast atom bombardment-mass spectrometry
-
- Hyp
-
- 4-trans-hydroxyproline
-
- μ-GIIIA
-
- μ-conotoxin GIIIA
-
- μ-GIIIB
-
- μ-conotoxin GIIIB
-
- HPLC
-
- high performance liquid chromatography
-
- MBHA
-
- 4-methyl benzhydrylamine
-
- MeBzl
-
- 4-methylbenzyl
-
- NMR
-
- nuclear magnetic resonance
-
- TFA
-
- trifluoroacetic acid
-
- Tos
-
- p-toluenesulfonyl
Venomous glands of cone snails (Conus) contain a number of biologically active peptide toxins, which are generally known as conotoxins 1. They are typically small disulfide-rich peptides containing 11–30 amino acid residues. Conotoxins can be classified into several families based on the number and pattern of disulfide bonds and their biological activities 2, 3. Members of a single family of conotoxins share similar protein folding but in some cases exhibit different biological activities 4-6. These differences in biological activities are due to their ability to bind with specific ion channels or receptors 7. Some of these conotoxins are used as tools in investigating receptor structure and function 3 and ion channel geometry 8. It is well known now that ω-conotoxin MVIIA, a specific blocker of the N-type calcium channel, can be used as an analgesic drug 9.
The particular conotoxins μ-conotoxin GIIIA (μ-GIIIA) and GIIIB (μ-GIIIB), isolated from the marine snail Conus geographus, are peptide toxins composed of 22 amino acid residues with three disulfide bridges 10-12. The amino acid sequences 13, 14 and the disulfide pairings 15 of the toxin were determined as shown in Figure 1. The toxins preferentially block skeletal muscle sodium channels 16 and are a promising tool for investigating sodium channels 17. The three-dimensional structures of μ-GIIIA 18 and μ-GIIIB 19 have been determined by nuclear magnetic resonance (NMR) spectroscopy. In our previous synthetic studies on μ-GIIIA, we found that [1] the three-dimensional structure formed by three disulfide bridges is essential for activity, [2] the molecular basicity is important for high inhibitory activity, [3] the guanidino group of Arg13 is the most important, and [4] two Hyp residues at the sixth and seventh positions are important for correct folding of the peptide 20. We also reported that the three-dimensional structure of [Ala13]μ-GIIIA was the same as that of native μ-GIIIA, as determined by NMR analysis, confirming that the loss of activity is not due to conformational change, but the removal of the essential guanidino group 21. The essential role of Arg13 in blocking sodium channels has been verified repeatedly 22-24. Recently, we reported that each of three disulfide bridges were essential for the conformation and activity of μ-GIIIA 25. We also reported on the roles of Hyp residues of μ-GIIIA 26.

The known sodium channel blocker of tetrodotoxin (TTX) is a small molecule and has strong basic moiety composed of Arg 27. TTX interacts with sodium channels through the interaction of its guanidinium moiety with acidic residues located at vestibule and inner pore of channel peptides, where the inner acidic residue is more crucial in the inhibitory action 28. Our previous work using avidin and biotin to limit the entry of toxin to channel pore also suggests some penetration of Arg13 protruded from μ-GIIIA into channel pore is essential to elicit inhibitory action 29. Thus, to study in detail the roles of basic amino acid residues in the activity of μ-GIIIA and μ-GIIIB, seven analogs of μ-GIIIA and two analogs of μ-GIIIB were synthesized as shown in Figure 1. μ-GIIIA analogs were synthesized by replacing systematically the three Arg residues (Arg1, Arg13, and Arg19) with one, two, and three Lys residues. μ-GIIIB analogs were synthesized by replacing simultaneously all four Lys residues (Lys9, Lys11, Lys16, and Lys19) with Arg residues and further replacement of acidic Asp residues with neutral Ala residues.
Materials and Methods
Materials
Boc-Ala-OH, Boc-Arg (Tos)-OH, Boc-Asp (OBzl)-OH, Boc-Cys (MeBzl)-OH, Boc-Gln-OH, Boc-Hyp (Bzl)-OH, Boc-Lys (2-Cl-Z)-OH, Boc-Thr (Bzl)-OH, and MBHA-resin were obtained from the Peptide Institute (Minoh, Japan). Other reagents were of peptide synthesis grade and were obtained from Kokusan Chemical Works, Ltd. (Tokyo, Japan) or Watanabe Chemical Industries, Ltd. (Hiroshima, Japan). Automated peptide synthesis was conducted using a Biosearch (Petaluma, CA, USA) model 9500 peptide synthesizer. Amino acid analyses were performed on a Beckman System Gold amino acid analyzer after hydrolysis in 6 m hydrochloric acid at 110 °C for 24 h and derivatization by 4-dimethyl aminoazobenzene-4′-sulphonyl chloride. FAB-Mass spectra were measured using a JEOL (Akishima, Japan) JMS-HX100 mass spectrometer. Analytical RP- high performance liquid chromatography (HPLC) was conducted using a Shimadzu LC-6A system with an ODS column (Shim-pack CLC-ODS (M), 0.46 × 25 cm). Preparative RP-HPLC was performed using a Shimadzu LC-8A system with an ODS column (Shim-pack PREP-ODS (H), 2 × 25 cm).
Peptide synthesis
A linear precursor of [Lys1,13,19]μ-GIIIA (K1,13,19) was synthesized using solid-phase methodology of Boc chemistry starting from MBHA-resin (0.47 g, 0.3 mmol equivalent). The Boc group was removed by the treatment with 45% trifluoroacetic acid (TFA) in CH2Cl2 containing 2.5% anisole. Coupling reaction was carried out with 0.4 m Boc-amino acid (5 equivalent) in DMF and 0.4 m DIPCDI (5 equivalent) in DMF for 2 h. Unreacted amino group was capped by the treatment with 0.3M 1-acetylimidazole in CH2Cl2. After 22 cycles of coupling, 1.47 g of protected peptide resin was obtained. The resin was treated with liquid HF (14.7 mL) in the presence of anisole (2.2 mL), ethyl methyl sulfide (0.37 mL), and ethanedithiol (0.37 mL) at 0 °C for 1 h. After removal of HF under reduced pressure, addition of diethyl ether produced white precipitate, which was collected by filtration, and the crude linear peptide was extracted with 2 m AcOH (150 mL). The solution was adjusted to pH 7.8 with aqueous NH4OH and diluted to a final peptide concentration of 0.2 mm (1500 mL). The reaction solution was stirred slowly at 5 °C to form the disulfide bonds. The oxidation reaction was monitored by HPLC, and the crude oxidized product (415 mg) was obtained after lyophilization of the solvent. The crude product dissolved in 30% AcOH (20 mL) was loaded onto a column (5 × 109 cm) of Sephadex G-50F and eluted with the same solvent. Fractions containing the desired product were collected and lyophilized to give 152 mg of peptide, which was loaded onto a column (1.8 × 20 cm) of carboxymethylcellulose CM-52 and eluted with a linear gradient from 0.01 m NH4OH (pH 4.5) to 0.8 m NH4OH (pH 6.5). Fractions containing the desired product were collected and lyophilized to give 23 mg of peptide, which was further purified by preparative HPLC with an ODS column (2 × 25 cm) by making use of an isocratic elution of 6% CH3CN in 0.1% aqueous TFA to give 16 mg of purified peptide as a TFA salt. The peptide was loaded onto a column (1.8 × 100 cm) of Sephadex G-10 and eluted with 0.5 m AcOH to give a purified peptide (13 mg) as an AcOH salt. Other peptides were synthesized using similar procedures. The concentrations of CH3CN in the final preparative HPLC purification were as follows: K1 (8%), K13 (8%), K19 (7%), K1,13 (8%), K1,19 (7%), K13,19 (8%), K1,13,19 (6%), 4R (10%), and 4R2A (5-35% in 30 min, linear gradient). Primary structures and the purity of the analogs were confirmed by analytical HPLC, amino acid analysis, and FAB-MS measurement. The mass data (M+H+) were as follows: K1 (2580), K13 (2580), K19 (2580), K1,13 (2552), K1,19 (2552), K13,19 (2552), K1,13,19 (2524), 4R (2751), and 4R2A (2671).
Circular dichroism measurements
Circular dichroism (CD) spectra were recorded with a JASCO (Hachioji, Japan) J-600 spectropolarimeter in H2O solution (0.01 m sodium phosphate, pH 7.0) at 20 °C, with a quartz cell of 1-mm path length. The results are expressed as molar ellipticity [θ].
Bioassay
Inhibitory effects of synthesized analogs on twitch contractile response of the isolated rat diaphragm to direct electrical stimuli were assayed using the methods described previously 20. Male rats (Wister, 200–300 g) were stunned and bled. Diaphragm muscles were excised and cut into four stripes, which were electrically stimulated with 5 msec pulses at 0.1 Hz (supramaximal voltage). Twitch-tension responses were recorded isometrically. The activity of the peptides was expressed as % inhibition of twitch response, and the values provided were the mean of two sets of experiments.
Results
Synthesis of peptides
Seven analogs of μ-GIIIA and two analogs of μ-GIIIB were successfully synthesized using a simple one-step oxidation reaction of linear precursors assembled by solid-phase method of Boc-chemistry. Figure 2 shows the analytical HPLC profiles of the crude and purified K1,13,19 as an example. Because of the highly hydrophilic nature of the peptide, as shown in Figure 2, preparative HPLC purification was troublesome in the isocratic elution condition. Only a small amount of the samples could be loaded onto the column. When we tried to load a larger amount of sample, most of the sample was eluted without being held on the column. The yields of peptides starting from MBHA-resin were as follows: K1 (3%), K13 (3%), K19 (2%), K1,13 (2%), K1,19 (2%), K13,19 (2%), K1,13,19 (2%), 4R (3%), and 4R2A (5%). Recently, many synthetic peptides have been purified using only RP-HPLC. Although the yields of our synthesis were not so high, the traditional three-step purification (gel filtration, ion exchange, and RP-HPLC) was useful for the isolation of pure peptides.
Circular dichroism
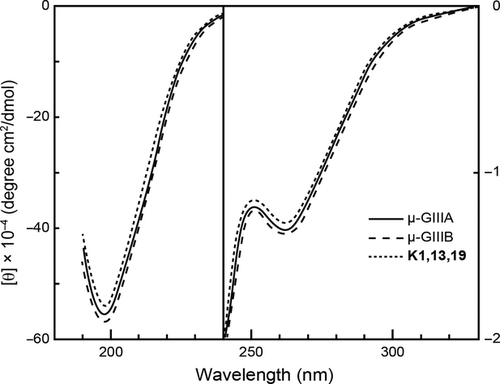
The CD spectrum of μ-GIIIA was not characteristic except for negative Cotton effects around 260 nm due to the disulfide bridges (Figure 3). All the synthetic analogs showed CD spectra similar to that of μ-GIIIA. The result clearly shows that the analogs have three-dimensional structures similar to that of μ-GIIIA.
Biological Activity
Figure 4 shows the inhibitory effects of μ-GIIIA and its analogs on the twitch response of the muscle to electrical stimuli. Native μ-GIIIA caused a dose-dependent inhibition of the response over a concentration range 0.03–1 μm with an IC50 value of approximately 0.1 μm. The twitch response of the muscle was abolished by 1 μm μ-GIIIA. K19 showed almost the same activity as that of native μ-GIIIA, while K1 exhibited slightly weaker activity than that of native μ-GIIIA. Among the analogs with single amino acid substitution, K13 indicated the largest reduction of activity. Among the analogs with double substitution, K1,19 were shown to cause the highest activity, in good agreement with the result of single mutation. K1,13,19 did not completely block the twitch response even at a high concentration of 30 μm.
Figure 5 shows the inhibitory effects of μ-GIIIB and its analogs on the twitch response of the muscle to electrical stimuli. As reported previously 13, μ-GIIIB was more potent than μ-GIIIA in inhibitory activity on the twitch response. Replacement of all four Lys residues with Arg residues (4R) resulted in the enhancement of activity. However, the analog 4R2A, which had the highest basicity among tested peptides, showed weaker activity than native μ-GIIIB.
Discussion
Referring to our previous results 20, we have studied in detail the roles of basic amino acid residues in μ-GIIIA and μ-GIIIB. The Lys substitution for Arg13 in μ-GIIIA largely reduced the activity in both single and double mutations, confirming the importance of the side chain guanidino group of Arg13. On the other hand, Arg19 could be replaced with Lys without reduction of activity, indicating that only the basicity is required at this position.
μ-GIIIB with eight basic residues (four Arg and four Lys) showed higher activity than μ-GIIIA with seven basic residues (three Arg and four Lys), suggesting again that the molecular basicity is important for the activity of μ-conotoxins. Therefore, we replaced all four Lys residues in μ-GIIIB with Arg residues to enhance the activity. As we expected, analog 4R exhibited higher activity than native μ-GIIIB. As replacement of acidic Asp2 and Asp12 in μ-GIIIA with neutral Ala resulted in the enhancement of activity 20, we examined similar substitution in the μ-GIIIB analog. However, 4R2A was a little weaker than the parent 4R in its activity. The activity of 4R2A was even weaker than that of native μ-GIIIB. Although the nature of this weaker activity is not known precisely, 4R may have the strong basicity all over the molecule that may raise the possibility of interaction of the toxin with sodium channel acidic sites, and the presence of acidic Asp at the 2nd position presumably neutralize the Arg1 basic residue, and thereby producing the polarity in the 4R molecule as more basic at protruding Arg13, thus conversely resulting in some loss of activity in 4R2A due to the loss of molecular polarity at the 2nd residue against Arg13 side where enough basicity is still retained even after the replacement at 12th residue. Therefore, the basicity as well as polarity of the toxin molecule might be pivotal in exerting the biological activity.
For the structure–activity relationship study of peptides with multiple disulfide bonds, it is important to know whether or not substitution affects the three-dimensional structure as well as disulfide bond pairings. All the isolated analogs showed similar CD spectra to that of native μ-GIIIA, demonstrating that the analogs have three-dimensional structures similar to that of μ-GIIIA. This means that the changes in activity associated with amino acid replacement are due to the changes in amino acid side chains.
The interaction of μ-GIIIA with sodium channels has been reported to be similar to that of guanidinium toxins like tetrodotoxin (TTX) and saxitoxin (STX) 24, 30-32, although μ-GIIIA selectively interacts with muscle-type sodium channels. The action of μ-GIIIA is strongly dependent on the transmembrane potential, and the blockade of sodium channels is lacking when applied to the intracellular side. NMR studies demonstrate a structural basis for the similarity of action between μ-GIIIA and guanidinium toxins 18, 20, 21. The guanidinium moiety of Arg13, the active center in μ-GIIIA, protrudes outward from the molecule. The size of the protruded peptide segment and its basicity may be closely comparable to those of TTX.
Chang et al. have analyzed the precise interaction of μ-GIIIA with sodium channels through thermodynamic mutant cycle analysis 24. Arg13 interacted strongly with domain II Glu758 with the association energy ascribed to both electrostatic and nonelectrostatic components, and less strongly with Glu403. They have further proposed that the guanidinium moiety of Arg13 binds to the channel mouth adjacent to the pore loops of domains I and II in sodium channels. The blocking of sodium channel activity is not achieved by direct interaction with the selectivity filter in the channel protein, and the toxin is more superficial than in the case of TTX and STX, although both the toxins are also capable of interacting with Glu758 and Glu403. Although the side chain of Arg13 is the most important binding site for μ-conotoxins, present results suggest that multiple interactions of several residues other than the essential Arg13 are also important for the binding to sodium channels.
Conclusions and Future Directions
We have very recently described the roles of individual disulfide bridges 25 and Hyp residues 26 in the conformation and activity of μ-GIIIA. In the present paper the roles of basic residues of μ-GIIIA and μ-GIIIB were studied. Combination of these data will shed light on the structure–activity relationships of μ-GIIIA and μ-GIIIB, the first μ-conotoxins isolated, and may enable the design of novel drugs based on the μ-conotoxins 33.
Acknowledgments
The authors wish to express their appreciation to Dr. Hideyoshi Higashi of Mitsubishi Kagaku Institute of Life Sciences for providing measurements of FAB-MS and also to Prof. Scott Pugh of Fukuoka Women's University for correction of English usage in this manuscript.
Conflict of Interest
All authors declare that they have no conflict of interest. All institutional and national guidelines for the care and use of laboratory animals were followed. This article does not include any studies using human subjects.




