Synthetic Substrates Specific to Activated Plasmin Can Monitor the Enzymatic Functional Status in Situ in Breast Cancer Cells
Abstract
We here strove to overcome the limitations of expression analyses such as PCR and IHC, based on molecular recognition between target and probe molecules, by designing synthetic substrates specific to the target molecules to directly estimate the enzymatic functionality in situ. The specific substrate contains a probing unit, which is an organic fragment for specific enzyme binding, and a reactive unit, which is a natural peptide subject to catalysis. In this study, the activation of plasminogen to plasmin was examined in MDA-MB231 breast cancer cells using the plasmin-specific synthetic substrates designed from their inhibitors. The localization and function of the activated plasmin were successfully visualized by fluorophore combined with the specific substrate concurrently. This would be the first time for activated plasmin at work in situ by direct observation. Our concept to directly monitor the functionality of target enzymes can be used straightforwardly for other proteases such as cathepsins or caspases. Also, this substrate concept as a ‘tailor-made substrate’ would be utilized as a novel functional molecular probe in vivo with appropriate detectable probes.
Various molecular probes are widely used to qualitatively or quantitatively analyze the status of target molecules in cells and tissues, such as oligonucleotides, antibodies, and fluorescent dyes 1. Among them, both oligonucleotide primers and specific antibodies have become standard probes in the expression analysis of molecular biology, as well as diagnostics by the PCR and IHC methods. However, because the expression analysis is based on molecular recognition between target and probe molecules, there are several limitations on the detectability, such that alterations in the primary sequence of the target molecules or post-translational modifications, such as phosphorylation, glycosylation, and intramolecular cleavage, impede correct molecular recognition. These alterations/modifications in the target molecules are commonly observed in disease-relevant proteins; for instance, the point mutations of EGFR have significant influences on the efficacy of molecular targeted drugs like the anti-EGFR antibody remedy 2.
One possible way to overcome these limitations would be to directly estimate the functionality of the target molecules. In this study, we designed synthetic substrates specific to a target enzyme as a functional molecular probe. The specific substrates are designed to consist of two distinct units in a molecule: a probing unit, which is a synthetic organic fragment capable of specifically binding to the target enzyme, and a reactive unit, which is a natural peptidic fragment subject to enzymatic catalysis (Scheme 1). High reactivity for the target enzyme and high selectivity against isozymes will be accomplished by the use of appropriate synthetic organic fragments in the probing unit, which can be designed by drug discovery technologies such as high-throughput screening, molecular modeling, and combinatorial chemistry. Natural peptidic fragments may not fulfill this property at higher levels due to the limited diversity of chemical structures in peptide side chains. To monitor enzymatic reaction, detectable probes such as chromogenic and fluorescent dyes (e.g. boron dipyrromethene: BODIPY) or MRI-sensitive moiety (e.g. Gd[III] chelates) are to be included within a specific synthetic substrate 3, 4.
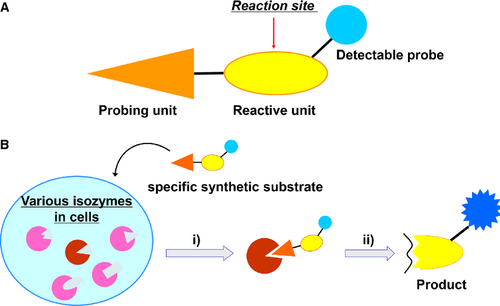
This substrate probe was examined for the plasmin–plasminogen system 5. Plasmin plays a central role in the invasion/metastasis of cancer cells through degrading the extracellular matrix and activating the latent proforms of invasive proteases, for example, matrix metalloproteases 6, 7. Activated plasmin is generated from its zymogen plasminogen mainly by the urokinase–urokinase-type plasminogen activator receptor (uPAR) system in cancer cells 8, 9. The activation by the urokinase–uPAR system is taken place on the surface of cancer cells, and then, the activated plasmin is vicinally tethered to plasminogen receptors on the cells to facilitate cell migration/locomotion during invasion/metastasis 10, 11. Using plasmin-specific synthetic substrates, we strove to visually obtain the information regarding the localization and function of the activated plasmin concurrently in breast cancer cells in situ.
Methods and Materials
Materials
All chemicals were purchased from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan) or Wako Pure Chemical Industries, Ltd. (Osaka, Japan) and used without further purification. All protected amino acids and coupling reagents were purchased from Watanabe Chemical Industries, Ltd. (Hiroshima, Japan) 1H-NMR experiments were recorded on a JMTC-600 (JEOL Ltd., Tokyo, Japan) 600-NMR spectrometer with CDCl3 or DMSO-d6 as solvents. Chemical shifts are expressed in parts per million (ppm, δ) and referred to the solvent signal. HRMS spectra were recorded on the AccuTOF (JMS-T100LC) equipped with an electrospray ion source (JEOL Ltd.). The preparative HPLC system consisted of a Hitachi L-2130 System Controller, a Hitachi In-Line Degasser, and a Hitachi L-2300 Absorbance Detector (Hitachi High-Technologies Co., Tokyo, Japan). The absorbance detector was operated at 254 nm. The mobile phase for preparation and analysis was a combination of water (A) and acetonitrile (B), both containing 0.1% TFA, and the flow rates were 8 and 1 mL/min, respectively. TSK gel ODS columns (21.5 × 300 mm for preparation and 4.6 × 150 mm for analysis; TOSOH Co., Tokyo, Japan) were used.
Human plasmin was purchased from Chromogenix AB (Mölndal, Sweden), and human urokinase was purchased from Midorijyuji Co. (Osaka, Japan). Bovine insulin and S-2251 MCA were purchased from Sigma (St. Louis, MO, USA). PBS and Hanks' balanced salt solution (HBSS) were obtained from Invitrogen (Carlsbad, CA, USA). Lys-plasminogen was purchased from Technoclone (Vienna, Austria).
Synthesis of specific synthetic substrates
Synthesis, yields, and characterization data of sub-1 and sub-2 depicted in Figure S1 are given in the Appendix S1.
Enzymatic kinetics of substrates
The enzyme reaction for human plasmin and urokinase was carried out in 0.05 m Tris–HCl buffer (pH 7.4 for plasmin and pH 8.8 for urokinase). Kinetic constants KM and kcat were determined by a method described previously, by measuring the p-nitroaniline (E405) released from p-nitroanilides 12.
Imaging of activated plasmin by plasmin-specific substrates in breast cancer cells
Human breast cancer cell line MDA-MB231 cells (ATCC, Manassas, VA, USA) were cultured in Leibovitz L-15 medium (Sigma) supplemented with 10% (v/v) fetal calf serum and antibiotics at 37 °C. Cells were grown overnight in glass-bottom chamber slides (Becton, Dickinson, Franklin Lakes, NJ, USA). The medium was discarded and the cells were washed with PBS. Then, the cells were incubated for 1 h in Hanks' balanced salt solution (HBSS) with 10 μg/mL Lys-plasminogen (Technoclone) and S-2251 MCA or sub-2 (each 50 μm). After washing the cells with HBSS, fluorescence was observed using confocal laser scanning fluorescence microscopy and measured (C-LSM510; Carl Zeiss, Jena, Germany).
Results
Design of plasmin-specific synthetic substrates
Plasmin-specific synthetic substrates were designed from plasmin-selective inhibitors. In our group, a novel plasmin-specific inhibitor has been developed 13. In the series of inhibitors, a potent plasmin inhibitor with a non-peptidic side chain and peptidic scaffold (inhibitor 1) was selected for the template structure of the specific substrate (Figure 1A, compound 3 in Ref. 13). This inhibitor showed sevenfold plasmin inhibitory activity over the parent peptidic inhibitor D-Ile-Phe-Lys-CN; the IC50 values of inhibitor 1 and the parent inhibitor for the plasmin were 11 and 78 μm, respectively 13. The inhibitory activity of this inhibitor for urokinase was much weaker (IC50 > 1000 μm) than plasmin inhibition, indicating good selectivity. The predicted binding mode of inhibitor 1 is shown in Figure 1B 13. The side chain of Lys residues of the inhibitor (P1 moiety) was deeply bound to the S1 site of the plasmin. The picolyl-o-Phe residue of the inhibitor (P2 moiety) was bound at the widely open area between the S2 and S3 sites. The P3 moiety D-Ile was placed on the surface of the plasmin in order to retain the overall conformation of binding. These favorable interactions between the inhibitor and plasmin realized high inhibitory activity. The favorable interactions were utilized by the probing unit of the plasmin-specific substrates in this study.
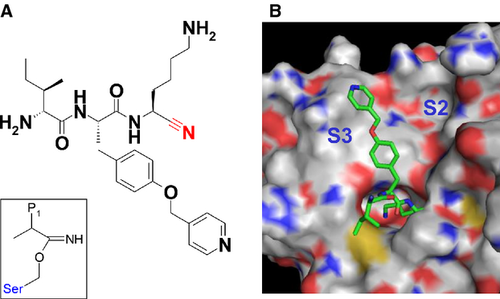
Our inhibitors were mechanistically based on a nitrile warhead that is reversibly covalently bound to the catalytic nucleophile of proteases at the intermediate state of the inhibition reaction (See the square box in Figure 1A). The structure of this intermediate mimics that of the intermediate in the protease hydrolysis reaction (Figure 2A). Because the inhibitory activity of the warhead inhibitors is known to correlate with substrate kinetics due to this similarity, the high inhibitory activity and selectivity of the inhibitor were assumed to be straightforwardly converted to high enzymatic reactivity and selectivity of plasmin-specific substrates 14. The para-nitroanilide (pNA) and methyl coumaryl amide (MCA) moieties were used as the reactive units for cleavage of the plasmin-specific substrates sub-1 and sub-2, respectively, so that both can be connected by a peptide bond with the probing unit (Figure 2B). The pNA moiety was used as a chromophore and the MCA moiety was used as a fluorophore group for the detection of the cleavage reaction (Figure 2C).
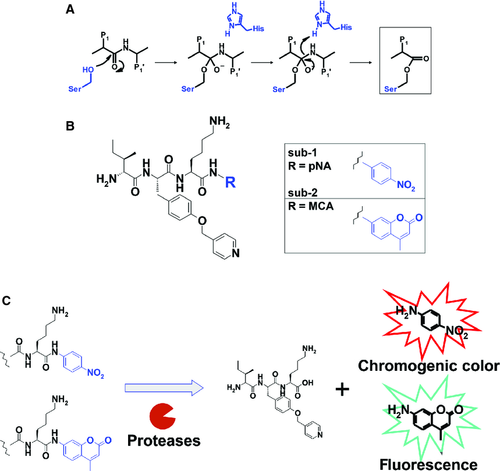
Synthesis of plasmin-specific substrates
The chromogenic and fluorogenic substrates were prepared by a conventional solution method, by assembling building blocks corresponding to the P1, P2, and P3 moieties, followed by deprotection with 20% piperidine/DMF and 32% HBr/acetic acid, respectively (Figure S1). The resulting crude products were purified by preparative HPLC to provide substrates sub-1 and sub-2. (See Appendix S1 for experimental details.)
Enzymatic kinetics of substrates
The enzymatic kinetics of the plasmin-specific substrate sub-1 was examined with plasmin and urokinase. S-2251 (D-Val-Leu-Lys-pNA), which is widely used as a plasmin-specific peptidic substrate in biochemical studies, was employed as a comparator. As a result, the plasmin reactivity of sub-1 (kcat/KM) was ca. seven times higher than that of S-2251 (Table 1). This enhancement was mainly due to an increase in binding affinity of sub-1 to plasmin; the KM values were 30 and 240 μm for sub-1 and S-2251, respectively. On the other hand, the reactivity of sub-1 to urokinase was not clearly determined due to the precipitation of the reaction mixture at a high concentration of the substrates. However, according to the kinetic reaction, sub-1 most probably has much weaker reactivity than S-2251, suggesting that plasmin-specific substrates have better selectivity to plasmin.
Probing of activated plasmin by plasmin-specific substrate in breast cancer cells
To visualize the localization and function of the activated plasmin in situ, sub-2 was examined in breast cancer cell line MDA-MB231 cells. MDA-MB231 cells are known to have a higher expression of proteins involved in the urokinase–uPAR system, to be highly invasive, and to have a proliferative feature 16-18. First, without plasminogen treatment, the cell surface of MDA-MB231 cells was monitored only with sub-2 or comparator peptidic substrate S-2251 MCA, which has a fluorescent MCA instead of pNA (Figure 3A). Neither of the substrates produced a strong fluorescent signal on the cell, although the urokinase–uPAR system is activated on MDA-MB231 cells (Figure S2), indicating that the selectivity to urokinase is high enough for either substrate. Next, the activated plasmin was generated by adding plasminogen to MDA-MB231 cells, and the substrates were simultaneously added to the medium as well. After incubation for 1 h, excess amounts of plasmin and reacted substrates were washed out. As seen in Figure 3B, a strong fluorescent signal was only observed from the cells with sub-2, which was cleaved by the activated plasmin and released the fluorescent MCAs. The relative intensity of fluorescence showed signals 10-fold stronger from sub-2 compared with those of S-2251 MCA (Figure 3C).
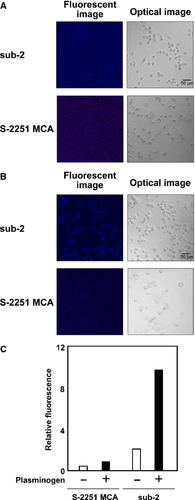
In addition to MDA-MB231 cells, another breast cancer cells, MCF-7, were examined for the same experiments using the plasmin-specific synthetic substrates as to confirm our method. MCF-7 cells are known to be latent in expressing the urokinase–uPAR system, as confirmed in Figure S2, and to be less invasive 17, 18. As expected, no strong fluorescent signals were observed from both of the substrates without or with plasminogen (Figure S3), suggesting that the activated plasmin was not generated from MCF-7 cells due to the latent urokinase–uPAR system.
Discussion
In this study, information on the localization and function of the activated plasmin was concurrently observed in situ in MDA-MB231 breast cancer cells using the plasmin-specific synthetic substrates. Although the information obtained in this study is already well known in this research field, it would be worthwhile mentioning that it was the first time for activated plasmin at work in situ by direct observation. Otherwise, the information on the localization and function has to be obtained from independent experiments; the localization information was obtained using fluorescence-tagged plasminogen or uPAR antibody 19, 20, and the activation of the plasminogen was measured by enzymatic activity using cell culture supernatant after plasminogen activation by incubation with cells 21.
Our concept to directly estimate the functionality of target molecules using substrate probes can be used for other proteases such as cathepsins or caspases, for which specific inhibitors with warheads are already known 22. In addition, this concept may be expanded for other types of enzymes if the products of enzymatic reactions are measurable or traceable by detectable probes.
From a technical point of view, the most significant key to success for the specific synthetic substrates will be to design a probing unit with high reactivity and selectivity. Theoretically, the stronger binding affinity and selectivity the specific substrates have, the better the information obtained. However, in practice, a certain amount of the substrates, such as 10–100 μm, is required to sense the signal of detectable probes in the experiments, so the binding affinity KM of the probing unit should be kept relatively weak, that is, in a μm range. This is because the effect of ‘product inhibition’, that is a product molecule derived from enzymatic reaction can tightly bind to the enzyme, likely occurs and this effect would lead to artificial observations, if the probing unit possesses too strong an affinity for the enzyme.
Conclusion
In conclusion, we have shown the first example that the specific synthetic substrate can be used to monitor the enzymatic functional status in situ for the plasmin–plasminogen system in breast cancer cells. This concept of specific synthetic substrate, as a ‘tailor-made substrate’, would be utilized as a novel functional molecular probe in vivo although detectable probes to overcome the photon attenuation in living tissue should appropriately be chosen 23. Further studies are warranted to well establish this concept of the specific substrates in various enzymatic systems.
Conflict of Interest
The authors have no conflict of interest to declare.




