Eribulin is an immune potentiator in breast cancer that upregulates human leukocyte antigen class I expression via the induction of NOD-like receptor family CARD domain-containing 5
Abstract
Eribulin inhibits microtubule polymerization and improves the overall survival of patients with recurrent metastatic breast cancer. A subgroup analysis revealed a low neutrophil to lymphocyte ratio (NLR) (<3) to be a prognostic factor of eribulin treatment. We thus hypothesized that eribulin might be related to the immune response for breast cancer cells and we analyzed the effects of eribulin on the immune system. Immunohistochemical staining revealed that human leukocyte antigen (HLA) class I expression was increased in clinical samples after eribulin treatment. In vitro assays revealed that eribulin treatment increased HLA class I expression in breast cancer line cells. RNA-sequencing demonstrated that eribulin treatment increased the expression of the NOD-like family CARD domain-containing 5 (NLRC5), a master regulator of HLA class I expression. Eribulin treatment increased the NY-ESO-1-specific T-cell receptor (TCR) transduced T (TCR-T) cell response for New York oesophageal squamous cell carcinoma 1 (NY-ESO-1) overexpressed breast cancer cells. The eribulin and TCR-T combined therapy model revealed that eribulin and immunotherapy using TCR-T cells has a synergistic effect. In summary, eribulin increases the expression of HLA class 1 via HLA class 1 transactivatior NLRC5 and eribulin combination with immunotherapy can be effective for the treatment of breast cancer.
Abbreviations
-
- ALC
-
- absolute lymphocyte count
-
- CARD
-
- caspase activation and recruitment domain
-
- ELISpot
-
- enzyme-linked immunospot
-
- HLA
-
- human leukocyte antigen
-
- IFN
-
- interferon
-
- IHC
-
- immunohistochemical
-
- MFI
-
- mean fluorescent intensity
-
- NLR
-
- neutrophil to lymphocyte ratio
-
- NLRC5
-
- NOD-like receptor family CARD domain-containing 5
-
- NOD
-
- nucleotide-binding olibgomerization domain
-
- qRT-PCR
-
- quantitative real-time PCR analysis
-
- RECIST
-
- response evaluation criteria in solid tumors
-
- siRNA
-
- small interfering RNA
-
- TCR
-
- T-cell receptor
-
- TCR-T
-
- TCR transduced T
-
- TTP
-
- time to progression
1 INTRODUCTION
Breast cancer is the most frequent malignancy in females, with 287,850 estimated new cases in the United States and 43,250 deaths in 2022.1 There are three major histological subtypes: hormone receptor-positive, HER2-positive, and triple-negative. Non-metastatic breast cancer can be treated by surgery and radiation therapy with a favorable prognosis, but systemic therapy for metastatic breast cancer is still limited, with a poor prognosis.2
Eribulin has been approved for the treatment of recurrent metastatic breast cancer and shows a significant improvement in overall survival.3, 4 Eribulin is an analog of the macrolide halichondrin B, which was identified in Halichondria okadai, a Japanese sponge.4 Eribulin has antitumor effects by inhibiting microtubule growth and inducing G2/M phase arrest.5, 6 Eribulin also has a role in telomerase reverse transcriptase inhibition, vascular remodeling, and inhibition of epithelial-mesenchymal transition by suppressing transforming growth factor-β signaling.7-11 Limited biomarkers for the prediction of the efficacy of eribulin have been described, but a low neutrophil to lymphocyte ratio (NLR) is a predictive marker for breast cancer and soft tissue sarcoma,12, 13 suggesting that the immune status of patients might be related to the antitumor effect of eribulin.
On the basis of this evidence, we hypothesized that the antitumor effect of eribulin might include an immune-related mechanism. We analyzed breast cancer cases who were treated with eribulin and found that human leukocyte antigen (HLA) class I expression was increased after eribulin treatment. To analyze its mechanism at the molecular level, we screened for candidate genes by RNA-sequencing (RNA-seq) and found that eribulin increased the expression of NLR family CARD domain-containing 5 (NLRC5), an HLA class I transactivator. We finally showed that the recognition of cancer cells by cytotoxic T lymphocytes was increased by eribulin treatment. These findings provide the rationale for the combination of eribulin and immunotherapy in the treatment of breast cancer.
2 MATERIALS AND METHODS
2.1 Patients, specimens, and immunohistochemical staining
The patients analyzed in this study were breast cancer cases treated with eribulin at a dose of 1.4 mg/m2 during a 2–5 min infusion on days 1 and 8 of a 21-day cycle at Sapporo Medical University Hospital from January 2012 to May 2021. Tissue samples were obtained from two patients treated with eribulin. This study was approved by the Institutional Review Board of Sapporo Medical University Hospital (#292–1044). Written informed consent was obtained from all patients in accordance with the guidelines of the Declaration of Helsinki.
Immunohistochemical (IHC) staining using an anti-HLA class I antibody (clone EMR8-5; Hokudo) was performed as described previously.14, 15 Formalin-fixed paraffin-embedded sections of surgical specimens from breast cancer patients (n = 2) were analyzed.
2.2 Cell lines and reagents
The human breast cancer cell lines MDA-MB-231 (HLA-A*02:01) and MCF7 (HLA-A*02:01) were purchased from the American Type Culture Collection (Manassas, VA, USA). MDA-MB-231 cells were cultured in Roswell Park Memorial Institute-1640 medium (Sigma-Aldrich), and MCF7 cells were cultured in Dulbecco's modified Eagle's medium (Sigma-Aldrich) supplemented with 10% fetal calf serum at 37°C in 5% CO2. Eribulin was purchased from Eisai Co. and paclitaxel was purchased from Bristol Myers Squibb.
2.3 Immunofluorescence staining
Approximately 20,000 cells/well were plated on glass-bottom dishes (AGC Techno Glass) overnight. The cells were fixed with 4% paraformaldehyde and washed with phosphate-buffered saline containing 1% bovine serum albumin. An anti-HLA class I antibody (clone EMR8-5) was used as a primary antibody, and an Fluorescein isothiocyanate (FITC)-labeled goat anti-mouse immunoglobulin G (IgG) heavy chain + light chain (H+L) polyclonal antibody (SeraCare, Milford, MA) was used as a secondary antibody. Images were obtained using an immunofluorescence microscope (BZ-X700; Keyence). Cell nuclei were identified by Vectashield Mounting Medium with 4',6-diamidino-2-phenylindole (DAPI) (Vector Laboratories) staining.
2.4 Flow cytometry and western blot analyses
Flow cytometry analysis using a Becton Dickinson (BD) FACSCalibur (Becton Dickinson) was performed as described previously.16 For the detection of HLA class I molecules, an anti-HLA class I antibody (clone W6/32) was used. For the detection of HLA class II molecules, an anti-HLA DR antibody (clone L243) was used. The W6/32 antibody and L243 were prepared from the supernatant of the hybridomas (American Type Culture Collection).
Western blot analysis was performed as described previously.16 The cells were washed in ice-cold phosphate-buffered saline and lysed by incubation on ice in a lysis buffer (50 mmol/L Tris–HCl [pH 8.0], 150 mmol/L NaCl, 5 mmol/L ethylenediaminetetraacetic acid (EDTA), 1% NP40). After sodium dodecyl sulfate-polyacrylamide gel electrophoresis, the proteins were transferred to a PVDF membrane (Immobilon-P; Millipore). After blocking, the membrane was incubated for 60 min with an anti-HLA class I antibody (EMR8-5) or mouse anti-β-actin monoclonal antibody (AC-15; Sigma-Aldrich). The membrane was reacted with a peroxidase-labeled goat antimouse IgG antibody or peroxidase-labeled goat antirabbit IgG antibody (KPL) for 30 min. Finally, the signal was visualized using an enhanced chemiluminescence (ECL) detection system (Amersham Life Science). The images were obtained by using Osyssey Imaging System (LI-COR). Raw images of Westen blots are presented in Figure S1.
2.5 Quantitative real-time PCR analysis
Quantitative real-time PCR analysis (qRT-PCR) was performed as described previously17 to determine the expression levels of human leukocyte antigen (HLA)-A, HLA-B, NLRC5, and GAPDH. Comprementary DNA (cDNA) was amplified via qRT-PCR with Taq DNA polymerase (Nippon Gene) and the StepOnePlus RT-PCR system (Thermo Fisher Scientific). The primer sequences were as follows: glyceraldehyde-3-phosphate dehydrogenase (GAPDH), Hs0160665_g1; HLA-A, Hs01058806_g1; HLA-B, Hs07292706_g1; and NLRC5, Hs01072155_g1 (Life Technologies; Thermo Fisher Scientific). The delta–delta Ct method was used for data analysis. Expression values for each sample were normalized to GAPDH, and the fold levels of the indicated genes represent the mean (±standard error of the mean) of replicate reactions.
2.6 RNA-seq
RNA quality was evaluated using an Agilent 4200 TapeStation (Agilent Technologies), and the concentration was measured using a Qubit Fluorometer (Thermo Fisher Scientific). Libraries for sequencing were constructed with 3000 ng of RNA from each sample using a TruSeq Stranded messenger RNA (mRNA) kit (Illumina). Library quantity was estimated using a KAPA Library Quantification kit (Roche). The average insert size of the libraries was 337–351 bp. High-throughput sequencing of the samples was performed using a NextSeq 500/550 High Output Kit v2.5 (75 cycles pair-end, 40/40 cycles; Illumina). There was an average of 18,364,442.5 sequence reads per sample. The bulk RNA-seq results were analyzed using CLC Genomics Workbench Version 12.0.2 (Filgen, Inc.). Raw and processed data have been deposited in the NCBI GEO database (GSE226836).
2.7 Small interfering RNA transfection
The cells were transfected with small interfering RNAs (siRNAs) using Lipofectamine RNAiMAX (Thermo Fisher Scientific). siRNA for human NLRC5 (4392420) was purchased from Thermo Fisher Scientific. Mock transfection consisted of transfection reagent only. The NLRC5 knock-down used equimolar concentrations of the siRNAs for a final siRNA pool concentration of 10 nM. All siRNA experiments were terminated after 72 h of transfection.
2.8 Cell proliferation assay
A cell proliferation assay was performed using a cell counting kit-8 (Dojindo). The cells were seeded on 96-well microtiter tissue culture plates at a density of 5.0 × 103 cells/well. After incubation with eribulin or paclitaxel for 48 h, 10 μL WST-8 reagent was added to the cells and the cells were incubated for 2 h at 37°C. The number of metabolically active cells in culture was quantified by measuring the absorbance at a wavelength of 450 nm using a microtiter plate reader.
2.9 Interferon-gamma ELISpot assay
An interferon-gamma (IFNγ) enzyme-linked immunospot (ELISpot) assay was performed as described previously.18 MCF7 cells expressing NY-ESO-1 were cultured for 48 h with 100 U IFNγ after the addition of 10 nM eribulin. MCF7 cells (10,000 cells/well) with or without eribulin were pre-incubated with or without 20 μM synthetic peptides for 2 h at 25°C. Then, 50,000 T cells were co-cultured with the MCF7 cells for 24 h at 37°C. After the addition of 2 μg/mL biotinylated antihuman IFNγ antibody and streptavidin-horseradish peroxidase, reactive spots producing IFNγ were visualized using ImageJ software.
2.10 Combination therapy model
NY-ESO-1/MCF7 cells were cultured in 12-well plates overnight at a density of 1.0 × 105 cells/well. The next day, eribulin was added to the cells. After 24 h, the cells were washed to remove eribulin. On day 2, 1.0 × 105 NY-ESO-1/T-cell receptor (TCR) transduced T (TCR-T) cells or CD8+ T cells isolated from peripheral blood mononuclear cells from healthy donors were co-cultured with the cancer cell lines. The cultures were washed on day 3 to remove the T cells, and cell counts and viability were measured on day 7.
2.11 Statistical analysis
Comparisons between two groups were performed using Student's t-test. Overall survival rates were calculated by the Kaplan–Meier method, and differences in survival curves were assessed by the log-rank test. The cutoff value for the baseline NLR was set as 3, as described previously.17, 18 Hazard ratios of the low-NLR group versus the high-NLR group were estimated from the stratified Cox proportional hazard model. All analyses were carried out with SPSS version 24.0 (SPSS, Inc.). A P value of less than 0.05 was regarded as statistically significant. All statistical tests were two-sided.
3 RESULTS
3.1 Patient characteristics and overall survival
The characteristics of the patients are summarized in Table 1. All patients had a metastatic lesion and were treated with eribulin. The median follow-up from initial eribulin treatment was 15 months (range 10.5–23.9 months). The median number of chemotherapy regimens prior to eribulin was two (zero to seven) cycles. Thirty-eight (82.6%) cases had metastatic lesions involving more than two organs. The number of patients in both groups was equal when an NLR of 3 was used as the cutoff value. Overall survival was significantly longer in the NLR-low group than in the NLR-high group (hazard ratio 0.323, 95% confidence interval 0.152–0.685; Figure 1A), as reported previously.12, 19 A previous study reported that the high absolute lymphocyte count (ALC) (≥1500/μL) group was associated with better prognosis than the low ALC (<1500/μL) group in the EMBRACE study,12 therefore we analyzed ALC in this study, but there was no significant relationship between the high ALC group and the low ALC group (Figure S2). Regarding the long-term benefits of eribulin in the NLR-low group, we hypothesized that an immunological mechanism had a role in its antitumor effect, especially in the NLR-low cases because long-term benefits are a characteristic feature of cancer immunotherapy.20, 21
| Characteristic | n | Range (%) | |
|---|---|---|---|
| Mean age | Years | 60 | (31–81) |
| Histological type | Invasive ductal | 40 | 87 |
| Invasive lobular | 4 | 8.6 | |
| Invasive micropapillary | 1 | 2.2 | |
| Mucinous | 1 | 2.2 | |
| ER, HER2 status | ER+/HER2- | 32 | 70 |
| ER-/HER2- | 14 | 30.4 | |
| Stage IV/MBC | Stage IV | 11 | 23.9 |
| MBC | 35 | 76.1 | |
| Number of prior chemotherapy regimens | 0 | 5 | 10.8 |
| 1 | 9 | 19.6 | |
| 2 | 15 | 32.6 | |
| ≧3 | 17 | 37 | |
| Number of metastatic site | 1 | 8 | 17.4 |
| ≧2 | 38 | 82.6 | |
| Site of disease | Non-visceral | 2 | 4.3 |
| Visceral | 44 | 95.7 | |
| Baseline NLR | ≦3 | 23 | 50 |
| >3 | 23 | 50 |
- Abbreviations: ER, estrogen receptor; HER2, human EGFR-related 2; MBC, metastatic breast cancer; NLR, neutrophil to lymphocyte ratio.
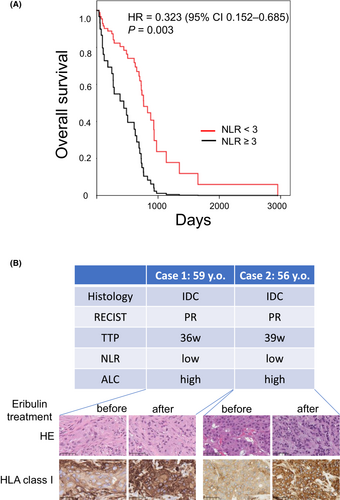
To address the immune microenvironment in eribulin-treated breast cancer lesions, we examined HLA class I expression by IHC staining using tissue from two eribulin-treated cases. Immunostaining revealed that HLA class I intensity and positive rates in the lesions were increased in the specimens obtained after eribulin treatment (Figure 1B).
3.2 Eribulin increases HLA class I expression in breast cancer cells
On the basis of the results of IHC staining, we hypothesized that eribulin can increase HLA class I expression in breast cancer cells. To assess this, we treated breast cancer cell lines (MCF7 and MDA-MB-231) with eribulin at several concentrations. Immunofluorescent staining revealed that HLA class I expression was increased in MCF7 cells following treatment with 10 nM eribulin (Figure 2A). Flow cytometry analysis demonstrated that HLA class I expression was increased by eribulin in MCF7 and MDA-MB-231 cells in a dose-dependent manner (Figure 2B–E). Western blot and qRT-PCR analyses also revealed that HLA class I expression was increased by eribulin in MCF7 and MDA-MB-231 cells (Figure 2F–I). These results indicate that HLA class I expression is increased in breast cancer cell lines at the mRNA level by eribulin.

3.3 RNA-seq analysis of eribulin-treated MCF7 cells
To address the molecular mechanism involved in the increase in HLA class I expression, we performed RNA-seq using mRNA isolated from eribulin-treated MCF7 cells and negative control untreated cells. The data are summarized in a dot plot (Figure 3A): 4888 genes were upregulated in eribulin-treated MCF7 cells (Table S1). Gene Ontology analysis revealed that antigen processing and presentation via HLA calss I, peptide antigen assembly with HLA class II, IFNγ-mediated signaling pathway, and several types of T cell responses were upregulated (Table S2). Among the genes upregulated in eribulin-treated MCF7 cells, HLA class I transactivator NLRC5 and HLA class 2 transactivatior CIITA were observed. CIITA downstream HLA class II (DR) was also increased in eribulin-treated MCF7 cells (Figure S3).

NLRC5 has been described as a transactivator of HLA class I and its overexpression increases HLA class I expression in breast cancer cells.22 We confirmed the expression of NLRC5. qRT-PCR analysis revealed that eribulin treatment increased NLRC5 mRNA expression by 1400-fold (Figure 3B). To address whether HLA class I expression depends on eribulin-induced NLRC5, we used siRNA specific for NLRC5. Knockdown of NLRC5 mRNA was confirmed by qRT-PCR (data not shown). Eribulin treatment did not increase HLA class I expression in NLRC5 siRNA-transfected cells, whereas eribulin treatment increased HLA class I expression in negative control siRNA-transfected cells (Figure 3C,D), suggesting that the increase in HLA class I expression by eribulin treatment depends on NLRC5.
3.4 Eribulin treatment enhances the cytotoxic T cell response
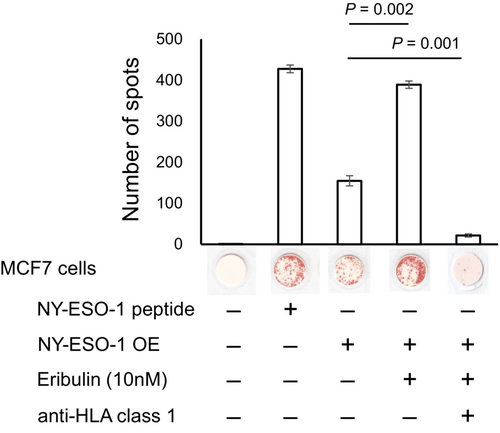
To confirm the function of HLA class I molecules increased by eribulin, we used NY-ESO-1-specific TCR-T cells, which were prepared as described previously using high-affinity HLA-A2 restricted anti-NY-ESO-1157-165 TCR sequence.16, 23 These cells recognized NY-ESO-1157-165 peptide-pulsed T2 cells and NY-ESO-1-overexpressing MCF7 cells (Figure 4). Eribulin treatment of NY-ESO-1-overexpressing MCF7 cells significantly increased the number of IFNγ spots compared with untreated NY-ESO-1-overexpressing MCF7 cells (Figure 4). Treatment with an anti-pan HLA class I antibody (W6/32) almost completely blocked the production of IFNγ spots in eribulin-treated NY-ESO-1-overexpressing MCF7 cells (Figure 4), suggesting that the IFNγ spots are specific for HLA class I expression.
3.5 Combination therapy model of eribulin and immunotherapy
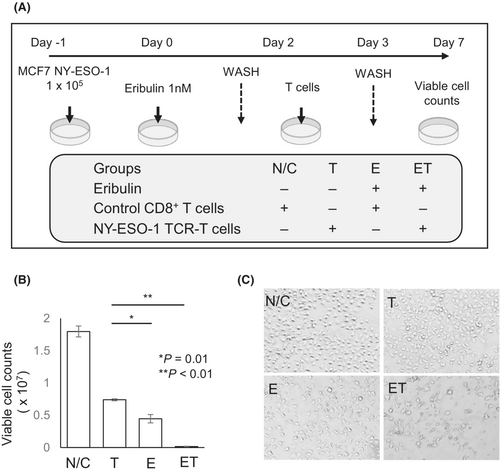
Since eribulin treatment increased HLA class I expression, we hypothesized that the combination of eribulin and immunotherapy may have a synergistic antitumor effect. To assess this hypothesis, we constructed a combination therapy model. The experimental design is summarized in Figure 5A. NY-ESO-1-overexpressing MCF7 cells were treated with eribulin, TCR-T cells, or eribulin and TCR-T cells. Eribulin monotherapy and TCR-T cell monotherapy significantly decreased the number of alive target cells (Figure 5B,C). The combination of eribulin and TCR-T cell treatment significantly decreased the number of alive target cells compared with the eribulin and TCR-T cell monotherapy groups (Figure 5B,C). These results indicate that the combination of eribulin and immunotherapy has a synergistic effect and might be a promising therapeutic approach for patients with breast cancer.
4 DISCUSSION
In this study, we analyzed breast cancer cases treated with eribulin. Cases with NLR <3 showed a better prognosis than those with a higher NLR, in agreement with previous observations,23 suggesting that the antitumor effect of eribulin depends on the cancer immune microenvironment. A previous study revealed that eribulin treatment increases the number of infiltrating CD8+ T cells,24 but the underlying mechanism was elusive. In the present study, we discovered that eribulin increased HLA class I expression in vitro and in vivo, and eribulin increased recognition by T cells in vitro. Eribulin treatment also increased the expression of HLA class II transactivator CIITA and its downstream HLA class II. Gene Ontology analysis revealed that antigen presentation, response to T cells, and response to IFN were listed for eribulin-treated MCF7 cells (Table S2), but not response to DNA damage or cell cycle. Thus, an improvement of the cancer immune microenvironment might be one of the major mechanisms by which eribulin exerts its antitumor effect in vivo.
Regarding the expression of HLA class I, we found that the expression of HLA class I transactivator NLRC5 was increased by eribulin. We confirmed by knockdown experiments using NLRC5 siRNA that a portion of the elevated HLA class I expression by eribulin is due to NLRC5. Eribulin was reported to reduce the tumor microenvironment by vascular remodeling.25 In fact, eribulin treatment increased the tumor oxygen saturation (SO2) in the tumors treated with eribulin.10 Hypoxia decreased the expression of major histocompatibility complex class I partly by hypoxia inducible factor 1-α (HIF1α),26 thus improvement of SO2 might also be related to an increase in HLA class I in vivo. The increase in NLRC5 by eribulin could be observed in the in vitro assay under normoxia, thus NLRC5 expression does not depend on HIF1α.
The molecular mechanism by which eribulin induces NLRC5 expression is still elusive, but eribulin has been shown to activate the cGAS/STING pathway through the cytoplasmic accumulation of mitochondrial DNA.27 Activation of the cyclic GMP-AMP synthase/stimulator of interferone genes (cGAS/STING) pathway induces the expression of type 1 IFNs.28 RNA-seq analysis of eribulin-treated MCF7 cells also showed an increase in IFNα and IFNβ expression in this study (Table S1), suggesting that eribulin activates the cGAS/STING signaling in our experiments. However, a recent study revealed that paclitaxel also activates the cGAS/STING pathway to activate interferone regulatory factor 3 (IRF3) and downstream programmed death-ligand 1 (PD-L1) expression.29 In addition, our results indicated that paclitaxel did not increase HLA class I expression (Figure S4). Thus, activation of the cGAS/STING pathway might be not sufficient for the induction of HLA class I expression. Moreover, IFNγ expression was also increased in eribulin-treated MCF7 cells. IFNγ induces NLRC5 expression through the Janus kinase 1 (JAK1)/signal transducer and activation of transcription 1 (STAT1) pathway,30 NLRC5 expression might depend on IFNγ, but the mechanism by which IFNγ is increased is also still elusive.
Eribulin inhibits epithelial-mesenchymal transition by suppressing transforming growth factor-β signaling,28 and epithelial-mesenchymal transition is related to resistance to cytotoxic T lymphocyte recognition.31, 32 Thus, eribulin might improve the immune-suppressive tumor microenvironment and augment cytotoxic T lymphocyte recognition. In this regard, a phase Ib/II clinical trial of eribulin combined with pembrolizumab (anti-PD1) immunotherapy for metastatic triple-negative breast cancer (ENHANCE1) has been reported.33 The therapy was tolerable and the objective response rate was 25.8%. Of note, PD-L1-positive cases showed a higher objective response rate than PD-L1-negative cases, suggesting that immunity has a role in the antitumor response. Another clinical trial of eribulin plus pembrolizumab for hormone receptor-positive and HER2-negative previously treated metastatic breast cancer reported an objective response rate of 40.9%.34 A randomized study revealed that the addition of pembrolizumab did not improve the efficacy of eribulin.35 Although the results were negative, whole exome sequencing and RNA-seq analyses revealed that immune infiltration and antigen presentation signatures could distinguish the cases that would respond to treatment, suggesting that the underlying immune status is critical for the antitumor effects of eribulin and pembrolizumab treatment.36 The findings of these clinical trials do not definitively demonstrate the efficacy of combination therapy with eribulin and immunotherapy, but the results are promising and further studies are expected.
In summary, we demonstrate that eribulin increases HLA class I expression through the induction of the HLA class I transactivator NLRC5 and increases T cell recognition. The eribulin and T cell combination therapy model revealed additional effects, therefore these results provide the rationale for the combined use of eribulin and immunotherapy in patients with breast cancer.
AUTHOR CONTRIBUTIONS
Aasaka Wada: Conceptualization; data curation; formal analysis; investigation; writing – original draft. Yoshihiko Hirohashi: Conceptualization; formal analysis; funding acquisition; investigation; project administration; writing – review and editing. Goro Kutomi: Investigation; supervision. Kenji Murata: Data curation; formal analysis; methodology. Sadahiro Iwabuchi: Data curation; formal analysis; methodology. Yuka Mizue: Data curation; formal analysis. Aiko Murai: Data curation; formal analysis. Daisuke Kyuno: Data curation; methodology. Hiroaki Shima: Data curation; investigation. Tomoyuki Minowa: Data curation; formal analysis. Kenta Sasaki: Data curation; formal analysis. Terufumi Kubo: Data curation; formal analysis. Takayuki Kanaseki: Formal analysis. Tomohide Tsukahara: Formal analysis. Munehide Nakatsugawa: Methodology. Shinichi Hashimoto: Data curation; formal analysis; funding acquisition; methodology. Makoto Osanai: Project administration; supervision. Toshihiko Torigoe: Conceptualization; funding acquisition; project administration; supervision; validation; writing – review and editing. Ichiro Takemasa: Conceptualization; investigation; project administration; supervision; validation; writing – review and editing.
ACKNOWLEDGMENTS
We thank ThinkSCIENCE Inc. (Tokyo, Japan) for English language editing.
FUNDING INFORMATION
This work was supported by KAKENHI grants from the Japan Society for the Promotion of Science (21K18257 to T. Torigoe and 20H03460 and 23H02696 to Y. Hirohashi). This work was also supported by grants from the Japan Agency for Medical Research and Development's Project for Cancer Research and Therapeutic Evolution (21cm0106309h0006 to T. Torigoe and 20cm0106352h0002 to T. Kanaseki), the Project for Promotion of Cancer Research and Therapeutic Evolution (22ama221317h0001 to Y. Hirohashi), and the Japan Science and Technology Agency's CREST program (JPMJCR15G3 to S. Hashimoto).
CONFLICT OF INTEREST STATEMENT
Toshihiko Torigoe is an editorial board member of Cancer Science. The other authors have no conflict of interest.
ETHICS STATEMENT
Approval of the research protocol by an Institutional Review Board: This study was performed by the guidelines established by the Declaration of Helsinki and was approved by the Ethics Committee of Sapporo Medical University.
Informed Consent: All patients provided written informed consent before participating in the study.
Registry and the Registration No. of the study/trial: N/A.
Animal Studies: N/A.




