Collagen XVII regulates tumor growth in pancreatic cancer through interaction with the tumor microenvironment
Abstract
Expression of the gene for collagen XVII (COL17A1) in tumor tissue is positively or negatively associated with patient survival depending on cancer type. High COL17A1 expression is thus a favorable prognostic marker for breast cancer but unfavorable for pancreatic cancer. This study explored the effects of COL17A1 expression on pancreatic tumor growth and their underlying mechanisms. Analysis of published single-cell RNA-sequencing data for human pancreatic cancer tissue revealed that COL17A1 was expressed predominantly in cancer cells rather than surrounding stromal cells. Forced expression of COL17A1 did not substantially affect the proliferation rate of the mouse pancreatic cancer cell lines KPC and AK4.4 in vitro. However, in mouse homograft tumor models in which KPC or AK4.4 cells were injected into syngeneic C57BL/6 or FVB mice, respectively, COL17A1 expression promoted or suppressed tumor growth, respectively, suggesting that the effect of COL17A1 on tumor growth was influenced by the tumor microenvironment. RNA-sequencing analysis of tumor tissue revealed effects of COL17A1 on gene expression profiles (including the expression of genes related to cell proliferation, the immune response, Wnt signaling, and Hippo signaling) that differed between C57BL/6-KPC and FVB-AK4.4 tumors. Our data thus suggest that COL17A1 promotes or suppresses cancer progression in a manner dependent on the interaction of tumor cells with the tumor microenvironment.
Abbreviations
-
- DEG
-
- differentially expressed gene
-
- KEGG
-
- Kyoto Encyclopedia of Genes and Genomes
-
- NK
-
- natural killer
-
- PAAD
-
- pancreatic adenocarcinoma
-
- qPCR
-
- quantitative PCR
-
- RNA-seq
-
- RNA-sequencing
-
- TME
-
- tumor microenvironment
1 INTRODUCTION
Tumor tissue comprises tumor cells and the surrounding tumor microenvironment (TME), which includes immune cells, blood vessel endothelial cells, fibroblasts, and ECM secreted from these various cell types.1 Interactions between tumor cells and nontumor cells as well as between tumor cells and ECM contribute to the regulation of tumor growth, invasion, and metastasis. Pancreatic adenocarcinoma (PAAD) is one of the most refractory cancer types, the poor treatment outcome of which is due in part to its characteristic TME.2, 3 An immunosuppressive TME results in the escape of cancer cells from immune attack,4 whereas abundant cancer-associated fibroblasts and excessive ECM, which give rise to a so-called desmoplastic stroma, contribute to epithelial–mesenchymal transition and anticancer drug resistance of cancer cells.5 The TME has thus attracted attention as a potential target for cancer therapy including the treatment of PAAD.6, 7
Collagen XVII, encoded by the COL17A1 gene, is a transmembrane-type collagen molecule that plays an important role in the anchoring of hemidesmosomes at the dermal–epidermal junction.8, 9 It forms homotrimers at the plasma membrane of basal cells, and, together with integrin α6β4, it binds to laminin 332, resulting in the stable association of basal cells with the underlying basement membrane. The loss of COL17A1 function, as a result either of the development of autoantibodies to the protein in bullous pemphigoid or of mutations of COL17A1 in junctional epidermolysis bullosa, gives rise to severe blistering skin diseases.10
In addition to such skin diseases, altered expression of COL17A1 has been implicated in various types of solid cancer, and COL17A1 has been suggested as a potential diagnostic and prognostic biomarker for PAAD.11-13 The role of COL17A1 in cancer progression, however, appears to be complex.8 Overexpression of COL17A1 in PAAD cells was found to promote cell proliferation in vitro and tumor growth in immunocompromised nude mice, whereas it was shown to inhibit the proliferation of invasive breast carcinoma (BRCA) cells.14-16 Furthermore, whereas COL17A1 expression was found to promote cell invasion in colorectal cancer and squamous cell carcinoma, it suppressed cell migration and invasion in PAAD and BRCA.17-20 COL17A1 thus appears to promote or suppress cancer progression in different settings, with the underlying mechanisms having remained unknown.
We have now examined the role and mechanism of action of COL17A1 in tumorigenesis by PAAD cells, in particular, in the context of immune cells present in the TME. We explored the effects of COL17A1 expression on cell proliferation in vitro with the mouse pancreatic cancer cell lines KPC and AK4.4. We also investigated the effects of COL17A1 expression on tumorigenesis in vivo with mouse homograft tumor models based on injection of these cell lines in syngeneic C57BL/6 and FVB mice, respectively. We found that COL17A1 promoted or suppressed the tumorigenicity of KPC and AK4.4 cells, respectively, in vivo without substantially affecting their proliferation in vitro.
2 MATERIALS AND METHODS
2.1 Cell culture and reagents
The mouse pancreatic cancer cell line KPC (C57BL/6 background) was established from a tumor induced in an LSL-KrasG12D/LSL-Trp53R172H/Pdx1-Cre mouse and was kindly provided by Ashok Saluja (University of Minnesota Medical School).21 The mouse pancreatic cancer cell line AK4.4 (FVB background) was established from a tumor induced in a Ptf1-Cre/LSL-KrasG12D/Trp53Lox/+ mouse and was kindly provided by Nabeel Bardeesy (Massachusetts General Hospital).22 The human pancreatic cancer cell lines PANC-1 (RRID: CVCL_0480) and MIA PaCa-2 (RRID: CVCL_0428) were obtained from RIKEN BioResource Research Center within the previous 3 years through the National BioResource Project of MEXT/AMED of Japan. Human pancreatic cancer AsPC-1 cells (RRID: CVCL_0152) were obtained from the American Type Culture Collection. All human cell lines were authenticated by short tandem repeat profiling within the last 3 years. KPC, AK4.4, PANC-1, and MIA PaCa-2 cells were cultured in DMEM (Sigma-Aldrich) and AsPC-1 cells in RPMI 1640 medium (Sigma-Aldrich), with each medium being supplemented with 10% heat-inactivated FBS (Thermo Fisher Scientific). All cell lines were maintained under a humidified atmosphere of 5% CO2 at 37°C, and all experiments were performed with mycoplasma-free cells.
2.2 In vitro cell proliferation assay
KPC and AK4.4 cells were plated at a density of 1 × 105 per 60-mm dish, and the cell number was determined every 2 days using an automated cell counter (Bio-Rad). The population doubling level (PDL) was calculated according to the formula ΔPDL = 3.322[log(Y) − log(I)], where Y is the number of cells at the end of the growth period and I is the number of cells plated. PANC-1, MIA PaCa-2, and AsPC-1 cells were plated at a density of 1 × 104 per well in a 96-well flat-bottom plate (Corning) and were cultured for 4 days. The relative cell number (absorbance at 450 nm) was measured every day with the use of a Cell Counting Kit-8 (Dojindo).
2.3 Mouse maintenance and tumorigenicity assay
C57BL/6J and FVB/N mice were obtained from CLEA Japan, and NOG [NOD.Cg-Prkdcscid Il2rgtm1Sug Tg (Pgk-EGFP)16/Jic] mice from the Central Institute for Experimental Animals (Kawasaki, Japan). Mice were maintained in a specific pathogen-free facility at the Institute of Animal Experimentation, Tohoku University Graduate School of Medicine. They were provided with water and rodent chow ad libitum and treated according to the Standards for Humane Care and Use of Laboratory Animals of Tohoku University and the Guidelines for Proper Conduct of Animal Experiments of the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
For assay of subcutaneous tumorigenicity, 5 × 105 KPC or AK4.4 cells in DMEM were injected into each of the left and right flanks of female mice at 6–10 weeks of age. KPC cells were injected into C57BL/6 mice and AK4.4 cells into FVB or NOG mice. The volume of the resulting tumors was measured every week. The mice were killed 4 weeks after cell injection, and the weight of tumors was measured. For assay of orthotopic tumorigenicity, 1 × 105 AK4.4 cells in DMEM were injected into the pancreas of female FVB mice at 6 weeks of age. The mice were killed 4 weeks after cell injection, and the volume and weight of tumors were measured.
2.4 Additional methods
Additional methods are described in Appendix S1.
2.5 Statistical analysis
Data are presented as means ± SEM for the indicated number (n) of biological replicates, unless indicated otherwise, and they were subjected to statistical analysis with GraphPad Prism 9 version 9.4.1, R software version 4.0.3, or Excel for Microsoft 365. A p-value of <0.05 was considered statistically significant.
3 RESULTS
3.1 Increased COL17A1 expression is associated with poor survival in patients with PAAD
To investigate the role of COL17A1 in cancer, we first examined COL17A1 mRNA abundance in 26 types of cancer using The Cancer Genome Atlas and Genotype-Tissue Expression RNA–sequencing (seq) databases. The abundance of COL17A1 mRNA was increased in some primary tumor types such as PAAD and lung squamous cell carcinoma, whereas it was reduced in others, including BRCA and skin cutaneous melanoma, or not changed in yet others, such as liver hepatocellular carcinoma and thyroid carcinoma, compared with corresponding normal tissue (Figure 1A,B).
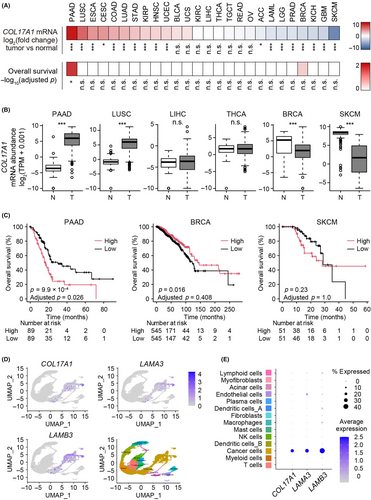
Survival analysis for cancer patients revealed that COL17A1 expression in tumor tissue was associated with overall survival in PAAD and BRCA (Figure 1A,C), consistent with previous observations,14, 16 although this association was not significant for BRCA after Bonferroni correction for multiple testing. Of note, a higher COL17A1 mRNA abundance was associated with a worse or better overall survival for patients with PAAD or BRCA, respectively, suggesting that COL17A1 may have different roles in cancer progression depending on cancer type.
Analysis of single-cell (sc) RNA-seq data for PAAD (GSE155698) revealed that COL17A1 and the genes for the COL17A1 binding partners LAMA3 and LAMB3 were predominantly expressed in cancer cells rather than in stromal cells (Figure 1D,E). Together, our database analyses thus indicated that higher COL17A1 expression in pancreatic cancer cells was associated with worse overall survival of PAAD patients.
3.2 COL17A1 expression does not affect proliferation of pancreatic cancer cells in vitro
To evaluate the potential role of COL17A1 in PAAD development, we studied two mouse pancreatic cancer cell lines, KPC and AK4.4, that express negligible levels of the endogenous protein (Figure 2A). These cells were infected with a recombinant lentivirus encoding mouse COL17A1 and subjected to drug selection. Given that only a small fraction of the selected cells expressed COL17A1 (data not shown), we obtained cell clones stably expressing the exogenous protein (KPC-Col17a1 or AK4.4-Col17a1 cells; Figure 2A). Cells harboring the empty vector (KPC-vector or AK4.4-vector cells) were examined as controls. Immunofluorescence analysis revealed that most KPC-Col17a1 and AK4.4-Col17a1 cells, but not the corresponding parental cells, expressed COL17A1 (Figure 2B). KPC-Col17a1 cell clone C6 showed a small but significant increase in the rate of cell proliferation in vitro compared with control cells (Figure 2C). Such an effect was not apparent, however, for KPC-Col17a1 clone B2 (Figure 2C) or for two clones, C4 and D2, of AK4.4-Col17a1 cells (Figure 2D).
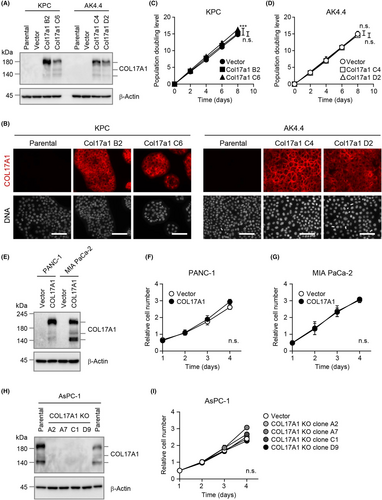
We also analyzed two human pancreatic cancer cell lines, PANC-1 and MIA PaCa-2, for which endogenous COL17A1 was not detected by immunoblot analysis (Figure 2E). Forced expression of human COL17A1 in these cells yielded polyclonal populations in which most of the selected cells expressed the exogenous protein (Figure 2E, data not shown), but it did not affect their proliferation rate in vitro (Figure 2F,G).
We further tested the effect of COL17A1 loss on cell proliferation in the human pancreatic cancer cell line AsPC-1, which expresses endogenous COL17A1 at a high level (Figure 2H). We obtained four AsPC-1 cell clones with homozygous deletion of COL17A1 and found that such COL17A1 knockout had no effect on cell proliferation (Figure 2I). Collectively, these results suggested that the expression level of COL17A1 did not markedly affect the ability of pancreatic cancer cells to proliferate in vitro.
3.3 COL17A1 expression promotes or suppresses tumor growth in mouse homograft models of pancreatic cancer
To examine the effects of COL17A1 expression on tumorigenesis in vivo, we established two murine homograft tumor models in which KPC or AK4.4 cells were injected subcutaneously into syngeneic C57BL/6 or FVB mice (C57BL/6-KPC and FVB-AK4.4 models), respectively (Figure 3A). KPC-Col17a1 cells formed larger tumors than did KPC-vector cells in recipient C57BL/6 mice (Figure 3B–D), consistent with a recent finding that COL17A1 expression promoted the growth of tumors formed by the human pancreatic cancer cell line AsPC-1 in immunodeficient nude mice.14 In contrast, tumors formed by AK4.4-Col17a1 cells were markedly smaller than those formed by AK4.4-vector cells in recipient FVB mice (Figure 3E–G). COL17A1 expression also suppressed the growth of tumors in the pancreas of FVB-AK4.4 orthotopic model mice (Figure S1A,B). We focused on the subcutaneous tumor models in subsequent experiments because no AK4.4-Col17a1 tumors were recognizable for molecular analysis in the orthotopic model (Figure S1C).
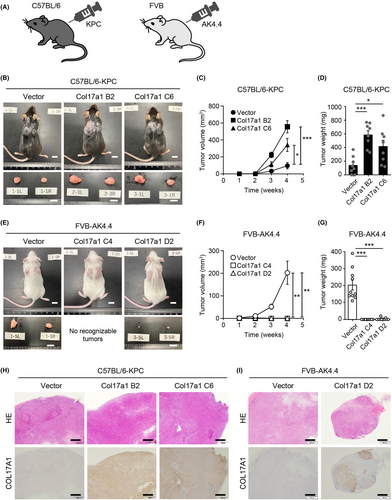
Immunohistochemical analysis of subcutaneous tumor tissue isolated from recipient mice revealed that almost all cells expressed COL17A1 in KPC-Col17a1 tumors (Figure 3H), whereas most cells did not express COL17A1 in AK4.4-Col17a1 tumors (Figure 3I). Given that COL17A1 expression was detected in all AK4.4-Col17a1 D2 cells used for the tumorigenicity assay (Figure S2A), it seemed unlikely that contamination with and overgrowth of COL17A1-negative AK4.4 cells were responsible for this loss of COL17A1 expression in the tumor tissue. More detailed microscopic analysis revealed that the COL17A1-negative cells in AK4.4-Col17a1 tumors appeared to be mesenchymal cells (Figure S2B). Indeed, COL17A1-negative regions of the tumors were strongly positive for vimentin, a mesenchymal cell marker (Figure S2C). The loss of the COL17A1 signal in AK4.4-Col17a1 D2 tumors thus appeared to be due to infiltration of host-derived mesenchymal cells. Together, our results thus suggested that, despite the lack of an obvious effect of COL17A1 expression on KPC and AK4.4 cell proliferation in vitro, such expression promoted or suppressed, respectively, the growth of tumors formed by these cells in vivo.
3.4 COL17A1 upregulates gene expression related to cell proliferation in C57BL/6-KPC tumors
To explore the mechanisms of COL17A1-induced tumor promotion and suppression, we performed RNA-seq analysis of tumors formed by KPC-Col17a1 clone B2, AK4.4-Col17a1 clone D2, and their corresponding vector control cells. We identified 398 and 1502 differentially expressed genes (DEGs) in the C57BL/6-KPC and FVB-AK4.4 models, respectively (Figure 4A). The larger number of DEGs in the FVB-AK4.4 model might be due to the increased infiltration of mesenchymal cells and immune cells (see below) in AK4.4-Col17a1 tumors. Only 24 (2%) upregulated DEGs and 19 (2%) downregulated DEGs associated with COL17A1 expression were common to both C57BL/6-KPC and FVB-AK4.4 models, indicating that COL17A1 differentially affects gene expression profiles in these models (Figure 4B). We also compared upregulated DEGs in each tumor model with downregulated DEGs in the other model to identify common genes with opposite regulation patterns (Figure S3A,B). Of note, 35 DEGs downregulated in C57BL/6-KPC tumors and upregulated in FVB-AK4.4 tumors were found to be highly enriched in genes related to the innate immune response (Figure S3B–D).
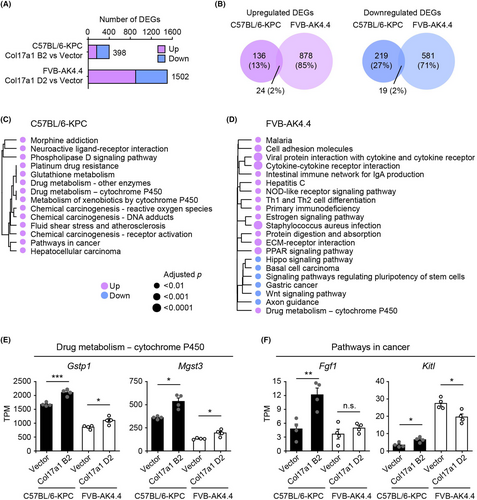
We then performed enrichment analysis of DEGs for Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways to identify biological pathways associated with regulation of tumor growth by COL17A1 (Figure 4C,D). The pathway “drug metabolism–cytochrome P450” was the only pathway enriched in both C57BL/6-KPC and FVB-AK4.4 models, with the expression of the associated genes Gstp1 and Mgst3 (both of which encode GST enzymes) being upregulated in Col17a1 tumors relative to vector tumors (Figure 4C–E). The COL17A1-induced expression of these enzymes might thus contribute to the suppression of mitochondrial membrane potential and reactive oxygen species production by COL17A1.23
Although COL17A1 was previously shown to promote PAAD progression through activation of the NF-κB signaling pathway,14 this pathway was not enriched in our analysis (Figure 4C,D). We next focused on “pathways in cancer,” which was enriched for DEGs upregulated in KPC-Col17A1 tumors relative to corresponding vector control tumors (Figure 4C), given that this pathway includes genes related to cell proliferation and might shed light on the mechanism of COL17A1-induced tumor promotion in the C57BL/6-KPC model. Expression of the component genes Fgf1 and Kitl, which encode ligands for receptor-type protein-tyrosine kinases, was significantly upregulated in KPC-Col17a1 tumors but was not affected or was downregulated in AK4.4-Col17a1 tumors (Figure 4F). These results thus indicated that gene expression related to cell proliferation was regulated differentially by COL17A1 between C57BL/6-KPC and FVB-AK4.4 tumor models.
3.5 COL17A1 promotes infiltration of natural killer cells into FVB-AK4.4 tumors
We next explored the mechanism by which COL17A1 expression suppresses tumor growth as observed in the FVB-AK4.4 model. Enrichment analysis of DEGs in this model revealed that upregulated genes were enriched in immune response pathways including “cytokine–cytokine receptor interaction” and “viral protein interaction with cytokine and cytokine receptor” (Figure 4D). These pathways were not enriched in the C57BL/6-KPC model (Figure 4C). We compared gene expression for the first of these pathways between C57BL/6-KPC and FVB-AK4.4 tumors by RT and quantitative PCR (qPCR) analysis. Expression of the cytokine genes Cxcl9 and Cxcl11 as well as that of the corresponding receptor gene Cxcr3 were significantly upregulated in AK4.4-Col17a1 tumors relative to AK4.4-vector tumors isolated from FVB mice (Figure 5A and Figure S4). The expression of these genes was not altered by COL17A1 expression in C57BL/6-KPC tumors (Figure 5A and Figure S4C). These results, combined with our observation that genes related to innate immunity were regulated by COL17A1 in opposite directions in C57BL/6-KPC and FVB-AK4.4 tumor models (Figure S3B–D), indicated that COL17A1 differentially regulates the immune response in these two models.
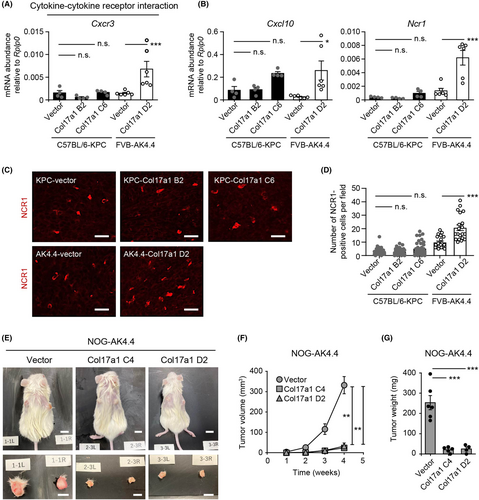
The observed changes in the expression of innate immune response–related genes prompted us to investigate tumor infiltration by innate immune cells. We first examined expression of the gene for CXCL10, a cytokine that regulates the chemotaxis of CXCR3-expressing cells such as natural killer (NK) cells, as well as the expression of Ncr1, a marker for NK cells, by RT-qPCR analysis.24, 25 The expression of both of these genes, most prominently that of Ncr1, was significantly increased in AK4.4-Col17a1 tumors relative to AK4.4-vector tumors, whereas it did not differ between KPC-Col17a1 and KPC-vector tumors (Figure 5B). Consistent with these observations, immunofluorescence analysis of tumor tissue revealed that both the CXCL10-positive area and number of NCR1-positive cells were significantly increased in AK4.4-Col17a1 tumors compared with AK4.4-vector tumors, whereas no such differences were apparent for the C57BL/6-KPC model (Figure 5C,D and Figure S5A,B). Most NCR1-positive cells were located in COL17A1-positive and vimentin-negative regions of AK4.4-Col17a1 tumors (Figure S5C,D), indicative of the infiltration of NK cells around pancreatic cancer cells. These results thus suggested that COL17A1 expression promoted the infiltration of NK cells in the FVB-AK4.4 tumor model but not in the C57BL/6-KPC model.
We also injected AK4.4 cells subcutaneously into immunodeficient NOG mice, which lack T cells, B cells, and NK cells and in which the function of macrophages and dendritic cells is attenuated,26 to examine whether immune cells are required for the suppression of AK4.4 tumor growth by COL17A1. Unexpectedly, we found that the growth of AK4.4-Col17a1 tumors was also suppressed relative to that of AK4.4-vector tumors in NOG mice (Figure 5E−G), suggesting that suppression of tumor growth by COL17A1 was not primarily due to increased infiltration of immune cells but rather to other unknown mechanisms.
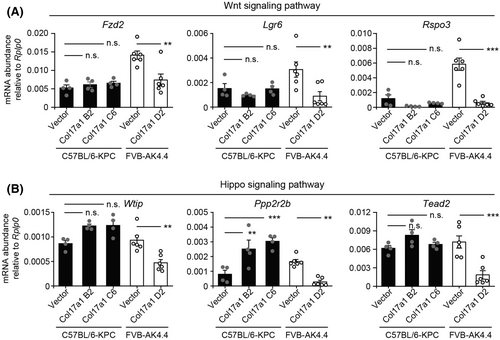
3.6 COL17A1 downregulates gene expression related to Wnt or Hippo signaling in FVB-AK4.4 tumors
Enrichment analysis for DEGs in the FVB-AK4.4 model revealed that downregulated genes in AK4.4-Col17a1 tumors were enriched in the “Wnt signaling pathway” and the “Hippo signaling pathway” (Figure 4D), with both of these pathways playing pivotal roles in pancreatic cancer development and progression.27, 28 Expression of genes related to these signaling pathways—including Lgr6 (encoding a cell surface receptor for the canonical Wnt pathway), Rspo3 (encoding a ligand for LGR4–6 receptors that promote canonical Wnt signaling), and Tead2 (encoding an effector transcription factor in the Hippo pathway)—was significantly downregulated in AK4.4-Col17a1 tumors but was not affected or was upregulated in KPC-Col17a1 tumors (Figure 6 and Figure S6). These results indicated that both the Wnt and Hippo signaling pathways are regulated differentially by COL17A1 in C57BL/6-KPC and FVB-AK4.4 tumors. Differential regulation of gene expression related to cell proliferation, Wnt signaling, and Hippo signaling might therefore contribute to the mechanisms by which COL17A1 promotes or suppresses tumor growth, as observed in our murine homograft tumor models, as well as by which it promotes or suppresses cancer progression in human PAAD and BRCA, respectively.
4 DISCUSSION
We found that expression of COL17A1 markedly promoted or suppressed the growth of tumors formed by pancreatic cancer cells in vivo without affecting the proliferation of these cells in vitro. These observations suggest that COL17A1 requires a tumor microenvironment, which is absent or limited in cell culture, for regulation of tumorigenesis by pancreatic cancer cells. We also found that changes in gene expression related to cell proliferation, Wnt signaling, and Hippo signaling might mediate the distinct effects of COL17A1 on tumor growth between the C57BL/6-KPC and FVB-AK4.4 mouse models.
COL17A1 appears to have pleiotropic functions. Whereas hemidesmosomal COL17A1 promotes the stable association of basal cells with the underlying basement membrane at the dermal–epidermal junction, a non-hemidesmosomal function of COL17A1 has also been suggested at intercellular spaces between basal keratinocytes.29, 30 In addition, an interplay between COL17A1 and various signaling pathways, including the Wnt and Hippo pathways, has been described.29-31 Furthermore, COL17A1 is subjected to proteolytic processing in its extracellular domain, with such processing having been found to modulate the motility and proliferation of keratinocytes.32, 33 Such multifaceted functions and regulation of COL17A1 might underlie its context-dependent effects on tumor growth.
Another possible factor that might contribute to the differential effects of COL17A1 on tumor growth in our two homograft models is the status of Trp53 in KPC and AK4.4 cells. COL17A1 is a downstream target of p53 in humans and mice.20 KPC cells harbor the Trp53R172H mutation, whereas AK4.4 cells are heterozygous for Trp53 deletion (Trp53Lox/+). The Trp53R172H gain-of-function mutation not only leads to loss of normal p53 function but endows the mutant protein with new protumorigenic activities.34, 35 Trp53R172H knockin mice thus develop tumors that are more malignant and metastatic compared with those of Trp53 null mice.36, 37 COL17A1 might therefore cooperate with other p53 targets that are differentially affected by the Trp53R172H mutation or Trp53 loss to exert tumor-promoting or tumor-suppressive effects. Genetic manipulation of Trp53 in KPC and AK4.4 cells as well as comprehensive analysis of the expression of p53 target genes in KPC-Col17a1 and AK4.4-Col17a1 tumors may reveal potential roles of p53 in COL17A1-mediated regulation of tumor growth.
COL17A1 increased the expression of immune response-related genes and the infiltration of NK cells in FVB-AK4.4 tumors but not in C57BL/6-KPC tumors. COL17A1 at the cell surface is the major autoantigen in the blistering skin disease bullous pemphigoid, and shedding of its ectodomain mediated by extracellular proteases generates neoepitopes on COL17A1.9, 38 COL17A1 might therefore be highly immunogenic in some tumor environments and trigger an enhanced immune response. Such an enhanced immune response and NK cell infiltration appear to be dispensable for COL17A1-induced tumor suppression, however, given that the growth of AK4.4-Col17a1 tumors was also suppressed relative to that of AK4.4-vector tumors in immunodeficient NOG mice. Thus, whereas differences in immune responses between C57BL/6-KPC and FVB-AK4.4 models might contribute to the differential regulation of tumor growth by COL17A1, other mechanisms must also play a role. Nonetheless, further analysis of the immune microenvironment of AK4.4-Col17a1 and KPC-Col17a1 tumors may provide insight into the immunosuppressive TME characteristic of pancreatic cancer.4
Our study has several limitations. First, although we found that COL17A1 regulated the growth of tumors formed by pancreatic cancer cells in vivo without affecting the proliferation of these cells in vitro, the environmental cues that interact with COL17A1 to influence the growth of such tumors remain unclear. Second, the effects of COL17A1 on tumor growth were investigated by forced expression of COL17A1 in KPC and AK4.4 cells. Although such overexpression experiments should be complemented by knockdown or knockout studies, we were not able to obtain mouse pancreatic cancer cells with high endogenous expression of COL17A1. Finally, for assay of tumorigenicity, we injected KPC or AK4.4 cells into mice at 6 to 10 weeks of age. Therefore, we cannot formally exclude the possibility that such a wide age range might affect the results, given that expression of COL17A1 in the epidermis declines with age.39, 40
AUTHOR CONTRIBUTIONS
R.K., R.F., S.A., and K.N.: study conceptualization, investigation, writing–original draft, and writing–review and editing. A.M., S.K., Y.S., T.S., and K. Murakami: investigation. K.I., M.I., K. Masuda, M.M., H.N., D.G.D., and M.U.: funding acquisition, resources, supervision, and writing–review and editing.
ACKNOWLEDGMENTS
We thank H. Miyoshi for providing the lentiviral vector CSII-EF-MCS-IRES-Puro; Y. Nagasawa, K. Kuroda, and M. Kikuchi for technical assistance; T. Konishi for help with preparation of the manuscript; other laboratory members for discussion; and the Biomedical Research Core of Tohoku University Graduate School of Medicine for technical support.
FUNDING INFORMATION
This study was supported by Japan Society for the Promotion of Science (JSPS) KAKENHI grants JP20K07560 and JP23K06626 (to R.F.), JP20K17672 (to S.A.), JP22K08816 (to M.M.), JP21K08725 (to K. Masuda), and JP17H04035, JP18H05215, and JP21H02458 (to K.N.).
CONFLICT OF INTEREST STATEMENT
M.U. is an Editorial Board Member of Cancer Science. The other authors declare no conflict of interest related to this study.
ETHICS STATEMENTS
Approval of the research protocol by an Institutional Review Board: N/A.
Informed consent: N/A.
Registry and the registration no. of the study/trial: N/A.
Animal studies: All animal experiments were performed according to protocols approved by the Institutional Animal Care and Use Committee (IACUC) of Tohoku University Graduate School of Medicine (2021MdA-004-03).




