Association of immune-related expression profile with sensitivity to chemotherapy in esophageal squamous cell carcinoma
Yoshiyuki Tsukamoto, Shusaku Kurogi, and Hajime Fujishima contributed equally to this work.
Abstract
Neoadjuvant chemotherapy (NAC) followed by surgery is one of the standard therapeutic approaches in Japan for patients with locally advanced esophageal carcinoma. Recently, the JCOG1109 study revealed that NAC with docetaxel, cisplatin and 5-fluorouracil (5-FU) (DCF-NAC) is superior to NAC with cisplatin and 5-FU, and has now become the standard preoperative chemotherapy. Using a microarray system, we have previously investigated the expression profiles of endoscopic biopsy samples from patients with esophageal squamous cell carcinoma (ESCC) before DCF-NAC (preNAC) and identified 17 molecules as biomarkers predictive of a pathologically complete response to DCF-NAC. Here, we re-grouped our previous dataset based on the histopathological response grade with the addition of several microarray profiles and conducted a re-analysis using bioinformatic web tools including DAVID, GSEA, UALCAN, and CIBERSORTx. We identified 204 genes that were differentially expressed between the highly resistant and sensitive groups. Some of these differentially expressed genes (DEGs) were related to the immune response and showed higher expression in the sensitive group. UALCAN showed that high expression of 28 of the top 50 DEGs was associated with a favorable prognosis (p < 0.25), and that this reached a significant (p < 0.05) level for 18 of them, suggesting that patients with high expression of these genes might have benefited from chemotherapy and thus had a better outcome. In preNAC biopsy tissues from a DCF-sensitive case, we demonstrated the presence of cells expressing mRNA for CXCL9, one of the prognosis-related DEGs. Our results highlight the association of immune-related expression profile in preNAC ESCC with the DCF-NAC efficacy.
Abbreviations
-
- 5-FU
-
- 5-fluorouracil
-
- DAVID
-
- Database for Annotation, Visualization, and Integrated Discovery
-
- DCF
-
- docetaxel, cisplatin and 5-fluorouracil
-
- DDR
-
- DNA damage response
-
- DEG
-
- differentially expressed gene
-
- ESCC
-
- esophageal squamous cell carcinoma
-
- FDR
-
- false discovery rate
-
- GEPIA2
-
- gene expression profiling and interactive analyses
-
- GSEA
-
- Gene Set Enrichment Analysis
-
- HNSCC
-
- head and neck squamous cell carcinoma
-
- JCOG
-
- Japan Clinical Oncology Group
-
- LM22
-
- leukocyte 22 data matrix
-
- NAC
-
- neoadjuvant chemotherapy
-
- pCR
-
- pathological complete response
-
- TCGA
-
- The Cancer Genome Atlas
-
- TIL
-
- tumor-infiltrating lymphocyte
-
- UALCAN
-
- The University of ALabama at Birmingham CANcer data analysis Portal
1 INTRODUCTION
Esophageal cancer (EC) is the eighth most common cancer and the sixth leading cause of cancer mortality worldwide.1 There are two major histological types of EC—adenocarcinoma and ESCC—which tend to be predominant in Western countries and Asia, respectively. In Japan, more than 90% of ECs are ESCC and its 5-year survival rate is 36%.2
Neoadjuvant chemotherapy followed by surgery is one of the standard therapeutic approaches for patients with locally advanced EC in Japan. Recently, the JCOG 1109 study revealed that NAC with DCF-NAC was superior to NAC with cisplatin and 5-FU (NAC-CF),3 and has now become the standard form of preoperative chemotherapy. However, some patients who have had DCF-NAC become inoperable due to the progression of their cancers during and after three courses of NAC, which usually takes 13–15 weeks. In addition, the incidence of adverse side effects is higher for DCF-NAC than for CF-NAC, remaining a possible option for CF-NAC to manage the excessive adverse effects.4 Therefore, to stratify patients according to their likely response, predictive biomarkers are needed.
Previously, we investigated expression profiles from 32 endoscopic biopsy samples of ESCC before NAC (preNAC) and identified a 17-molecule set that was predictive of a pathologically complete response to DCF-NAC.5 For that study, we divided tumor tissues into two groups according to their histopathological response grade: grades 0, 1a, 1b, 2, and 3, among which grades 0 and 3 indicate no response and a complete response, respectively.6, 7 Among the 32 cases, 9 were considered to show pCR (grade 3) and 23 were included in a non-pCR group (grades 0, 1a, 1b, and 2). However, we realized that some of the non-pCR cases were highly resistant to DCF-NAC, and hypothesized that regrouping the cases into “highly resistant” and other cases (hereafter referred to as “sensitive”) might enable us to identify biomarkers that would classify tumors as being highly resistant. Here, we re-analyzed our expression microarray dataset with the addition of five non-tumor and seven ESCC tissues by comparing the resulting three groups: non-tumor (n = 5), highly resistant tumor (n = 12; grade 0 and 1a) and sensitive tumor (n = 27; grade 1b, 2, and 3) tissues.
2 MATERIALS AND METHODS
2.1 Patients, neoadjuvant chemotherapy and response evaluation
Details of patients enrolled in this study were described in our previous study.5 Briefly, this cohort study included ESCC patients who received DCF-NAC followed by surgery at Oita University Hospital between June 2013 and October 2016. In total, 39 patients met the following inclusion criteria: (i) histological diagnosis of primary ESCC; (ii) stage IB/II/III according to UICC 7th edition8; (iii) age ≤80 years; (iv) performance status 0–1; and (v) no previous chemotherapy, thoracic radiotherapy (RT), or thoracic surgery. ESCC samples were collected by biopsy during endoscopic examination before administration of the first course of chemotherapy. The biopsy specimens were each collected from an elevated part at the proximal side of the tumor in a uniform manner. Matched non-tumor tissues (at least 5 cm from the tumor site) from five of the 39 cases were also collected as control samples. The specimens were then frozen and preserved in a freezer maintained at −80°C. This study was approved by the Ethics Committee of Oita University Faculty of Medicine (Approval No. 1081), and all patients included in the study provided written informed consent.
The DCF-NAC regimen consisted of a 1-h i.v. infusion of docetaxel (70 mg/m2) on day 1, a 2-h infusion of cisplatin (70 mg/m2) on day 1, and continuous i.v. infusion of 5-FU (750 mg/m2) on days 1–5. This regimen was administered every 3 weeks, and three scheduled courses were administered before esophagectomy. Surgery was scheduled to be carried out within 4–6 weeks after the last day of preoperative chemotherapy, when curative resection was considered possible. Clinicopathological characteristics are shown in Table 1.
| Highly resistant | Sensitive | p-value | |
|---|---|---|---|
| n = 12 | n = 27 | ||
| Age (years); mean ± SD | 67 ± 10 | 65 ± 7 | 0.53 |
| Sex | 0.53 | ||
| Male | 11 | 26 | |
| Female | 1 | 1 | |
| cT | 0.48 | ||
| 1b | 0 | 2 | |
| 2 | 4 | 5 | |
| 3 | 6 | 18 | |
| 4 | 2 | 2 | |
| cN | 0.65 | ||
| 0 | 5 | 7 | |
| 1 | 2 | 6 | |
| 2 | 4 | 13 | |
| 3 | 1 | 1 | |
| cStage | 0.39 | ||
| II | 4 | 6 | |
| IIIA | 6 | 19 | |
| IIIB | 2 | 2 | |
| pT | 0.0067 | ||
| 0 (Complete response) | 0 | 10 | |
| 1a/1b | 2 | 10 | |
| 2 | 3 | 3 | |
| 3 | 5 | 3 | |
| 4 | 2 | 1 | |
| pN | 0.036 | ||
| 0 | 3 | 17 | |
| 1 | 3 | 7 | |
| 2 | 3 | 2 | |
| 3 | 3 | 1 | |
| pStage | 0.013 | ||
| 0 | 1 | 12 | |
| I | 0 | 2 | |
| II A/B | 4 | 9 | |
| III A/B | 2 | 3 | |
| IV A | 5 | 1 | |
| Histopathological response grade | 0.00 | ||
| 0 | 1 | 0 | |
| 1a | 11 | 0 | |
| 1b | 0 | 6 | |
| 2 | 0 | 11 | |
| 3 | 0 | 10 | |
| Histology (post NAC) | 0.0045 | ||
| Well | 2 | 1 | |
| Moderate | 6 | 9 | |
| Poor | 4 | 5 | |
| No malignancy | 0 | 10 | |
- Note: The difference between the two groups was assessed using Mann–Whitney U-test or Fisher's exact test.
The pathological response was evaluated according to the Japanese Classification of Esophageal Cancer 11th edition as follows: grade 0, no recognizable cytological or histological therapeutic effect; grade 1a, viable cancer cells accounting for two-thirds or more of the tumor tissue; grade 1b, viable cancer cells accounting for between one-third and two-thirds of the tumor tissue; grade 2, viable cancer cells accounting for less than one-third of the tumor tissue; grade 3, no viable cancer cells apparent (pCR).6, 7 Patients were divided into highly resistant (n = 12; grade 0 and 1a) and sensitive (n = 27; grade 1b, 2, and 3) groups according to the pathological response (Table 2).
| Case ID | Histopathologic response gradea | Classification 1 previous studyb | Classification 2 present study |
|---|---|---|---|
| 1 | 3 | pCR | S |
| 3 | 1a | Non-pCR | HR |
| 4 | 3 | pCR | S |
| 5 | 1a | Non-pCR | HR |
| 6 | 2 | Non-pCR | S |
| 7 | 3 | pCR | S |
| 8 | 1a | Non-pCR | HR |
| 9 | 2 | Non-pCR | S |
| 10 | 3 | pCR | S |
| 11 | 2 | Non-pCR | S |
| 12 | 3 | pCR | S |
| 14 | 2 | Non-pCR | S |
| 15 | 1b | Non-pCR | S |
| 16 | 2 | Non-pCR | S |
| 17 | 3 | pCR | S |
| 18 | 1b | Non-pCR | S |
| 19 | 1a | Non-pCR | HR |
| 20 | 1a | Non-pCR | HR |
| 21 | 1a | Non-pCR | HR |
| 22 | 1a | Non-pCR | HR |
| 24 | 3 | pCR | S |
| 25 | 0 | Non-pCR | HR |
| 26 | 1a | Non-pCR | HR |
| 27 | 2 | Non-pCR | S |
| 28 | 2 | Non-pCR | S |
| 29 | 3 | pCR | S |
| 30 | 1b | Non-pCR | S |
| 31 | 1b | Non-pCR | S |
| 32 | 1b | Non-pCR | S |
| 33 | 2 | Non-pCR | S |
| 34 | 1b | Non-pCR | S |
| 35 | 3 | pCR | S |
| 36 | 1a | – | HR |
| 37 | 2 | – | S |
| 38 | 1a | – | HR |
| 39 | 2 | – | S |
| 41 | 1a | – | HR |
| 42 | 2 | – | S |
| 43 | 3 | – | S |
| 25 N | Non-tumorc | – | N |
| 28 N | Non-tumorc | – | N |
| 29 N | Non-tumorc | – | N |
| 33 N | Non-tumorc | – | N |
| 35 N | Non-tumorc | – | N |
- a According to Japanese Classification of Esophageal Cancer, 11th Edition.
- b Fujishima et al., PLOS ONE, 2017 (PMID; 29,136,005).
- c Tissues at least 5 cm from the tumor site in cases 25, 28, 29, 33, and 35.
- Abbreviations: HR, highly resistant; N, non-tumor; pCR, pathologically complete response; S, sensitive.
2.2 Gene expression microarray and data analysis
Details are described in our previous study.5 Frozen specimens were homogenized, and total RNA was extracted using a QIAGEN RNeasy mini kit (QIAGEN) in accordance with the manufacturer's protocol. Total RNA (200 ng) was reverse transcribed to cDNA using murine leukemia virus reverse transcriptase (Invitrogen Crop.).
A human 8 × 60 K whole-genome oligo DNA microarray chip (SurePrint G3 Human Gene Expression v3 Microarray Kit, G4851C, Agilent Technologies, Santa Clara, CA) was used for global gene expression analysis, in accordance with the manufacturer's protocol. Cyanine (Cy)-labeled cRNA was prepared using T7 linear amplification, in accordance with the Agilent Low RNA Input Fluorescent Linear Amplification Manual (Agilent Technologies). Labeled cRNA was fragmented and hybridized to the oligonucleotide microarray (Agilent Technologies). The fluorescence intensities were determined with an Agilent DNA Microarray Scanner and analyzed using Feature Extraction v.10.7.3.1 (Agilent Technologies). Expression levels were converted to log2 values and normalized to the median of the entire spot array using GeneSpring GX11 (Agilent Technologies). All data are available at the Gene Expression Omnibus (GEO) via NCBI under Accession No. GSE225178 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE225178). The probe set data were filtered based on signal intensity and flagged values, resulting in 33,632 remaining probes. Genes showing differential expression between the three groups were identified by one-way ANOVA (p < 0.05) and post hoc testing correction (Tukey). The Benjamini and Hochberg correction was used for false-positive reduction (multiple testing correction). The genes differentially expressed between the highly resistant and sensitive groups were identified as DEGs, and these comprised 204 genes. Hierarchical clustering with Euclidean distance was performed to stratify patients according to their expression profiles of the 204 DEGs. GeneSpring GX11 was used for these statistical and clustering analyses.
DAVID, a web bioinformatic resource system (https://david.ncifcrf.gov/home.jsp)9, 10 was used to reveal enriched functional annotations among the DEGs. The data from the Functional Annotation Chart with the default setting was expressed in a table format and modified.
To identify the molecular signatures in each sample group, we performed GSEA (https://www.gsea-msigdb.org/gsea/index.jsp).11, 12 For this, microarray data for the 33,632 filtered probes in 39 cancer samples were ranked based on differential expression and compared with the Hallmark gene sets, composed of predefined 50 gene sets. Enriched gene sets with an FDR of less than 0.05 were considered significant.
To estimate the proportion of immune cell infiltration in each sample, microarray data were analyzed using CIBERSORTx (https://cibersortx.stanford.edu/index.php)13-15 with a validated LM22, representing the expression profiles of 547 immune-related genes in 22 leukocyte lineages.13 We set the job parameters for Batch correction, Disable quantile normalization, Run mode and Permutations as “disabled,” “true,” “absolute,” and “100,” respectively.
The impact of the expression of the top 50 DEGs on patient survival was investigated using the UALCAN web tool (http://ualcan.path.uab.edu/)16, 17 with an HNSCC dataset from TCGA. We used the HNSCC dataset because all of our cases were squamous cell carcinoma while the EC dataset in TCGA included many adenocarcinomas. Furthermore, fluoropyrimidine-based and platinum-based chemotherapies are also standard in HNSCC.18 High-expression patients showed more than one-third quartile expression levels.
2.3 RNAScope in situ hybridization
The distribution of CXCL9 mRNA on formalin-fixed paraffin-embedded sections of biopsied tissues was investigated using RNAScope in situ hybridization in accordance with the manufacturer's instructions (Advanced Cell Diagnostics).
2.4 Immunohistochemistry
After using RNAScope for analysis of CXCL9, the distribution of epithelial cells and macrophages was detected by immunohistochemistry using antibodies against pan-cytokeratin (clones AE1/AE3, DAKO) or CD68 (clone KP1, Arigo Biolaboratories), respectively. The sections were visualized using Histofine Simple Stain AP (Nichirei) and Perma Red substrate (Diagnostic Biosystems) in accordance with the manufacturer's instructions.
2.5 Statistics
All statistical information is indicated in the figure legends for each set of data.
3 RESULTS
3.1 Involvement of immune-related genes in sensitivity to DCF chemotherapy
In this study, we re-analyzed our previous dataset (Case ID: 1–35) after the addition of the expression profiles for five non-tumor tissues (Case ID: 25 N–35 N) and seven tumor tissues (Case ID: 36–43; Table 2). The expression profiles of, in total, 44 samples were then divided into three groups; non-tumor group (5 cases), sensitive group (27 cases, therapeutic effect grades 1b, 2, and 3) and highly resistant group (12 cases, therapeutic effect grades 0 and 1a). Genes differentially expressed among the three groups were identified by one-way ANOVA (p < 0.05) and post hoc testing correction (Tukey) (Figure 1A). The Benjamini and Hochberg correction was used for false-positive reduction (multiple testing correction). We found that 4347 and 6282 probes, including redundant and non-coding genes, exhibited differential expression in the highly resistant and sensitive groups compared with the non-tumor group, respectively (Figure 1A). We identified 204 probes, corresponding to 172 genes, as DEGs between the sensitive and highly resistant groups (Figure 1A and Table S1). Among the 204 probes, the top 50 DEGs showing the most differential expression are listed in Table 3. Interestingly, most of these DEGs were upregulated in the sensitive group (184 upregulated and 20 downregulated). To investigate the impact of expression of the top 50 DEGs on patient survival, we used the UALCAN online tool for prognostic analysis in TCGA HNSCC datasets. We chose the HNSCC datasets because all of our cases were squamous cell carcinomas while the EC dataset in TCGA included many adenocarcinomas. Furthermore, fluoropyrimidine-based and platinum-based chemotherapies are also standard for HNSCC.18 Among the top 50 DEGs, 28 showed a tendency for higher expression to be associated with better prognosis (p < 0.25; Table 3), and this association was significant (p < 0.05) for 18 of them (Figure S1), suggesting that these genes could be used as biomarkers of patient survival as well as sensitivity to chemotherapy. Although the sample size was small, we also investigated the impact of the top 50 DEGs on patient survival in our cohort. We found that most of the genes showed a tendency for higher expression to be associated with better outcomes (data not shown), although the p-value was less than 0.3 for only 10 of those genes (CXCL13, LGALS2, linc-LRRC10-1, IKZF3, SEMA5B, MIR4697HG, ZNF831, PTPRC, TIGIT, and LAMP3; Table 3). Furthermore, in the DEG list, there are some genes functionally related to immune response such as chemokines, complement components and Toll-like receptors (Table 3). For example, CXCL13 is a chemokine that preferentially attracts B lymphocytes in comparison with T cells and macrophages.19 CXCL9 and CXCL10 are induced by IFNγ and their increased expression is associated with infiltration of activated T cells through their cognate receptor, CXCR3,20 which is also listed in Table 3. LGALS2 (galectin 2) was recently reported to be an immunotherapy target in triple-negative breast cancer.21 Granzyme K (GZMK) is one of the serine proteases from cytoplasmic granules of cytotoxic lymphocytes, such as CD8+ T cells and NK cells.22 As shown in Figure 1B and Table S1, most of the immune-related genes, including CXCL13, CXCL9, CXCL10, LGALS2, GZMK, CD8B, CYBB, IRF8, C1QB, TLR4, TIGIT, and CXCR3, were specifically upregulated in the sensitive group compared with the non-tumor group, while the expression levels of these genes were less changed in the resistant group. As a consequence, most of the resistant (11/12) and non-tumor (5/5) samples were clustered in the same branch, while seven of the 10 cases showing a complete response (therapeutic effect grade 3) were clustered in a different branch from resistant and non-tumor samples in hierarchical clustering using the expression profiles of 204 DEGs (Figure 2A). DAVID analysis indicated that the DEGs were strongly associated with the term “immunity” (Figure 2B). GSEA also showed that the Hallmark gene sets of “allograft rejection” and “interferon γ response,” both of which are related to immune response, were enriched in the sensitive group (Figure 2C). None of the gene sets in the Hallmark gene sets (ver. 7.5) were enriched in the highly resistant group (data not shown). Taken together, our data suggested that the immune response-related expression profile in preNAC biopsy samples is associated with sensitivity to DCF chemotherapy.

| Sensitive/Resistant | Impact on prognosis | |||||
|---|---|---|---|---|---|---|
| Probe namea | Gene symbol | Gene name | Fold change | Regulation | Based on UALCANb | Based on our cohortc |
| A_23_P121695 | CXCL13 | Chemokine (C–X–C motif) ligand 13 | 6.66 | Up | Higher is better (p = 0.0033) | Higher is better (p = 0.28) |
| A_23_P18452 | CXCL9 | Chemokine (C–X–C motif) ligand 9 | 6.23 | Up | Higher is better (p = 0.041) | p = 0.66 |
| A_24_P303091 | CXCL10 | Chemokine (C–X–C motif) ligand 10 | 5.76 | Up | Higher is better (p = 0.23) | p = 0.62 |
| A_23_P120902 | LGALS2 | Lectin, galactoside-binding, soluble, 2 | 5.15 | Up | Higher is better (p = 0.033) | Higher is better (p = 0.18) |
| A_33_P3343175 | CXCL10 | Chemokine (C–X–C motif) ligand 10 | 4.45 | Up | Higher is better (p = 0.23) | p = 0.62 |
| A_23_P156218 | GZMK | Granzyme K (granzyme 3; tryptase II) | 4.17 | Up | Higher is better (p = 0.012) | p = 0.65 |
| A_23_P159335 | CD8B | CD8b molecule | 3.97 | Up | Higher is better (p = 0.11) | p = 0.7 |
| A_23_P19182 | REEP2 | receptor accessory protein 2 | 3.40 | Up | Lower is better (p = 0.17) | p = 0.33 |
| A_32_P56249 | USP30-AS1 | USP30 antisense RNA 1 | 3.24 | Up | p = 0.62 | p = 0.77 |
| A_33_P3294533 | PRKCB | Protein kinase C, beta | 3.23 | Up | Higher is better (p = 0.026) | p = 0.81 |
| A_22_P00009273 | lnc-LRRC10-1 | lnc-LRRC10-1:1 | 3.22 | Up | n.a. | Higher is better (p = 0.28) |
| A_24_P365767 | CYBB | Cytochrome b-245, beta polypeptide | 3.16 | Up | p = 0.27 | p = 0.62 |
| A_23_P47704 | UCP2 | Uncoupling protein 2 (mitochondrial, proton carrier) | 3.14 | Up | p = 0.65 | p = 0.69 |
| A_33_P3343120 | IRF8 | Interferon regulatory factor 8 | 3.12 | Up | Higher is better (p = 0.0078) | p = 0.62 |
| A_21_P0007219 | lnc-AP000769.1–1 | lnc-AP000769.1–1:1 | −3.11 | Down | n.a. | p = 0.43 |
| A_23_P166797 | RTP4 | Receptor (chemosensory) transporter protein 4 | 3.08 | Up | p = 0.83 | p = 0.69 |
| A_32_P30905 | WDFY4 | WDFY family member 4 | 3.06 | Up | Higher is better (p = 0.00095) | p = 0.62 |
| A_23_P137366 | C1QB | Complement component 1, q subcomponent, B chain | 3.05 | Up | p = 0.75 | p = 0.83 |
| A_23_P434919 | RAB42 | RAB42, member RAS oncogene family | 2.99 | Up | Higher is better (p = 0.085) | p = 0.61 |
| A_33_P3404601 | C2 | Complement component 2 | 2.99 | Up | p = 0.91 | p = 0.57 |
| A_33_P3333960 | LINC00426 | Long intergenic non-protein coding RNA 426 | 2.97 | Up | Higher is better (p = 0.0035) | p = 0.81 |
| A_23_P140876 | ABCA3 | ATP-binding cassette, subfamily A (ABC1), member 3 | 2.97 | Up | p = 0.53 | p = 0.65 |
| A_22_P00009941 | IKZF3 | IKAROS family zinc finger 3 (Aiolos) | 2.95 | Up | Higher is better (p = 0.001) | Higher is better (p = 0.26) |
| A_23_P121374 | SEMA5B | Sema domain, seven thrombospondin repeats (type 1 and type 1-like), transmembrane domain (TM) and short cytoplasmic domain, (semaphorin) 5B | 2.94 | Up | p = 0.7 | Higher is better (p = 0.29) |
| A_33_P3253394 | LAIR1 | Leukocyte-associated immunoglobulin-like receptor 1 | 2.93 | Up | Higher is better (p = 0.26) | p = 0.83 |
| A_23_P125977 | C1QC | Complement component 1, q subcomponent, C chain | 2.93 | Up | p = 0.97 | p = 0.74 |
| A_33_P3292769 | NFAM1 | NFAT activating protein with ITAM motif 1 | 2.92 | Up | Higher is better (p = 0.019) | p = 0.66 |
| A_21_P0012030 | TEX41 | Testis expressed 41 (non-protein coding) | −2.89 | Down | p = 0.57 | p = 0.74 |
| A_19_P00320101 | MIR4697HG | MIR4697 host gene (non-protein coding) | 2.87 | Up | n.a. | Higher is better (p = 0.24) |
| A_24_P82749 | CD37 | CD37 molecule | 2.87 | Up | Higher is better (p = 0.013) | p = 0.77 |
| A_33_P3231414 | LILRB1 | leukocyte immunoglobulin-like receptor, subfamily B (with TM and ITIM domains), member 1 | 2.87 | Up | Higher is better (p = 0.065) | p = 0.62 |
| A_24_P237036 | TNFSF14 | Tumor necrosis factor (ligand) superfamily, member 14 | 2.85 | Up | p = 0.3 | p = 0.57 |
| A_24_P362193 | CD84 | CD84 molecule | 2.80 | Up | Higher is better (p = 0.13) | p = 0.66 |
| A_32_P206479 | ZNF831 | zinc finger protein 831 | 2.79 | Up | Higher is better (p = 0.0012) | Higher is better (p = 0.26) |
| A_23_P200138 | SLAMF8 | SLAM family member 8 | 2.78 | Up | p = 0.43 | p = 0.31 |
| A_32_P66881 | TLR4 | Toll-like receptor 4 | 2.78 | Up | p = 0.95 | p = 0.79 |
| A_33_P3364811 | PTPRC | Protein tyrosine phosphatase, receptor type, C | 2.78 | Up | Higher is better (p = 0.0034) | Higher is better (p = 0.26) |
| A_23_P75786 | SLC15A3 | Solute carrier family 15 (oligopeptide transporter), member 3 | 2.76 | Up | Higher is better (p = 0.099) | p = 0.64 |
| A_23_P323761 | TRAF3IP3 | TRAF3 interacting protein 3 | 2.75 | Up | Higher is better (p = 0.0015) | p = 0.74 |
| A_33_P3342056 | TIGIT | T-cell immunoreceptor with Ig and ITIM domains | 2.73 | Up | Higher is better (p = 0.0047) | Higher is better (p = 0.28) |
| A_23_P151307 | RAPGEF3 | Rap guanine nucleotide exchange factor (GEF) 3 | −2.72 | Down | p = 0.45 | p = 0.87 |
| A_24_P222655 | C1QA | Complement component 1, q subcomponent, A chain | 2.72 | Up | p = 0.83 | p = 0.31 |
| A_23_P26994 | GNGT2 | Guanine nucleotide binding protein (G protein), gamma transducing activity polypeptide 2 | 2.71 | Up | Higher is better (p = 0.088) | p = 0.85 |
| A_23_P64661 | ARHGAP9 | Rho GTPase activating protein 9 | 2.70 | Up | Higher is better (p = 0.0096) | p = 0.66 |
| A_23_P36120 | MS4A6A | Membrane-spanning 4-domains, subfamily A, member 6A | 2.70 | Up | p = 0.81 | p = 0.74 |
| A_23_P114299 | CXCR3 | Chemokine (C–X–C motif) receptor 3 | 2.69 | Up | Higher is better (p = 0.0029) | p = 0.77 |
| A_24_P237443 | SASH3 | SAM and SH3 domain containing 3 | 2.68 | Up | Higher is better (p = 0.033) | p = 0.62 |
| A_33_P3234546 | CD8B | CD8b molecule | 2.68 | Up | Higher is better (p = 0.11) | p = 0.74 |
| A_33_P3368014 | HVCN1 | hydrogen voltage gated channel 1 | 2.67 | Up | p = 0.37 | p = 0.31 |
| A_23_P29773 | LAMP3 | Lysosomal-associated membrane protein 3 | 2.64 | Up | p = 0.6 | Higher is better (p = 0.056) |
- a All the DEGs are shown in Table S1.
- b http://ualcan.path.uab.edu/
- c Cases with higher expression (26 cases) and those with lower expression (13 cases) in our cohort were compared.
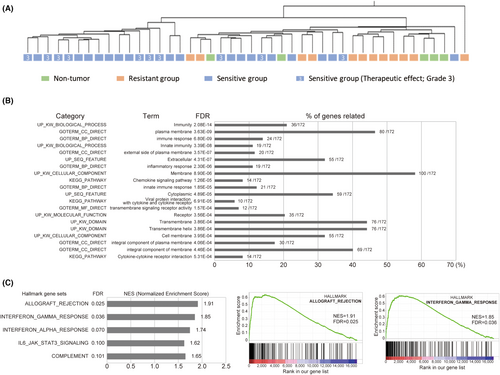
To exclude the possibility that biopsy samples in the highly resistant group accidentally contained far fewer tumor cells than the sensitive group, we compared the expression of genes upregulated in HNSCC, chosen based on the data set from GEPIA2,23 between the non-tumor, highly resistant and sensitive groups in our microarray data (Table S2). We chose HNSCC datasets for the same reason as that described above. Among the eight most upregulated genes in the HNSCC data set, six were also significantly upregulated in tumor tissue relative to non-tumor tissue in our microarray data (Table S2). Furthermore, all of the six genes were upregulated in both the resistant and sensitive groups, and showed no significant difference in expression levels between the two groups (Figure S2), suggesting it was unlikely that biopsy samples from the highly resistant group contained far fewer tumor cells than the sensitive group.
3.2 Potential involvement of M1 macrophage infiltration in sensitivity to DCF chemotherapy
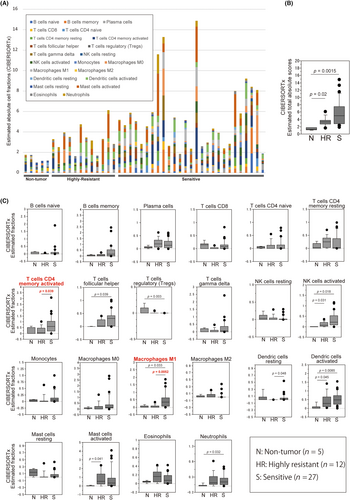
Because our data implicated immune cell infiltration in tumor tissues of DCF-NAC-sensitive cases, fractions of 22 immune cell types in each sample tissue were estimated using CIBERSORTx, which is a web bioinformatic tool that can assess the proportions of various types of infiltrating immune cells. The estimated-total absolute scores and absolute fractions of 22 immune cell types were compared between the non-tumor, highly resistant, and sensitive groups (Figure 3 and Table S3). The estimated-total absolute score was significantly higher in both of the tumor groups than in the non-tumor group (p = 0.02; and 0.0015 in the highly resistant and sensitive groups, respectively) but showed no significant difference between the highly resistant and sensitive groups (p = 0.14; Figure 3A,B), suggesting an immune-expression profile in both of the tumor groups. The estimated absolute cell fractions of CD4+ memory-activated T cells (p = 0.039) and M1 macrophages (p = 0.0052) in the sensitive group were significantly higher in those in the highly resistant group (Figure 3C), indicating possible infiltration of these immune cell types in the sensitive group. In particular, M1 macrophages exhibited the most significant difference between the highly resistant and sensitive groups (p = 0.0052), suggesting that M1 macrophage infiltration or expression of its related genes could be diagnostic biomarkers of DCF-NAC sensitivity.
3.3 Distribution of CXCL9-expressing cells in DCF-NAC-sensitive tumor tissues
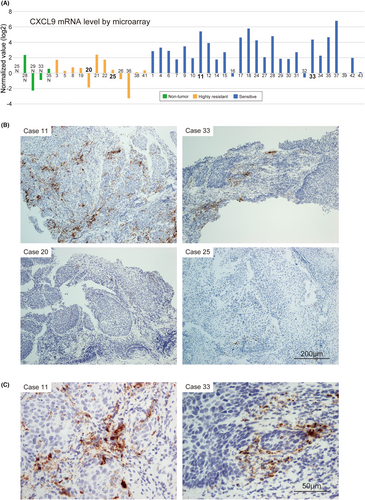
Consistent with our microarray data, CXCL9 and CXCL10, two of the most upregulated genes in the sensitive group (Figure 1B and Table 3), were highly expressed in M1 macrophages among the 22 leukocyte types in the LM22 expression profile,13 which is often referred to as a representative profile of 22 individual leucocyte lineages in CIBERSORTx. However, in contrast with CIBERSORTx estimation, a few of the previous studies reveal overexpression of CXCL9 in cancer epithelial cells as well.24, 25 Therefore, we next sought to determine the source of CXCL9 by in situ hybridization using two sensitive cases with high CXCL9 expression (cases 11 and 33) and two highly resistant cases with low CXCL9 expression (cases 20 and 25) in the microarray data (Figure 4A). Consistent with the microarray data, CXCL9 signals were clearly detectable in both two sensitive samples but barely detectable in the resistant samples (Figure 4B). We also investigated the distribution of CXCL13 using two sensitive cases with high CXCL13 expression (cases 28 and 37) and two highly resistant cases with low CXCL13 expression (cases 5 and 38) in the microarray data, and demonstrated consistency of the data between microarray and in situ hybridization (Figure S4). CXCL13 was clearly expressed in the stromal cells.
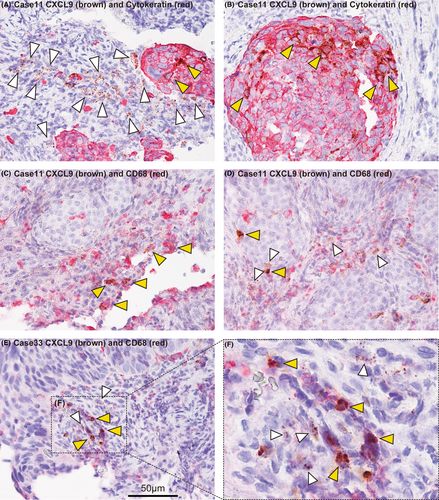
Next, we compared the distribution of CXCL9 with that of an epithelial cell marker (anti-cytokeratin antibody) or a macrophage marker (anti-CD68 antibody) by double staining using RNAScope and immunohistochemistry. Most of the CXCL9 signals were not detected in cytokeratin-positive cells, although only a small proportion of cytokeratin-positive cells expressed CXCL9 (Figure 5A,B). In contrast, many, but not all of the CD68-positive cells expressed CXCL9 (Figure 5C). To determine whether or not each of the CXCL9-positive macrophages are really M1 macrophages, further studies will be required. Interestingly, we also found that some CD68-negative stromal cells (non-macrophage stromal cells) also expressed CXCL9 (Figure 5D–F). Considering that previous reports have suggested T cells as the source of CXCL9,25, 26 it is possible that T cells in the biopsy samples also expressed CXCL9. Further studies are required to address this possibility. Taken together, our data suggest that CXCL9 is expressed mainly by tumor-surrounding stromal cells, including macrophages, and also slightly expressed by a small proportion of cancer cells, while another stromal cell type(s) not identified here could also be involved in CXCL9 expression.
4 DISCUSSION
In this study, we explored the gene expression profiles of preNAC biopsy samples and identified 204 genes as being differentially expressed between ESCCs that were highly resistant to DCF-NAC and those that were sensitive. Initially, we assumed that cancer cell-intrinsic alterations in the expression of efflux pump (e.g., ABCB1 and ABCC1),27 anti-apoptotic (e.g., BCL2 and MCL1)28 or DNA repair genes (e.g., ERCC1 and MLH1)29 would be involved in the resistance. However, such genes were not included in our DEGs. Unexpectedly, only a few genes were specifically upregulated or downregulated in the highly resistant group (Table S1). In contrast, some immune system-related genes were specifically upregulated in the sensitive group, suggesting infiltration of immune cells into DCF-NAC-sensitive tumor tissues. As a consequence, unsupervised hierarchical clustering analysis using 204 DEGs revealed that the expression profiles of highly resistant ESCCs were more closely related to non-tumors than those of sensitive ESCCs. Therefore, it is possible that the expression profiles obtained using bulk tumor tissues were due to the admixture of such profiles from not only cancer cells, but also immune cells and/or fibroblasts. Taken together, our data suggested that lower expression of immune system-related genes may be associated with high resistance to DCF chemotherapy in ESCCs. To reveal the cancer cell-intrinsic mechanism responsible for DCF-NAC resistance, it would be more reasonable to use cancer cells collected by microdissection or to perform single-cell RNA sequence analysis.
Several previous studies have also demonstrated the association of chemotherapy efficacy with immune system-related expression profiles or immune cell infiltration in preNAC biopsy samples. Fang et al. identified immune-related biomarkers predictive of NAC efficacy by using gene expression profiles of breast cancer patients.26 The genes identified in their report, such as CXCL9, CXCL10, CXCL11, CXCL13, GZMB, IDO1, and LYZ, were very similar to those in our study, suggesting a shared biological mechanism for chemosensitivity between esophageal and breast cancers. Choi et al. also focused on immune-related gene expression and tumor-infiltrating lymphocytes in the chemosensitivity of ovarian cancer.30 They identified several genes, including IRF1, CXCL9, LTB, CCL5, IL-8 (CXCL8), GZMA, PSMB9, CD38, and VCAM1, and TILs, as predictive biomarkers of adjuvant chemotherapeutic response. In addition, Sasagawa et al. have recently reported that the tumor genome and immune features are valuable biomarkers for the prediction of NAC efficacy in ESCC.31 Noma et al. have proposed the immunoscore signature in surgical specimens and tumor-infiltrating lymphocytes in preNAC biopsy as predictive biomarkers of NAC efficacy in ESCCs.32 Thus, an association between the immune profile of pre-chemotherapy tissue and chemosensitivity is becoming more evident based on data from several research groups.33, 34 However, the mechanism underlying interactions between the immune microenvironment and cancer cells is very complicated, and therefore more data will be required to establish a predictive method and suitable targeting therapy in a clinical setting. For example, details of the main source of chemokines are still unclear. Furthermore, the relationship between aberrant expression of immune-related genes and patient survival has not yet been fully investigated. The present study showed that the expressions of 18 out of the top 50 DEGs were significantly correlated (p < 0.05) with patient survival in the HNSCC dataset from TCGA. Our data thus support the previous reports and should help to facilitate the clinical application of immune-related profiles to ESCC chemotherapy.
The DEGs in this study included some immune-related genes, leading us to speculate that it might be possible to estimate the dominant infiltrating immune cells in DCF-NAC-sensitive ESCCs by applying our expression profile data. To test this, we utilized CIBERSORTx software, which is an analytical tool from the Alizadeh Laboratory and Newman Laboratory, to impute gene expression profiles and estimate the abundance of member cell types in a mixed cell population using gene expression data.13-15 CIBERSORTx estimated that M1 macrophages were more dominant in DCF-NAC-sensitive tissues. It has been reported that polarization of M1 macrophages is triggered by lipopolysaccharide (LPS) and interferon gamma (IFN-γ) stimulation,35 involved in anti-tumor immunity, and can drive Th1 T-cell responses through their expression of cytokines and chemokines, such as IL-12, CXCL9, and CXCL10.36 Indeed, CXCL9 and CXCL10 were highly expressed in M1 macrophages in the LM22 expression profile, which represents the expression profiles of 22 leukocyte types,13 and were also identified as DEGs in our dataset (Figure 1B and Table 3). In addition, previous reports have shown a dominant expression of these chemokines in cancer cells.24, 25 To determine the cellular source of these chemokines, we performed RNAScope in situ hybridization for CXCL9 and found that CXCL9 mRNA was expressed mainly in the stromal compartment surrounding tumor islets, probably including M1 macrophages. Thus, our data indicated that CXCL9 plays an important role in the tumor immune environment at the boundary between the stromal compartment and tumor islets in DCF-NAC-sensitive ESCC.
Previous studies have demonstrated that DDR deficiency sensitizes cells to chemotherapy with DNA damage-inducing reagents, such as cisplatin, 5-FU, and etoposide.37-39 Interestingly, DDR deficiency also reportedly causes immune response through two mechanisms. One is high production of neoantigens as a result of increased genomic instability and tumor mutation burden at the whole-genome level. Neoantigens are recognized by T cells and cause an adaptive immune response in which IFNγ plays a key role. The other is the activation of the cGAS/STING pathway, which senses cytosolic double-stranded DNA and triggers an innate immune response in which IFNα plays a key role.40-43 In either of these immune responses, CXCL9 and CXCL10 can be induced by IFNα and IFNγ. Interestingly, GSEA analysis using our dataset suggested allograft rejection-related and IFNγ-related expression profiles in DCF-NAC-sensitive tissues (FDR = 0.025 and 0.036, respectively, in Figure 2C). Both expression profiles are associated with the adaptive immune response. In addition, an innate immune response may also be involved in DCF-NAC sensitivity, as GSEA analysis also implicated the IFNα-related expression profile (FDR = 0.07; Figure 2C). Thus, our expression microarray data suggested that IFNγ-related and IFNα-related intact immunity is involved specifically in DCF-NAC-sensitive tissues. It has been reported that IFNγ and/or IFNα induced CXCL9 and CXCL10, which attract both cytotoxic and helper T cells.20, 35, 44 Consistent with these previous reports and our own speculation, T-cell-specific genes, such as CD8B and GZMK, were also identified as DEGs in our study. Therefore, our data suggested that the mechanisms responsible for enhancement of chemosensitivity and increased expression of immune-related genes may share DDR deficiency as a common feature in DCF-NAC-sensitive ESCCs, allowing the application of such immune-related genes as biomarkers for the prediction of chemosensitivity. Indeed, Mulligan et al. have demonstrated the feasibility of using DDR deficiency (DDRD)-related genes in preNAC biopsy samples, including several immune-related genes, as biomarkers of NAC efficacy.45 They used expression profiles of these 44 genes for prediction, which they named the DDRD assay. Although we were unable to adapt our microarray data to the DDRD assay, we compared the expression levels of the 44 DDRD-related genes between highly resistant and sensitive groups. We found that four of the 204 DEGs (CXCL10, ITGAL, IKZF3, and PTPRC) were included in the 44 genes, and five more genes showed differential expression (CD2, APOL3, RAC2, CD274, and ETV7) when we applied the Mann–Whitney U-test with non-corrected p-values for comparison analysis (data not shown).45 Their predictive method has also been evaluated by several other groups.46-49 In contrast, it has not been fully investigated whether cancer immunity evident in preNAC biopsy samples can directly make cancer cells more susceptible to chemotherapy. If this is the case, then it would be possible to enhance the efficacy of chemotherapy by modulating immune reactions. Our present data appear to be informative, not only for the development of biomarkers predictive of DCF-NAC sensitivity, but also for the maximization of DCF-NAC efficacy by combination treatment with immune-modulating agents.
ACKNOWLEDGMENTS
We would like to thank Mami Kimoto, Mayumi Takeda, Kanako Ito, Kei Shimizu, and Yuiko Aso for their excellent technical assistance. We are also grateful to the late Dr. Tsuyoshi Noguchi for his critical advice on the research.
FUNDING INFORMATION
This work was supported by the Oita University President's Strategic Discretionary Fund (2021 and 2022), by JSPS KAKENHI Grant Numbers JP21H02703 and 21 K12730 and by Takeda Science Foundation (2020).
CONFLICT OF INTEREST STATEMENT
The authors have no conflict of interest.
ETHICS STATEMENT
Approval of the research protocol by an Institutional Reviewer Board; This study was approved by the Ethics Committee of Oita University Faculty of Medicine.
Informed Consent; All patients included in the study provided written informed consent.
Registry and the Registration No. of the study/trial; N/A.
Animal Studies; N/A.




