Luteolin suppresses bladder cancer growth via regulation of mechanistic target of rapamycin pathway
Abstract
Luteolin is a natural flavonoid with strong anti–oxidative properties that is reported to have an anti–cancer effect in several malignancies other than bladder cancer. In this study, we describe the effect of luteolin on a human bladder cancer cell line, T24, in the context of the regulation of p21, thioredoxin-1 (TRX1) and the mechanistic target of rapamycin (mTOR) pathway. Luteolin inhibited cell survival and induced G2/M cell-cycle arrest, p21 upregulation and downregulation of phospho(p)-S6, which is downstream of mTOR signaling. Luteolin also upregulated TRX1 and reduced intracellular reactive oxygen species production. In a subcutaneous xenograft mouse model using the rat bladder cancer cell line, BC31, tumor volumes were significantly decreased in mice orally administered luteolin compared to control. Immunohistochemical analysis revealed that increased p21 and decreased p-S6 expression were induced in the luteolin treatment group. Moreover, in another in vivo N-butyl-N-(4-hydroxybutyl) nitrosamine (BBN)-induced rat bladder cancer model, the oral administration of luteolin led to a trend of decreased bladder tumor dimension and significantly decreased the Ki67-labeling index and p-S6 expression. Furthermore, the major findings on the metabolism of luteolin suggest that both plasma and urine luteolin-3ʹ-O-glucuronide concentrations are strongly associated with the inhibition of cell proliferation and mTOR signaling. Moreover, a significant decrease in the squamous differentiation of bladder cancer is attributed to plasma luteolin-3ʹ-glucuronide concentration. In conclusion, luteolin, and in particular its metabolized product, may represent another natural product-derived therapeutic agent that acts against bladder cancer by upregulating p21 and inhibiting mTOR signaling.
1 INTRODUCTION
Bladder cancer is the tenth most common cancer in the world, with an estimated 549 000 new cases and 200 000 deaths annually.1 Approximately 75% of bladder cancer patients are diagnosed with non–muscle-invasive bladder cancer (NMIBC); however, the remaining 25% have muscle-invasive bladder cancer (MIBC) or metastatic disease at the time of initial diagnosis. More than half of patients with NMIBC have disease recurrence and 10%-15% develop disease progression. Chemotherapy and immuno-oncology drugs can be partially effective against recurrence and metastasis of MIBC after cystectomy; however, overall survival is still less than a year.2 Therefore, alternative or supportive treatments for bladder cancer are needed.
Oxidative stress arises from an imbalance between reactive oxygen species (ROS) production and redox systems. It can indicate carcinogenesis and promote cell proliferation in various organs. Smoking is the greatest risk factor for death from bladder cancer as well as its incidence.1, 3 Excessive ROS production in response to cigarette smoke acts as an oxidative stress that can induce genomic instability and promote tumorigenesis.4, 5
Flavonoids are polyphenolic compounds found in fruits or vegetables that are believed to provide a health benefit. Luteolin, a flavonoid found in perilla, parsley and green peppers, scavenges ROS.6 Luteolin shows an anti–cancer effect in a variety of cancers, including colon, pancreatic, prostate and lung cancer, and has anti–oxidative, anti–inflammatory and anti–allergic properties.6, 7 We have previously found that luteolin has a preventive role in non–alcoholic steatohepatitis-related hepatocarcinogenesis through suppression of hepatic ROS accumulation and NF-κB signaling.8 However, the anti–cancer effect of luteolin in bladder cancer has received little attention in the literature.
Thioredoxin-1 (TRX1) is 12-kDa thiol redox-active protein that is believed to protect individuals from oxidative stress-induced damage by scavenging ROS. TRX1 is translocated into the nucleus to regulate the activity of transcription factors such as activator protein-1, NF-κB and p53, and plays an important role in the regulation of cell viability and activation.9-11 An elevated level of TRX1 is found in various human cancers, such as lung, liver, colorectal and prostate cancers, as compared to their respective normal tissues.11, 12 In addition, enhanced TRX1 levels are often reported in many types of cancers that have progressed, metastasized or become resistant to chemotherapy.9, 10 For instance, castration-resistant prostate cancer cells showed enhanced TRX1 expression under androgen-deprived conditions.11 Elevated TRX1 expression may be the consequence of accumulated ROS generated by cancer cells after exposure to antineoplastic agents.5, 13 However, TRX1 itself may not always have oncogenic functions.11 To date, the role of TRX1 expression in bladder cancer remains to be elucidated.
The mechanistic target of rapamycin (mTOR), a highly conserved serine–threonine kinase, acts as an anti–cancer agent to inhibit cellular growth or proliferation as well as an immunosuppressant in several cancers, including bladder cancer cells.14-16 The intraperitoneal administration of an mTOR inhibitor reduced the proliferation and tumor incidence of bladder cancer in an N-butyl-N-(4-hydroxybutyl) nitrosamine (BBN)-induced mouse bladder cancer model.16 A single treatment with an mTOR inhibitor, everolimus, in patients with metastatic urothelial carcinoma (UC) resulted in a 31% objective response rate (ORR), in the form of tumor regression, including a near-complete response and a partial response.17 Moreover, another phase II study of dual treatment with phosphoinositol-3-kinase and mTOR inhibitors in locally advanced or metastatic patients with UC demonstrated that the ORR was 15%, with 50% of patients experiencing grade 3-4 adverse events.18 These studies indicate that mTOR inhibition has the potential to suppress bladder cancer, although the benefit is not as strong as expected.
The relationships among oxidative stress, TRX1 and the mTOR pathway have been outlined in previous studies. Cysteine oxidants, having an opposing function to anti–oxidants, stimulate mTOR complex 1 (mTORC1) activity via Rheb.19 British anti–lewisite, which can reverse oxidation reactions, strengthened the interaction between mTORC1 and raptor, and inhibited ribosomal protein S6 kinase1 (S6K1) phosphorylation.20 Furthermore, TRX1 had a protective role in cardiomyocytes by interacting directly with mTOR.21
In this study, we describe an anti–proliferative effect of luteolin in human bladder cancer cell lines. We also describe a suppressive effect of dietary luteolin in rat bladder cancer tumor and BBN-induced bladder carcinogenesis models. The inhibitory effect of luteolin was due to p21 upregulation and decreased phospho(p)-S6, which is a substrate of mTORC1. Furthermore, we identified a metabolite of luteolin in plasma and urine that strongly contributed to its chemopreventive activity.
2 MATERIALS AND METHODS
2.1 Cell culture and reagents
The human bladder cancer cell lines, T24, 5637 with a p53 mutation and RT-4 with wild-type p53, were acquired from the ATCC and maintained in DMEM and RPMI 1640, respectively (Thermo Fisher Scientific), supplemented with 10% FBS. All cells were authenticated by DNA profiling using short tandem repeat analysis. A rat bladder cancer cell line, BC31 with a p53 mutation, was derived from a BBN-induced rat bladder cancer and cultured as previously described.22-24 All cells were cultured at 37°C in a humidified atmosphere of 5% CO2. Luteolin (3,4,5,7-tetrahydroxy flavone) and 1-methylpropyl 2-imidazolyl disulfide (PX-12) were purchased from Tokyo Chemical Industry and Sigma–Aldrich, respectively.
2.2 Cell viability assay
T24 and 5637 cells were seeded in 96-well plates at a density of 3 × 103 cells/well. The cells were grown for 24 hours and treated with luteolin for 48 hours. Cell viability was evaluated using Cell Counting Kit-8 (Dojindo Laboratories). All experiments were performed in triplicate.
2.3 Flow cytometry of apoptosis and cell-cycle analysis
Cells were treated with luteolin for 48 hours and collected in 6-well plates. A FACSCalibur system (BD) was used for both apoptosis and cell-cycle assays. We used a phycoerythrin Annexin V Apoptosis Detection Kit with 7-aminoactinomycin D (BioLegend) for apoptosis assays, and a Cell Cycle Phase Detection Kit (Cayman Chemical) to evaluate the cell cycle by propidium iodide according to the manufacturer’s instructions. All experiments were performed in triplicate.
2.4 Western blot analysis
All proteins were collected from cells at 70%-80% confluency in 6-well plates. A total of 30 µg protein per lane was resolved on 12% acrylamide gels and transferred onto Hybond ECL membranes (GE Healthcare UK). Primary antibodies and their dilution ratios are listed in Table S1. All experiments were performed in triplicate.
2.5 In vitro siRNA transfection
Small interfering (si)RNAs targeting human TP53 sequences were obtained from Sigma-Aldrich. RT4 cells (1.5 × 105/well) were seeded in 6-well plates and transfected with 20 nmol/L siRNA using Lipofectamine RNAiMAX (Thermo Fischer Scientific) according to the manufacturer’s protocol.8 Non–targeting siRNA, with no significant homology to any known rat and human genes, was also used as a negative control (NC). Confirmation of TP53 knockdown and treatment with luteolin were performed 24 hours after transfection. All experiments were performed in triplicate.
2.6 TRX1 gene expression analysis
The Cancer Genome Atlas (TCGA) bladder cancer datasets (http://cancergenome.nih.gov/) were analyzed as previously described.11 The unit is Fragments Per Kilobase of transcript per Million mapped reads Upper Quartile (FPKM-UQ).
2.7 TRX1 and DCFH-DA assay
TRX1 levels were evaluated using a Human Thioredoxin Assay Kit (IBL). Briefly, T24 cells were seeded into 6-well plates followed by a 48-hour exposure to luteolin. After treating the medium of each plate according to the manufacturer's instructions, we measured absorbance at 450 nm using a microplate reader. A dichloro-dihydro-fluorescein diacetate (DCFH-DA) assay was performed to evaluate intracellular ROS production, as previously described.22 All experiments were performed in triplicate.
2.8 Cancer patient tissue samples
A total of 166 patients who underwent radical cystectomy or transurethral resection for bladder cancer and whose pathology was UC or its variants, as diagnosed by experienced pathologists, were eligible for this study. This study was approved by the institutional review board at Nagoya City University Hospital and the approval number was NCU-893. All enrolled patients provided written informed consent.
2.9 Animals
Three or four experimental rats or mice were housed in each plastic cage on wood-chip bedding in an air-conditioned specific pathogen-free animal room at 22 ± 2°C and 55% ± 5% humidity with a 12-hour light/dark cycle. Food and tap water were available ad libitum. The present experiments were performed under protocols approved by the Institutional Animal Care and Use Committee of Nagoya City University School of Medical Sciences.
2.10 Xenograft mouse model
Seven-week-old male KSN nude mice (Japan SLC) were inoculated subcutaneously in the back with 5 × 104 BC31 cells. Two days after injection, 24 mice were randomly divided into two treatment groups and fed either an AIN76A control diet (Oriental Bio Science) or AIN76A with 100 ppm luteolin for 5 weeks. Tumor size was monitored weekly. Tumor volume was calculated using the following formula: 1/2 × Length ×Width × Height.
2.11 N-butyl-N-(4-hydroxybutyl) nitrosamine-induced rat bladder carcinogenesis model
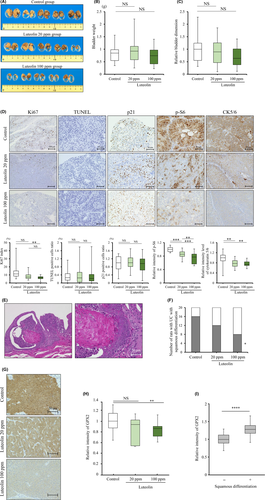
Six-week-old male F344 rats (Charles River Laboratories Japan) were given 0.05% BBN (Tokyo Chemical Industry) in drinking water for 10 weeks. A total of 60 rats were then randomly divided into three groups and received either a control diet, or 20 or 100 ppm luteolin for 20 weeks. A 24-hour specimen of urine was collected in the 29th week. All rats were killed, and blood, bladders, livers and kidneys were collected in the 30th week. Bladder tissues were divided equally into four portions and fixed by formalin, and H&E stains of all specimens were evaluated by experienced pathologists (N.A-I, SS) according to previously described instructions.25 Each tumor dimension was quantified using a BZ-9000 multifunctional microscope and associated analysis software (Keyence).
2.12 DNA extraction and direct sequencing for p53 gene in N-butyl-N-(4-hydroxybutyl) nitrosamine-induced rat bladder cancers
Genomic DNA was extracted from BBN-induced rat bladder tumors histologically diagnosed as cancer from the control group (n = 10) and the luteolin 100 ppm group (n = 10). The extraction from formalin-fixed paraffin-embedded tissues was performed using NucleoSpin DNA FFPEXS (MACHEREY-NAGEL). Exons 5, 6-7 and 8 of rat p53 gene were analyzed as previously reported.26 The primer sequences were 5′-CGCTGACCTTTGATTCTTTC-3′ and 5′-AGTTCTAACCCCACAGCAGT-3′ for exon 5, 5′-GTTAGAACTGGTTGTCCAGGG-3′ and 5′-CCCAACCTGGCACACAGCTTCCT-3′ for exon 6-7, and 5′-CTGTGCTCCTCTTGTCCCG-3′ and 5′-CCTCCACCTTCTTTGTCCTG-3′ for exon 8. PCR products were purified with NucleoSpin Gel and PCR Clean-up (MACHEREY-NAGEL). Direct sequencing was performed using 2 µL of aliquots by Applied Biosystems 3130xl Genetic Analyzer (Thermo Fischer Scientific) according to the manufacture’s instructions. Sequencing primers for exon 5 were 5′-GATTCTTTCTCCTCTCCTACAG-3′ and 5′-AGTTCTAACCCCACAGCAGT-3′, for exon 6-7 were 5′-GCCTCTGACTTATTCTTGCT-3′ and 5′-AACCTGGCACACAGCTTCCT-3′, and for exon 8 were 5′-TGCCTCCTCTTGTCCCGGGT-3′ and 5′-CACCTTCTTTGTCCTGCCTG-3′.
2.13 Immunohistochemistry and histological analysis
Deparaffinized formalin-fixed human and rat tissues were incubated with primary antibodies (listed in Table S2). A TUNEL assay was performed using an In situ Apoptosis Detection Kit from Takara, as previously described.22 Immunostaining was done according to the manufacturer’s instructions.27
In the analysis of positive cell rates for Ki67, TUNEL and p21, five microscopic fields were randomly selected and quantified with the same threshold nuclear intensity at a ×400 magnification according to analysis software (Keyence). The cytoplasmic intensities of p-S6 and cytokeratin 5/6 (CK5/6) were randomly selected in five cancerous areas at ×400 magnification and quantified with analysis software.
2.14 Metabolism of luteolin
Plasma and urine luteolin levels in F344 rats (control: n = 10, luteolin 100 ppm: n = 18) were analyzed by HPLC-tandem mass spectrometry at the Biodynamic Plant Institute. All samples were analyzed using the API 3200 System (ABSciex) with an HPLC system (Shimazu, Kyoto, Japan). Chromatographic separation was achieved on a YMC-Pack ProC18 (5 µm, 3.0 mm × 150 mm; YMC) and maintained at 40°C at a flow rate of 0.3 mL/min. The concentrations of luteolin-aglycone, luteolin-7-glucoside and luteolin-3ʹ-glucuronide were measured.
2.15 Statistical analysis
Data were analyzed using EZR software (Saitama Medical Center, Jichi Medical University) and Fisher’s exact test, Student’s t test or ANOVA, where appropriate. Spearman’s rank correlation coefficient was used to evaluate the correlation of two variables. P < 0.05 was considered a statistically significant difference.
3 RESULTS
3.1 Luteolin has a cancer suppressive effect and upregulates p21Waf1/Cip1 in bladder cancer cells
As shown in Figure 1A, luteolin had an inhibitory effect on the cell viability in a dose-dependent manner against human bladder cancer cell lines. Figure 1B,C and Figure S1A show that luteolin clearly induced apoptosis in bladder cancer cells. Next, we found that luteolin induced G2/M arrest in a dose-dependent manner (Figure 1D,1 and Figure S1B). We further explored changes in cell-cycle-related protein expression in response to treatment with luteolin. We found upregulated p21Waf1/Cip1 and p27Kip1, and downregulated cyclin A and D1 in luteolin-treated T24 cells, which were derived from a high-grade human bladder cancer (Figure 1F). The upregulated expression of p21Waf1/Cip1 and decreased expression of cyclin D1 with luteolin treatment were also evident in a low-grade human bladder cancer cell line, 5637 (Figure 1F). To explore the effect of p53 function on p21 Waf1/Cip1 regulation by luteolin, we further demonstrated an effect on a p53 wild-type human bladder cancer cell line, RT4. The expression level of p21 Waf1/Cip1 was higher in RT4 cells compared to T24 and 5637 cells. Luteolin increased the expression of p21Waf1/Cip1 in RT4 cells (Figure 1G). TP53 knockdown clearly downregulated p21Waf1/Cip1, indicating that p21Waf1/Cip1 expression was p53-dependent. Luteolin still upregulated p21Waf1/Cip1 in TP53-silenced RT4 cells (Figure 1H). Taken together, luteolin induces p21Waf1/Cip1 in bladder cancer cells with both p53 wild-type and mutation.
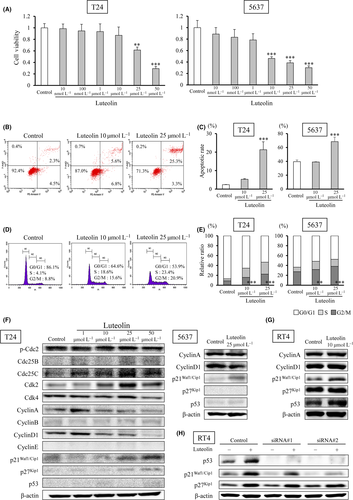
3.2 Luteolin inhibits the mechanistic target of rapamycin pathway in bladder cancer
The mTOR regulates cell growth, proliferation, metabolism and autophagy. S6K is a major substrate of the mTOR kinase. The activity of S6K1, an isoform of S6K, is controlled with phosphorylation by mTOR, with the position of Thr389 being the most critical site of phosphorylation for kinase activity. S6 is a substrate of S6K regulated by phosphorylation and is involved in cell-cycle progression, ribosomal proteins, and elongation factors necessary for translation.14, 28, 29
Western blot analysis showed that levels of phospho(p)-Akt, phospho(p)-p70S6K and phospho(p)-S6 in bladder cancer cell lines were decreased by luteolin in a dose-dependent manner (Figure 2A,B). These effects were obtained 12 and 24 hours after exposure to luteolin (Figure 2C). We then compared the effect of luteolin to the mTOR inhibitor, Torin1. The inhibition of mTOR significantly reduced the survival rate at a concentration of more than 10 nmol/L, and induced G1/S arrest and apoptosis in T24 cells (Figure S2A,C-F). Western blot analysis indicated that Torin1 treatment of T24 cells did not induce p21 expression, in contrast to luteolin (Figure S2B). These results suggest that the anti–tumor effect of luteolin was not phenocopied by an mTOR inhibitor and was affected by p21 in bladder cancer. (Figure S2A-D). Next, we evaluated the role of the mTOR pathway in patients with bladder cancer by analyzing the immunohistochemistry of p-S6 and Ki67. Although no statistical differences were found in the relative intensity of p-S6 between pTa low-grade and T1 plus Ta high-grade tumors, significant differences were found between more than T2 and pTa low-grade or T1 plus Ta high-grade tumors (P < 0.001, respectively; Figure 2D,E). A statistical correlation between the Ki67-labeling index and the relative intensity of p-S6 was evident (P < 0.001, r = 0.46; Figure 2F). These results indicate that mTOR activity may be related to cell proliferation in bladder cancer.
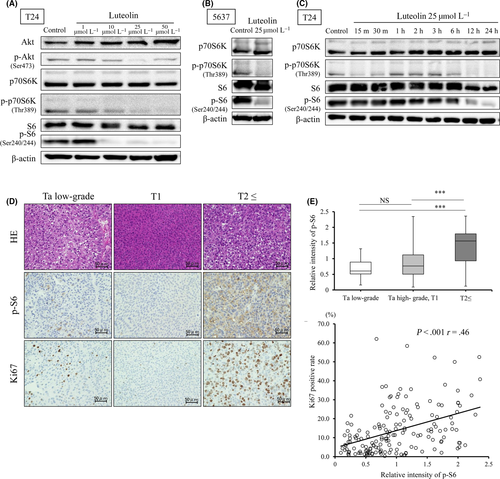
3.3 The upregulation of TRX1 by luteolin suppresses cell proliferation in bladder cancer cells
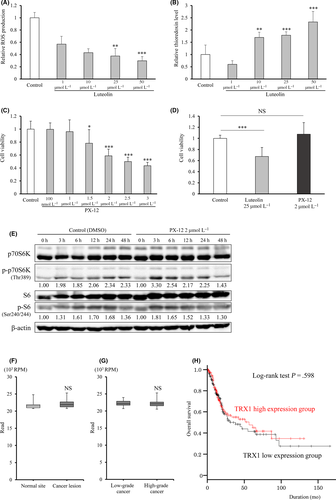
We next evaluated whether luteolin affected the TRX1 level or the relationship between mTOR and TRX1. Intracellular ROS decreased and TRX1 increased in a concentration-dependent manner in luteolin-treated T24 cells (Figure 3A,B). To explore whether the suppressive effect of luteolin on cell viability can be attributed to TRX1 upregulation and whether TRX1 is associated with the mTOR pathway, we treated cells with the TRX1 inhibitor, PX-12.11 PX-12 decreased T24 cell proliferation in a dose-dependent manner. This suggests that an imbalance in oxidative stress occurred that adversely affected cell viability (Figure 3C). T24 cells were harvested after 48-hour exposure to luteolin, followed by another 48-hour exposure to luteolin, DMSO control or 2 µmol/L PX-12 (nearly 40% decreased cell proliferation). Cells treated with luteolin followed by DMSO recovered from the decrease in cell survival induced by luteolin (P < 0.001; Figure 3D). PX-12 treatment also led to an increase in cell viability, despite the growth-suppressive concentration (Figure 3D). This indicates that TRX1 played a role in the inhibition of ROS production and cell proliferation in bladder cancer cells. Western blot analysis revealed that levels of p-p70S6K and p-S6 recovered with PX-12 treatment after luteolin-induced mTOR suppression quicker than those after DMSO treatment (exposure post–3-hour to PX-12 vs 12-24-hour to DMSO; Figure 3E). We further analyzed TRX1 expression and also prognosis by using a TCGA dataset of 338 patients with bladder cancer. Figure 3F,G shows no difference in the TRX1 level between normal and cancer tissues, and between low-grade and high-grade tumors. Furthermore, a significant difference in overall survival between high and low TRX1 expression groups was not observed (Figure 3H). These data indicate that TRX1 is not necessarily a negative prognostic factor in bladder cancer, unlike for other cancers,9-11 and luteolin prevents the accumulation of intracellular ROS levels and mTOR signaling by upregulating the TRX1 level in bladder cancer.
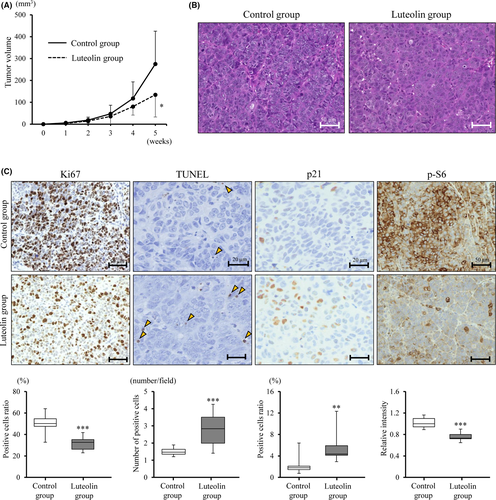
3.4 Anti–tumor effect of luteolin in a subcutaneous BC31 xenograft model
We next examined the anti–tumor effect of dietary luteolin in a subcutaneously transplanted BC31 xenograft model. Of 24 mice, the tumors of 10 out of 12 mice in the control group and 9 out of 12 mice in the luteolin 100 ppm group were successfully engrafted; and these 19 mice were subsequently used for analyzing the effect of luteolin. Significant differences in the amount of dietary intake, and body and organ weights between the two groups were not noted (Figure S3A-C). Histological analyses revealed that no toxic effect was induced by luteolin. The tumor volumes of mice of the luteolin 100 ppm group were significantly smaller than those of the control group of mice (P < 0.05; Figure 4A,B). Ki67-labeling index was significantly decreased and rates of TUNEL-positive cells were significantly increased by luteolin (P < 0.001 for both; Figure 4C). These results suggest that luteolin inhibited proliferation and induced apoptosis in bladder cancer cell xenografts (Figure 4C). The percentage of p21-positive cells was significantly higher (P < 0.01) and p-S6 intensity was significantly lower (P < 0.001) in the luteolin 100 ppm group compared to the control group, which was consistent with in vitro results (Figure 4C).
3.5 Luteolin metabolites decrease mechanistic target of rapamycin signaling and cell proliferation in an N-butyl-N-(4-hydroxybutyl) nitrosamine-induced rat bladder cancer model
Finally, we investigated the anti–tumor effect of the oral intake of luteolin using a BBN-induced rat bladder cancer model. A rat in the 100-ppm group died because of bladder cancer without metastasis or hydronephrosis the day before it was due to be killed. Significant differences in the amount of BBN water, dietary intake, and body and organ weights among the three groups were not observed (Figure S4A-D). No histological change was observed in livers and kidneys.
All rats developed superficial bladder cancer without invasion. DNA sequencing analysis revealed that point mutations of p53 gene were observed in rat bladder cancers (Table S3). Mutations in codon 195 (GTG → ATG) and codon 251 (ACC → ATC) were similar to those reported in a previous study.26 Bladder weight and tumor dimension tended to be reduced by luteolin treatment (Figure 5A-C). Immunohistochemical analyses revealed a significantly decreased Ki67-labeling index (P < 0.01) and p-S6 level (P < 0.001) in the high-dose luteolin group, but differences in positive rates of TUNEL and p21 staining were not observed (Figure 5D). We further analyzed any change in squamous differentiation, which relates to worse recurrence and progression rates30 with luteolin, because we previously found that the oxidative stress-regulated gene glutathione peroxidase 2 (GPX2) was upregulated in several carcinomas, including UC,22, 27, 31, 32 and promoted squamous differentiation.22 Moreover, we recently found that luteolin downregulated GPX2 in the rat prostate tumor and in a human prostate cancer cell line.33 The frequency of squamous differentiation in the tumors was significantly decreased in the luteolin-treated groups (P < 0.05; Figure 5E,5). Consistent with this result, the level of CK5/6, a marker of squamous differentiation,30, 34 was decreased by luteolin treatment (P < 0.01; Figure 5D,E). Immunohistochemistry revealed that the expression of GPX2 was induced in bladder cancer with squamous differentiation, compared to pure UC, and was significantly reduced by luteolin in rat BBN-induced bladder cancer (P < 0.01; Figure 5G,H). Moreover, the expression level of GPX2 and the incidence of squamous differentiation were significantly correlated in the model (P < 0.001; Figure 5I). GPX2 expression was shown in RT4 and BC31 cells but not in T24 and 5637 cells, and was not affected by luteolin treatment in vitro (Figure S5). These results indicate that luteolin reduces squamous differentiation through the suppression of GPX2 in rat bladder cancer in vivo.
Next, we analyzed the metabolism of luteolin and whether it is involved in the anti–cancer effect. Previous reports described the oral administration of luteolin-aglycone or glucoside and their conjugation to glucuronide in rats occurring mainly in plasma and tissues.35, 36 Consistent with this, luteolin-aglycone and glucoside were not detected in plasma. Instead, concentrations of luteolin-3ʹ-glucuronide in plasma (1.66 ± 1.30 ng/mL) and urine (25.1 ± 18.5 ng/mL), respectively, were significantly increased after luteolin treatment (Figure 6A). Concentrations of luteolin-3ʹ-glucuronide showed a mild correlation between urine and plasma (r = 0.25, P = 0.20; Figure 6B).
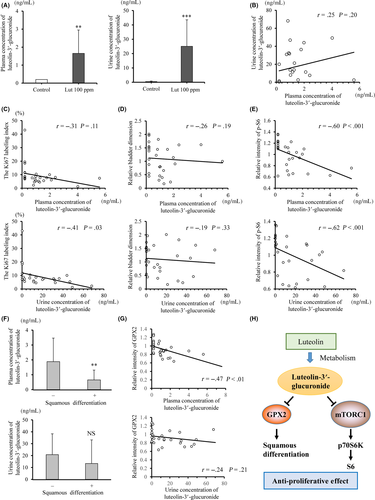
The luteolin-3ʹ-glucuronide concentration in urine (r = −0.41, P = 0.03), as well as that in plasma (r = −0.31, P = 0.11), was significantly correlated with the Ki67-labeling index of the tumors (Figure 6C), even though it did not correlate with tumor dimension (Figure 6D). Moreover, the relative p-S6 intensity of the tumors and luteolin-3ʹ-glucuronide concentration in plasma, as well as that in urine, showed a strong correlation (r = −0.60, P < 0.001 and r = −0.62, P < 0.001, respectively; Figure 6E). With respect to the preventive effect of luteolin on squamous differentiation, luteolin-3ʹ-glucuronide concentrations in plasma were significantly correlated with a lack of squamous differentiation and low GPX2 intensity (P < 0.01; Figure 6F, P < 0.01; Figure 6G).
4 DISCUSSION
Luteolin inhibits cell growth by regulating the cell cycle in several cancers.6-8 A member of the Cip/Kip family, p21 shares an N-terminal domain that binds cyclins and cyclin-dependent kinases (CDK).37 In bladder cancer, decreased p21 expression was associated with reduced survival and increased recurrence after radical cystectomy, suggesting that p21 has tumor-suppressive roles in bladder cancer.38-40 In the present study, luteolin upregulated p21 and induced G2/M arrest in human bladder cancer cells. The p21 protein is well-known as a regulator of G1/S; however, G1 and G2 arrest are induced by p21 through the inhibition of cyclin/CDK2 complexes.41 This suggests that p21 affects multiple phases of the cell cycle, depending on the cellular context.
When a “p21-positive bladder tumor” was defined as more than 10% of the bladder cancer cells were expressing p21, the incidence of p21-positive bladder tumor cases was 33%-64%.38-40 As similarly defined in a BBN-induced rat bladder cancer model in the present study, more than 98% of rats (58/59 rats) were p21-positive, with p21-positive rates in each rat being 27.8 ± 10.5%. The higher expression of p21 in the rat may have led to an insignificant change in p21 by luteolin treatment in the present rat BBN bladder cancer model.
Both in vitro and in vivo studies showed that luteolin inhibited the mTOR pathway in bladder cancer. Reduced levels of p-S6 downregulated mTOR in human bladder cancer specimens and this was significantly correlated with disease progression and disease-specific survival.42, 43 The present study demonstrates that p-S6 was upregulated during stage progression and was correlated with the Ki67-labeling index in human bladder cancers. This suggests that p-S6 may reflect not only the prognosis but also the ability of tumors to proliferate or invade in bladder cancer.
We focused on TRX1 function in bladder cancer because it is an anti–oxidative gene that is involved in mTOR signaling. Luteolin upregulated TRX1 and downregulated mTOR signaling in human bladder cancer, while an TRX1 inhibitor induced recovery from mTOR activation. These results suggest TRX1 is a tumor-suppressive gene in bladder cancer. A significant difference between TRX1 expression and prognosis in bladder cancer cases is not found, meaning that TRX1 may be an inducible suppressor of proliferation by reacting with an anti–cancer agent such as luteolin. Indeed, Yokomizo et al indicated that TRX1 levels are associated with drug sensitivity in T24 cells.44 However, further study is needed to elucidate the function of TRX1 as an anti–cancer agent in bladder cancer.
We investigated the anti–tumor activity of orally administered luteolin using a rat BC31 xenograft and a BBN-induced bladder carcinogenesis model. The Ki67-labeling index and mTOR activation represented by p-S6 expression were significantly decreased by dietary luteolin consumption in both rodent models. However, significant tumor suppression was detected only in the BC31 xenograft model. In the BBN-induced rat bladder cancer model, the incidence and size of tumors have been reported to be in accordance with concentration, duration and post–duration of BBN intake.45, 46 The present study set a BBN intake for 10 weeks followed by 20 weeks of luteolin administration. This induced bigger tumors and individual variability in all treatment groups due to the difficulty in the analysis of preneoplastic foci-like hyperplasia. This is one reason why tumors in luteolin-treated animals were not significantly different from those of controls. In line with a decreased Ki67-labeling index and mTOR activity, luteolin may also have a suppressive role in BBN-induced rat bladder carcinogenesis.
We further determined the relationship between level of lueolin in the body and a suppressive role in bladder carcinogenesis. Luteolin-aglycone is absorbed directly into the intestine, but luteolin-7-glucoside is absorbed after hydrolysis to form luteolin-aglycone. The most abundant metabolite of luteolin is luteolin-3ʹ-glucuronide in rats and occurs because luteolin-aglycone and glucoside are conjugated to glucuronide in the small intestine, liver and kidneys by phase II enzymes.35 In comparison, the major metabolite of luteolin in human plasma is luteolin-3ʹ-sulfate, which is believed to be converted by phenol sulfotransferase in the intestine.35, 47 The half maximal concentration of luteolin-3ʹ-glucuronide in rat plasma after the oral administration of luteolin-aglycone occurred at 12 hours, while that of luteolin-3ʹ-sulfate in humans occurred at approximately 6 hours. This difference is explained by the enterohepatic recycling of glucuronide.48 Flavonoid glucuronide is excreted into feces and urine, while flavonoid sulfates are mainly excreted in urine.35, 36
In this study, the amount of daily feed intake was 12.6 g/d/rat in the BBN model (Figure S3B), which was converted into luteolin-aglycone at 1.26 mg per day (3.5 mg/kg) in the 100-ppm luteolin group. The plasma and urine concentrations of luteolin-3ʹ-glucuronide were 1.66 ± 1.30 and 25.1 ± 18.5 ng/mL, respectively, in rats treated with 100 ppm luteolin. This were equivalent to 3.6 ± 2.8 µmol/L and 54.3 ± 40.0 µmol/L, respectively, in vitro. These were almost the same concentrations as found in in vitro experiments, after 10-25 µmol/L luteolin treatment, in this study.
Several studies on the relationship between concentrations of luteolin metabolites and anti–inflammatory effects have been reported.30, 34-36 However, few studies have focused on the concentrations of luteolin metabolites and their anti–cancer effects in vivo. The major findings of the present in vivo study were as follows (Figure 6H). First, both plasma and urine concentrations of luteolin-3ʹ-glucuronide were significantly correlated with decreased Ki67-labeling indices and p-S6. Second, the plasma level of luteolin-3ʹ-glucuronide is related to the suppression of squamous differentiation of bladder tumors, which led to a poor prognosis in human bladder cancer.30, 34 Our study provides new insight into how luteolin alleviates not only proliferation but also squamous differentiation of bladder cancer. Therefore, luteolin may have potential as an anti–cancer agent for bladder cancer, although further studies are warranted.
ACKNOWLEDGMENTS
This work was supported by a Grant-in-Aid from the Ministry of Education, Culture, Sports Science, and Technology of Japan, Ministry of Health, Labour and Welfare (grant number 18K16705), a grant from the TOBE MAKI Scholarship Foundation (grant number 18-JA-501) and the Aichi Health Promotion Foundation (grant number 30127).
DISCLOSURE
The authors have no conflict of interest to declare.




