Transduced caudal-type homeobox (CDX) 2/CDX1 can induce growth inhibition on CDX-deficient gastric cancer by rapid intestinal differentiation
Abstract
Intestinal metaplasia induced by ectopic expression of caudal-type homeobox (CDX)2 and/or CDX1 (CDX) is frequently observed around gastric cancer (GC). Abnormal expression of CDX is also observed in GC and suggests that inappropriate gastrointestinal differentiation plays essential roles in gastric tumorigenesis, but their roles on tumorigenesis remain unelucidated. Publicly available databases show that GC patients with higher CDX expression have significantly better clinical outcomes. We introduced CDX2 and CDX1 genes separately into GC-originated MKN7 and TMK1 cells deficient in CDX. Marked suppression of cell growth and dramatic morphological change into spindle-shaped flat form were observed along with induction of intestinal marker genes. G0-G1 growth arrest was accompanied by changed expression of cell cycle-related genes but not with apoptosis or senescence. Microarray analyses additionally showed decreased expression of gastric marker genes and increased expression of stemness-associated genes. Hierarchical clustering of 111 GC tissues and 21 non-cancerous gastric tissues by selected 18 signature genes based on our transcriptome analyses clearly categorized the 132 tissues into non-cancer, “CDX signature”-positive GC, and “CDX signature”-negative GC. Gene set enrichment analysis indicated that “CDX signature”-positive GC has lower malignant features. Immunohistochemistry of 89 GC specimens showed that 50.6% were CDX2-deficient, 66.3% were CDX1-deficient, and 44.9% were concomitant CDX2/CDX1-deficient, suggesting that potentially targetable GC cases by induced intestinal differentiation are quite common. In conclusion, exogenous expression of CDX2/CDX1 can lead to efficient growth inhibition of CDX-deficient GC cells. It is based on rapidly induced intestinal differentiation, which may be a future therapeutic strategy.
1 INTRODUCTION
Despite the gradually decreased prevalence in most countries, gastric cancer is still the third most common cause of death from cancer worldwide.1 Although the risk of gastric cancer is becoming reduced by improved cancer detection and decreased prevalence of Helicobacter pylori (H. pylori) infection, prognosis of advanced gastric cancer is still very poor as a result of insufficient treatment options.2 It is broadly accepted that gastric cancer usually develops from gastric mucosa with atrophic change and intestinal metaplasia, both of which are mostly caused by chronic H. pylori infection.3, 4 Despite the rather homogeneous condition of H. pylori-induced chronic gastritis, gastric cancer presents various clinicopathological features.5-7 Such diversity makes it difficult to plan a strategy against gastric cancer, and consequently leads to poor prognosis of the disease.
Intestinal metaplasia frequently observed around gastric cancer suggests that inappropriate gastrointestinal differentiation plays essential roles in gastric tumorigenesis.8, 9 Our previous studies indicated that a disrupted balance between gastric and intestinal differentiation affects gastric oncogenesis.5, 6, 10, 11 Ectopic expression of caudal-type homeobox genes (CDX families) is thought to be indispensable for “intragastric intestinal metaplasia,” because CDX2 and/or CDX1 almost always express on the gastric mucosa with intestinal metaplasia, and also because exogenous CDX transduction induces intestinal metaplasia in the stomach of model mice.8, 12-15 Of the three genes of human CDX families, CDX4 expresses only at embryonic stage and its function is poorly understood.16 On the contrary, CDX2 and CDX1 are well known to play important roles not only in early embryonic development but also in regulating proliferation and differentiation of intestinal epithelial cells in adults.14 Both CDX2 and CDX1 function as transcription factors, and they are thought to be able to compensate each other.14, 16 Although neither CDX2 nor CDX1 is originally expressed in the stomach; they are often induced in a morbid condition such as chronic atrophic gastritis and intestinal metaplasia.8, 17
It remains controversial as to how ectopic expression of CDX influences the initiation and progression of stomach cancer. In a mouse model, after long-term observation, Mutoh et al18 showed that intestinal-type adenocarcinoma frequently arose from intestinal metaplasia in the CDX2 transgenic mice. In Mutoh's study, most gastric tumors had some mutation of p53 and/or APC, which suggests that overexpression of CDX2 is one of the multiple steps in gastric carcinogenesis. On the contrary, Liu et al19 showed that CDX2 expression in gastric dysplasia/cancer progressively decreased over time. Mizoshita et al20 reported that CDX2-positive gastric cancer showed a significantly better outcome compared with CDX2-negative gastric cancer. These pathological studies suggest a tumor-suppressive activity of CDX2, which is contradictory to Mutoh's result.18 We have previously reported that CDX2 and Brm-type SWI/SNF chromatin remodeling complex cooperatively regulate villin1 expression in gastrointestinal cells,10 and also found that Brm deficiency in gastric cancer is negatively associated with differentiation status of gastric malignancy.5 According to the accumulated results, including ours, we believe that CDX plays pivotal roles through interaction with the SWI/SNF complex upon determining differentiation status of gastric cancer. We also speculate that CDX expression can promote intestinal differentiation in gastric cancer and consequently reduce the malignant properties.
Based on this background, we tried to evaluate the effect of exogenous CDX (CDX2 and CDX1) expression in gastric cancer cells. Recently, Dang et al21 reported that disruption of CDX2 did not significantly affect tumorigenic potential in MKN45, a gastric adenocarcinoma cell line strongly expressing CDX2. In the present study, we used other gastric cancer-originated cell lines, MKN7 and TMK1, both of which lack expression of CDX2 and CDX1.10, 22 We believe our results can shed light on the controversial effect of CDX on gastric tumorigenesis, and further lead to a new therapeutic approach of gastric malignancy based on the control of disrupted gastrointestinal differentiation.
2 MATERIALS AND METHODS
2.1 Kaplan-Meier plot analyses
Publicly available KM plotter23 and TCGA (The Cancer Genome Atlas) data set at the cBioPortal24, 25 were used to plot disease-free survival curves and overall survival curves of gastric cancer patients. Regarding the TCGA data, the patients were divided into two groups based on the level of CDX2 and CDX1 expression which was shown as Z-scores (>0.65 for “CDX high” group and ≤0.65 for “CDX low” group). According to the log-rank test, P values <0.05 were considered statistically significant.
2.2 Cell culture, retrovirus vectors, and cell proliferation assay
For the stable transduction of CDX genes, we used VSVG-pseudotyped pMXs-IRES-puro retrovirus vectors.10 To evaluate cell proliferation, we used MTT assay. Detailed information of cell lines used and cell-related experimental procedures are described in Doc S1.
2.3 Western blot analysis and reverse transcriptase-PCR analysis
Western blotting and RT-PCR were carried out as we previously reported.26 Antibodies used and primer sequences of 16 gene transcripts are described with detailed experimental procedures in Doc S1.
2.4 Tumor samples and immunohistochemistry
We randomly selected 89 gastric adenocarcinoma samples surgically resected at the Fujita Health University Hospital. This study was approved by the ethics committees of the University of Tokyo and the institutional ethical review board for human investigation at Fujita Health University. Immunohistochemistry to examine expression of CDX2 and CDX1 is described in detail in Doc S1.
2.5 Cell cycle analysis
MKN7 and TMK1 cells were grown semi-confluent in 100-mm culture dishes and infected with CDX-expressing retrovirus vectors (CDX2, CDX1, and mock) at appropriate MOI. After confirming the morphological changes in both cells infected with CDX expression vectors (6 days post-infection for MKN7 and 9 days post-infection for TMK1), staining with propidium iodide was carried out (using Cycletest plus DNA reagent kit; Becton Dickinson Immunocytometry Systems, San Jose, CA, USA). Cell cycle was then analyzed using FACS Calibur flow cytometer (Becton Dickinson Immunocytometry Systems) and ModFit LT version 3.0 software (Verity Software House, Topsham, ME, USA).
2.6 Microarray gene expression analysis and selection of gene probes for CDX2/CDX1 common signatures, CDX2 signature, and CDX1 signature
We examined total RNA obtained from MKN7 cells at 6 days post-infection and from TMK1 cells at 8 days post-infection with CDX-expressing retrovirus vectors. After extraction with RNeasy Plus Mini Kit (Qiagen, Venlo, the Netherlands), total RNA was analyzed on GeneChip Human Genome U133 Plus 2.0 Array (Affymetrix, Santa Clara, CA, USA) according to the manufacturer's protocols. After normalization of the raw data, fold changes in gene expression in CDX-transduced cells were calculated relative to the mock-infected control cells. Raw data are available in NCBI's Gene Expression Omnibus (GEO; series accession number is GSE102208).
To decide signature gene sets for CDX2/CDX1 combined, CDX2, and CDX1 expression, genes were selected based on more than threefold upregulation or downregulation compared with mock-infected control.
2.7 Latest comprehensive gene expression data of 132 gastric tissues
Latest comprehensive gene expression data of 111 gastric cancer tissues and 21 noncancerous gastric tissues were obtained from GSE54129 from NCBI's GEO.
3 RESULTS
3.1 Gastric cancer patients with a higher level of CDX expression have significantly better clinical outcomes
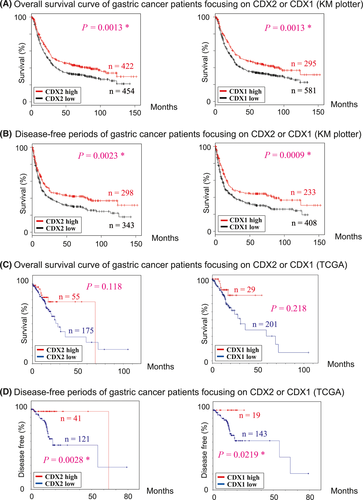
Using the data set from KM plotter23 and TCGA,24 clinical outcomes of gastric cancer patients were analyzed focusing on the expression of CDX2 and CDX1 (Figure 1). According to the data of KM plotter,23 overall survival curves and disease-free survival curves showed that gastric cancer patients with higher expression of CDX have significantly longer overall survival and disease-free periods compared to those without (Figure 1A,B). According to the data of TCGA,24 disease-free survival curves also showed that gastric cancer patients with higher expression of CDX have significantly longer disease-free periods (Figure 1D). For overall survival curves based on TCGA data (Figure 1C), gastric cancer patients with a high level of CDX expression have better survival, although we could not detect a significant association between overall survival and CDX expression. In total, higher expression of CDX in gastric cancer generally shows a tendency to be associated with better prognosis.
3.2 Exogenous expression of CDX2 and CDX1 induces dramatic morphological change and severe growth inhibition in CDX-deficient gastric cancer cells
To evaluate the effect of CDX transcription factors on gastric cancer cells, we introduced human CDX2 and CDX1 genes separately into MKN7 and TMK1 cells which are both deficient in CDX expression.10, 22 After infection with retrovirus vectors and selection with puromycin, we discovered that transduction of either CDX2 or CDX1 gave rise to dramatic morphological change on these CDX-deficient gastric cancer cells (Figure 2A). At 7 days post-infection, both MKN7 and TMK1 showed a flatter morphology accompanied by marked suppression of cell growth. Transformation of MKN7 occurred earlier and more drastically compared with that of TMK1. Namely, we observed complete growth arrest with no surviving cells for CDX-transduced MKN7 cells. On the contrary, a very small fraction of TMK1 cells survived 10 or more days after transduction of CDX, but the growth speed of surviving cells was much slower than for mock-infected cells. Such CDX-induced arrest or severe delay of cell growth was validated by MTT assay (Figure 2B), in which the effects of CDX2 and CDX1 were almost equivalent in both cell lines. Such suppressive effects on cell growth could not be detected in CDX-transduced MKN74 (Figure 2B), AGS, SW480, and LoVo cells (data not shown), all of which originally express CDX.10
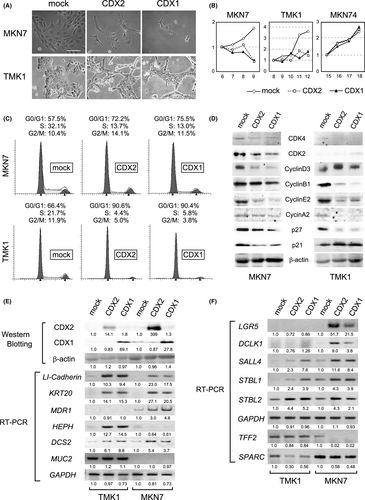
3.3 CDX-induced growth inhibition in gastric cancer cells occurs through G0-G1 cell cycle arrest
To elucidate the growth suppression in CDX-transduced MKN7 and TMK1, cell cycle analysis was carried out by flow cytometry and western blotting. As shown in Figure 2C, exogenous expression of CDX2 and CDX1 caused a substantial decrease in S-phase populations and a concomitant increase in G0/G1-phase populations, indicating that the transduced CDX gene led to G0-G1 growth arrest in both cell lines. Western blot analyses of cell cycle-related proteins showed that expression of cyclin E2, CDK4 and p27/Kip1 was markedly decreased in both CDX-transduced cell lines (Figure 2D). In contrast, reduction of cyclin D3, CDK2 and p21/Waf1/Cip1 was observed in MKN7 only, and reduction of cyclin A2 and cyclin B1 was observed in TMK1 only (Figure 2D). Although there was some difference between the two CDX-deficient gastric cancer cell lines, expression of several cell cycle-related proteins was mostly decreased after exogenous expression of CDX. This was probably as a result of suppressed intracellular metabolism and reduced protein production, which simultaneously occurred with G0/G1 growth inhibition. We speculate that these are the reasons why p27 in CDX-transduced MKN7/TMK1 and p21 in CDX-transduced MKN7 were reduced, both of which usually increase along with G0/G1 growth arrest.
We further carried out a senescence-associated β-galactosidase assay (Figure S1) and two independent assays to detect apoptosis (cleaved caspase-3 immunohistochemistry in Figure S2A and TUNEL assays in Figure S2B), but we could not detect any difference between the CDX-transduced MKN7/TMK1 and the mock-infected control cells. It is indicated that severe growth suppression observed in CDX-transduced MKN7 and TMK1 was neither due to apoptosis nor rapid senescence.
3.4 Differentiation status is considerably changed in CDX-deficient gastric cancer cells after transduction with CDX2/CDX1 expression vector
Reverse transcription-PCR was next carried out to evaluate transcription of several genes related to intestinal differentiation.12, 27-30 As shown in Figure 2E, LI-cadherin and KRT20 (cytokeratin 20) transcripts were strongly upregulated in CDX-transduced MKN7 and TMK1. Induction of these typical intestinal marker genes indicates that exogenous expression of CDX2 and CDX1 can lead to intestinal differentiation in gastric cancer cells. Furthermore, we also found that other known downstream genes of CDX such as HEPH,27 MDR1,29 and DCS2 (desmocollin2)30 were similarly transactivated by exogenous expression of CDX2 and/or CDX1 (Figure 2E). Although we could not detect activation of MUC2 transcription,31 we concluded that retrovirally transduced CDX2 and CDX1 certainly functioned as transcription factors12, 14, 28 and induced intestinal differentiation through transactivation of gut-associated genes.
We further carried out RT-PCR to evaluate expression of genes related to stemness and gastric differentiation. Expression of LGR5 and DCLK1 (DCAMKL1), both of which are well-known marker genes of intestinal stemness,32-34 was induced in CDX-transduced MKN7 cells (Figure 2F). Although such induction was not detected in CDX-transduced TMK1 cells, possible transactivation of intestinal stemness-related genes by CDX should be worthy of note. Furthermore, we found that expression of several stemness-related genes such as Sall4,35 Satb2,36 and Satb136 was upregulated in response to exogenous expression of CDX (Figure 2F), although the meaning of induced stemness in gastric cancer cells remains unclear. Regarding expression of gastric marker genes, TFF2/SP (trefoil factor 2)37, 38 was downregulated in MKN7 cells, and SPARC (osteonectin)39, 40 was downregulated in TMK1 cells. Changed status of differentiation in CDX-transduced gastric cancer cells must have some association with the observed severe G0/G1 growth arrest in them.
To investigate the altered gene expression accompanied by CDX-induced growth arrest more broadly, mRNA microarray analyses using CDX-transduced MKN7 and TMK1 cells were carried out (Tables S1 and S2 for upregulated genes; Tables S3 and S4 for downregulated genes). From the view of gut differentiation, microarray data expectedly showed that exogenous CDX induced several intestinal marker genes such as KRT20,28 DSC2,30 TSAPN8,41 CEACAM6,42 and MME (CD10)43 (Tables S1 and S2). Microarray data also showed that several gastric marker genes such as TFF2/SP,37, 38 TFF1/pS2,44 KCNJ15,45 SPARC,39, 40 and CDA (cytidine deaminase)46 were contrastively downregulated (Tables S3 and S4). These results reinforced our hypothesis that rapidly induced “intestinal differentiation” accompanied by weakened “gastric differentiation” is a critical mechanism underlying the G0-G1 growth arrest of CDX-transduced MKN7 and TMK1.
3.5 Signature genes related to CDX expression based on our transcriptome analysis clearly categorize gastric tissues into non-cancerous mucosa, “CDX signature”-positive cancer, and “CDX signature”-negative cancer
To examine the meaning of CDX expression in gastric cancer, the signatures related to CDX2/CDX1 expression were analyzed using the latest comprehensive gene expression data of 132 gastric tissues (111 gastric cancer tissues and 21 noncancerous gastric tissues). Using our microarray gene expression data of MKN7 and TMK1 with exogenous CDX2 and CDX1 expression, 18 common signature genes were selected based on more than threefold upregulation or downregulation (common for CDX2 and CDX1) compared with mock-infected control. The 18 selected signature genes for CDX2/CDX1 expression were then applied to the gene expression data of a total of 132 total gastric cancerous/non-cancerous tissues (Figure 3A). Hierarchical clustering showed that this “CDX2/CDX1 (common) signature” can clearly distinguish gastric cancer from non-cancerous gastric mucosa. In addition, 111 gastric cancer lesions were plainly categorized into two categories: positive for “CDX2/CDX1 signature” and negative for “CDX2/CDX1 signature.” The gene expression pattern of non-cancerous gastric mucosa is obviously close to that of “CDX2/CDX1 signature”-positive gastric cancer rather than “CDX2/CDX1 signature”-negative” gastric cancer (Figure 3A).

We additionally selected 112 signature genes for CDX2 expression and 118 signature genes for CDX1 expression based on more than threefold up/downregulation in CDX-transduced MKN7 and TMK1 cell lines and carried out hierarchical clustering in the same way (Figures S3 and S4). Similar to the “CDX2/CDX1 (common) signature” (Figure 3A), both “CDX2 signature” and “CDX1 signature” can clearly distinguish gastric cancer from non-cancerous gastric mucosa and can also clearly categorize the 111 gastric cancer cases into “CDX signature”-positive and “CDX signature”-negative tumors.
3.6 Gene set enrichment analysis indicates a significant association between cancer-associated properties and expression status of CDX in gastric cancer
We further carried out gene set enrichment analysis (GSEA) to understand the molecular background of “CDX signature” in gastric cancer.47 For “CDX2/CDX1 signature”-positive gastric cancer (left panel of Figure 3B,C), we identified several pathways related to tumor-suppressive function including p53_PATHWAY, KRAS_SIGNALING_DN, G2M_CHECKPOINT, and DNA_REPAIR. For “CDX2/CDX1 signature”-negative gastric cancer (right panel of Figure 3B,D), we conversely identified several oncogenic pathways such as WNT_BETA_CATENIN_SIGNALING, IL2_STAT5_SIGNALING, EPITHELIAL_MESENCHYMAL_TRANSITION, ANGIOGENESIS, NOTCH_SIGNALING, TGF_BETA_SIGNALING, IL6_JAK_STAT3_SIGNALING, and KRAS_SIGNALING_UP. These results could partly explain the meaning of CDX2/CDX1 expression in gastric cancer, which was suggested by hierarchical clustering analyses (Figure 3A, Figures S3 and S4). In short, CDX-positive gastric cancer tends to have fewer malignant features, and CDX-negative gastric cancer conversely tends to have more malignant features.
3.7 Approximately half of gastric cancer lesions surgically resected neither express CDX2 nor CDX1
To examine expression of CDX in gastric cancer, immunohistochemistry was carried out using 89 randomly selected gastric cancer specimens surgically resected. As shown in Figure 4A, 50.6% of gastric cancer lesions did not express CDX2, and 66.3% of them did not express CDX1. Concerning CDX2, our result was similar to the previous report showing that about half of gastric cancer tissues lack CDX2 expression.20 Expression of CDX1 has not been adequately evaluated so far, but our result indicated that the rate of CDX1-deficient gastric cancer is similarly high (Figure 4A, Table S5). Our immunohistochemical analysis also showed that decrease or absence of CDX expression was observed not only in intestinal-type gastric cancer but also in diffuse-type gastric cancer (Figure 4A, Table S5).

Our data also showed that expression of CDX2 and CDX1 tends to be concomitantly reduced in gastric cancer lesions regardless of histological type (Figure 4B). Of the total 89 gastric cancer cases, as many as 40 lesions (44.9%) expressed neither CDX2 nor CDX1. Correlation coefficients of CDX2 and CDX1 expression were rather high (Figure 4B; all P values <0.001), which indicated that coexpression or codeficiency of CDX2 and CDX1 is frequently observed in gastric cancer. The high prevalence of CDX2/CDX1 double-negative tumors suggested that induced intestinal differentiation may be a novel strategy against gastric cancer in the future.
3.8 Clinicopathological and molecular features of gastric cancer focusing on CDX2/CDX1 expression
Using the TCGA data set, we further analyzed CDX2-positive (N = 55), CDX2-negative (N = 175), CDX1-positive (N = 29), and CDX1-negative (N = 201) gastric cancer patients. As shown in Figure 4D, we analyzed associations between the expression status of CDX and 24 various variables and found four clinicopathological/molecular features significantly associated with expression of CDX in gastric cancer.
First, Epstein-Barr virus (EBV)-positivity is negatively associated with CDX-positivity with statistical significance, probably because CDX-positive gastric cancer mostly originated from H. pylori-induced chronic gastritis. Second, DNA hypermethylation status is frequently observed in CDX-positive gastric cancer compared with CDX-negative cancer, probably reflecting the fact that chronic infection of H. pylori induces intestinal metaplasia of gastric mucosa with high levels of aberrant DNA methylation.48 Third, microRNA expression clusters denote significantly different patterns between CDX-positive gastric cancer and CDX-negative gastric cancer, possibly reflecting the transcription of miRNAs regulated by CDX2/CDX1. And, finally, lymphocyte infiltration was more frequently observed in CDX-negative gastric cancer, probably reflecting the abundant lymphocyte infiltration of CDX-negative and EBV-associated gastric cancer.25
4 DISCUSSION
Although intestinal metaplasia of gastric mucosa is broadly accepted as a premalignant condition of the stomach,8, 9 how to understand the progression of ectopic intestinal differentiation is a matter of debate. Abnormal expression of CDX2/CDX1 is an essential trigger of intestinal metaplasia in the stomach,8, 13, 15 but many results including ours (Figure 1) showed that CDX-expressing gastric cancer tends to have less malignant potential and better clinical outcome.19, 20 Hierarchical clustering and GSEA also indicated that signatures related to CDX expression denote lower malignant features among the gastric cancer cases (Figure 3, Figures S3 and S4). Intestinal metaplasia is typically classified into two categories: mixed gastric-and-intestinal type (incomplete type expressing both gastric and intestinal marker mucin) and solely intestinal type (complete type expressing intestinal but not gastric marker mucin).7 Filipe's monumental study reported that the solely intestinal type showed a smaller risk of gastric oncogenesis compared with the mixed gastric-and-intestinal type.9 Together, these results suggested that CDX-induced intestinal differentiation may reduce the malignant potential of gastric mucosa.
Taking this into consideration, intestinal metaplasia (ectopic intestinal differentiation) cannot simply be regarded as an unfavorable event. Unstable differentiation status between “gastric” and “intestinal” property probably shows a higher risk of malignancy in the stomach.6, 9 Therefore, properly accomplished intestinal differentiation could decrease the risk of gastric cancer. In the present study, we discovered that some gastric cancer cells can be treated by forced induction of intestinal differentiation (Figure 2). Of the various strategies against malignancy, regulating the disordered differentiation status of cancer is the smartest one. Because maturation of tumors generally leads to the lower malignant property of cancer, we hypothesize that CDX-induced intestinal differentiation can attenuate the malignant potential of gastric cancer. We hope that a personalized therapy for gastric cancer based on induced intestinal differentiation may be realized in the future.
Many previous studies showed that CDX directly controls transcription of various intestinal marker genes,10, 12, 28, 31 which was reproduced in the present study (Figure 2E). In addition, we found a tendency that expression of gastric marker genes is suppressed in CDX-transduced gastric cancer cells (Figure 2F, Tables S3 and S4). Although associations between CDX and transcription of non-intestinal genes are probably indirect, such associations can partly explain the decreased expression of gastric markers accompanied by ectopic CDX expression observed in chronic gastritis.
Our results also suggest that CDX has the potential to promote de-differentiation by induction of stemness-associated genes (Figure 2F, Tables S1 and S2). Recently, Fujii et al49 reported that CDX1 confers an intestinal phenotype on gastric epithelial cells based on increased expression of two stemness-associated factors. Simmini et al50 reported that CDX2 is essential to maintain identity of the intestinal stem cell. These reports suggested that CDX not only promotes intestinal differentiation but also works to maintain the stemness of the intestine. Such paradoxical functions of simultaneous differentiation and de-differentiation may be one of the reasons for the controversial aspects concerning CDX expression and gastric malignancy.18-21 The meaning of induced stemness in gastric cancer cells remains unclear. It may undesirably function by diminishing intestinal differentiation and consequently leading to instability of the gastric mucosa. Conversely, it may play preferable roles in recovery from the unstable differentiation status and subsequent appropriate reprogramming of the disordered gastric mucosa.
We are now planning to establish the dozens of primary culture cells from gastric cancer lesions surgically resected and will try to examine the effects of CDX transduction on these cells. The difference between CDX-expressing and CDX-deficient primary gastric cancer cells can elucidate the above-described disputable matters10, 18-20 and also give us more accurate guiding principles concerning our hypothesis. In addition, we are searching new reagents which induce intestinal differentiation in CDX-deficient gastric cancer cells. On the basis of controlling disrupted gastrointestinal differentiation, such reagents may be a novel therapeutic choice against gastric cancer in the future. To avoid the confusing effect regarding differentiation and de-differentiation, we hope to identify novel reagents that can give rise to rigid intestinal differentiation without inducing unstable stemness.
In conclusion, we discovered that exogenous expression of CDX2/CDX1 can lead to G0-G1 growth arrest in CDX-deficient gastric cancer cells, accompanied by induction of intestinal genes and decreased expression of gastric genes. Publicly available databases showed that gastric cancer with CDX (CDX2 and/or CDX1) expression has significantly better clinical outcomes. Both hierarchical clustering of 132 gastric tissues based on our transcriptome analyses and gene set enrichment analyses indicated that “CDX signature”-positive gastric cancer has significantly lower malignant features compared with “CDX signature”-negative gastric cancer. A novel therapy against gastric cancer based on induced intestinal differentiation may be possible in the future.
ACKNOWLEDGMENTS
We are grateful to Mr Takeshi Shimamoto for assistance in statistical analyses and are also grateful to Mrs Mitsue Yamamichi-Nishina for evaluating the densitometry. This work was supported in part by a Grant-in-Aid for Scientific Research (C) from the Japan Society for the Promotion of Science (Grant number: 25460381), in part by a grant-in-aid for pioneering basic research from the Ministry of Health, Labour and Welfare (H20-genome-g-006), and in part by National Cancer Center Research and Development Fund in Japan (H23-A-2).
CONFLICTS OF INTEREST
Authors declare no conflicts of interest for this article.




