Effect of a hypoxic microenvironment after radiofrequency ablation on residual hepatocellular cell migration and invasion
Abstract
Clinical observations have shown that the boundary of tumor ablation is often less than safe border and that the use of radiofrequency ablation (RFA) in the treatment of hepatocellular carcinoma (HCC) may probably accelerate its recurrence and metastasis. RFA can cause the formation of a transition zone between normal liver tissues and necrotic coagulation, where blood stagnation and thrombosis expose residual cancer cells to a hypoxic microenvironment. As the blocked vessels are slowly reperfused, the oxygen supply is gradually restored. Here, HCC cells underwent heat treatment and were cultured under hypoxic conditions to mimic the aforementioned situation, and morphological changes were observed in the surviving cells. Compared with their parental cells, hypoxic HCC cells showed changes that include enhanced invasive, metastatic, and chemoresistant abilities as well as mesenchymal characteristics. There was also a higher percentage of stem-like cells. However, either improving the hypoxic microenvironment or silencing hypoxia inducible factor (HIF)-1α signaling significantly reduced the invasive, metastatic, and chemoresistant potential and reversed the epithelial-mesenchymal transition to varying degrees. Together, these results indicated that a sustained hypoxic microenvironment after RFA may exert a negative impact on the prognosis of HCC patients, and minimizing exposure to a hypoxic microenvironment and targeting HIF-1α signaling might be effective strategies for patients who experience insufficient RFA therapy.
Hepatocellular carcinoma (HCC) ranks as the third most frequent cause of cancer-related death.1 Although surgical operation and transplantation have been recognized as the primary curative therapies for patients with HCC, the advanced neoplastic stage, severe liver dysfunction and shortage of available liver grafts restrict their application.2 Radiofrequency ablation (RFA) therapy has emerged as an alternative due to its minimal invasiveness, safety, and shorter hospital stays. The survival of patients with HCC <3 cm treated by RFA competes with that of surgical candidates.3 However, suboptimal RFA treatment of HCC has been reported as a risk factor of early diffuse recurrence,4 and large tumor size is an independent risk factor of local recurrence because of its poorly defined margins.5 The necrotic lesion generated by RFA is surrounded by a rim of chronically hypoxic liver tissue, where aggressive outgrowth of residual hepatic micro-metastases has been observed.6 A hypoxic microenvironment contributes to the development of a malignant phenotype, which includes local invasion, metastatic progression, and chemoresistance.7
Hypoxia inducible factor (HIF)-1α is a key mediator of cellular adaption to hypoxia8 and can facilitate the progression of various cancer cells by promoting cell migration, tumor angiogenesis, and metastasis.9 The epithelial-mesenchymal transition (EMT) has long been recognized as an important step in the progression of primary tumors to distant metastasis.10 At the molecular level, EMT is marked by the loss of epithelial markers such as E-cadherin and the acquisition of mesenchymal markers such as N-cadherin and vimentin.11 Accompanied with these changes, HCC cells that undergoes EMT gains mesenchymal characteristics such as migration, invasiveness, and apoptotic resistance.12 HIF-1α can regulate EMT either directly or indirectly by activating its downstream transcription factors, namely Snail, Slug, and Twist.13 Previous studies have mostly focused on the effect of RFA itself regarding the progression of residual cancer cells. However, the role of the hypoxic microenvironment that manifests following RFA in more aggressive phenotypes and whether altering the biological characteristics of the microenvironment could influence the invasion and distant metastasis of HCC remain unclear.
In this study, we investigated the effect of the hypoxic microenvironment following insufficient RFA on the in vitro and in vivo progression of residual cells; we also sought to determine the underlying mechanisms involved in this transition. Furthermore, we aimed to ascertain whether interfering with the development of hypoxia or choosing strategies aimed at inhibiting HIF-1α expression could invert EMT and downregulate the elevated invasiveness and metastatic potential of the residual cells.
Materials and Methods
Cell culture and cell treatment
The human HCC cell lines MHCC97H and SMMC7721 were cultivated in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum (FBS; Invitrogen, Carlsbad, CA, USA) in a humidified atmosphere of 5% CO2 at 37°C. Insufficient RFA and the subsequent hypoxic microenvironment were simulated in vitro as follows. Cells were seeded in the flasks and incubated for 24 h, after which the flasks were submerged in a 47°C water bath for 5 min and subjected to an anaerobic incubator (1% O2, 5% CO2 at 37°C) to mimic the post-RFA hypoxic environment. Two days later, the flasks were divided into two groups: flasks in the first group were sustained in the anaerobic incubator (hypoxia group), and the remaining flasks were placed in the normal oxygen incubator (reperfusion group). The control group was submerged in the 37°C water bath for 5 min and cultured under normoxic conditions. The cell morphology was observed under a microscope (Nikon, Tokyo, Japan).
Chemicals and reagents
Primary antibodies against E-cadherin, N-cadherin, and vimentin were purchased from Cell Signaling Technology (Danvers, MA, USA). Antibodies recognizing MMP2, MMP9 and Snail were purchased from Abcam (Cambridge, MA, USA); antibodies against HIF-1α and β-actin were acquired from ProteinTech (Wuhan, China). YC-1 (a HIF-1α inhibitor) and SB431542 (an inhibitor of the TGF-β type I receptor) were obtained from Sigma-Aldrich (St. Louis, MO, USA), and human recombinant TGF-β1 was purchased from PeproTech (Rocky Hill, NJ, USA).
Invasion and migration assays
A transwell assay (Corning, Corning, NY, USA) was performed to assess the invasive and migratory abilities of HCC cells. Briefly, the upper chamber was coated with Matrigel (BD Biosciences; Bedford, MA, USA). Approximately 2 × 104 cells were seeded into the upper inserts in serum-free medium, with Dulbecco's modified eagle medium (DMEM) containing 10% FBS used as a chemoattractant in the lower chamber; the cells were incubated in either normoxic or hypoxic conditions for 48 h. Cells on the upper surface of the chamber were removed using a cotton swab, and the invaded cells were fixed with 4% paraformaldehyde, stained with crystal violet, and photographed under a microscope. The migration assay was performed under identical conditions except that the upper chambers were not coated.
RT-PCR
Total RNA was extracted using an RNAiso Plus kit (Takara; Dalian, China), and reverse transcription was performed using an RT-PCR kit. Quantitative real-time polymerase chain reaction (qPCR) was performed by using a LightCycler 96 Instrument (Roche, Basel, Switzerland) using the primers listed in Table 1. The housekeeping gene β-actin served as an internal control to normalize the mRNA expression levels of the target genes.
| Gene | Primers | Sequence (5′-3′) |
|---|---|---|
| Snail | Forward | ACTGCAACAAGGAATACCTCAG |
| Reverse | GCACTGGTACTTCTTGACATCTG | |
| Slug | Forward | CCTGGTTGCTTCAAGGACAC |
| Reverse | AGCAGCCAGATTCCTCATGT | |
| Twist1 | Forward | GCCGGAGACCTAGATGTCATTG |
| Reverse | CTATCAGAATGCAGAGGTGTGAGG | |
| Vimentin | Forward | GTGAATACCAAGACCTGCTCAATG |
| Reverse | AGGGAGGAAAAGTTTGGAAGAGG | |
| VEGFA | Forward | AGGGCAGAATCATCACGAAGT |
| Reverse | AGGGTCTCGATTGGATGGCA | |
| β-actin | Forward | CATGTACGTTGCTATCCAGGC |
| Reverse | CTCCTTAATGTCACGCACGAT |
Western blotting
Total protein was isolated from lysed cells, and equivalent amounts of protein were separated on a gel via sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to polyvinylidene fluoride membrane, and probed with primary antibodies overnight at 4°C. After the blots were incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies, they were visualized with an ECL reagent (Millipore; Billerica, MA, USA).
Immunohistochemistry
Tumor tissues were fixed, embedded and sliced into 6-μm sections, which were stained for E-cadherin, N-cadherin, vimentin, Snail, MMP2, and MMP9 by using immunohistochemistry as previously described.14 Staining results were viewed under a light microscope.
Flow cytometry analysis, tumor growth in xenograft mice, HIF-1α shRNA transfection, cell viability, ELISA, tail vein metastasis assay, and soft agar colony formation
Flow cytometry analysis, tumor growth in xenograft mice, HIF-1α shRNA transfection, cell viability, ELISA, tail vein metastasis assay, and soft agar colony formation were conducted as described in the Supporting Information Materials and Methods (Appendix. S1).
Statistical analysis
All values are expressed as the mean ± SD. Using SPSS 13.0 for Windows (Chicago, IL, USA), either Student's t-test or anova was used to analyze differences between the groups. A P-value <0.05 was considered statistically significant.
Results
The formation of a hypoxic microenvironment following insufficient RFA promotes HCC cell migration and invasion
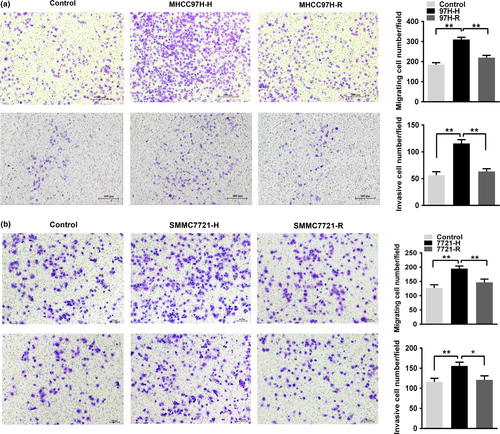
To evaluate the effect of the hypoxic microenvironment following insufficient RFA on HCC cells, MHCC97H and SMMC7721 cells were subjected to heat intervention followed by incubation in a constant anaerobic environment. The surviving cells were designated as MHCC97H-H and SMMC7721-H, respectively. MHCC97H-H cells displayed elevated migratory and invasive abilities than those in the parent control group (Fig. 1a). As mentioned above, cells treated with heat and hypoxia for two days followed by exposure to normoxic conditions were designated MHCC97H-R cells. The migratory and invasive abilities of MHCC97H-R cells were lower compared with MHCC97H-H cells (Fig. 1a). Similar results were also observed among the SMMC7721-H, SMMC7721-R and control cells (Fig. 1b), which suggest that a hypoxic microenvironment due to insufficient RFA could promote HCC cell migration and invasion.
A hypoxic microenvironment following insufficient RFA confers mesenchymal characteristics to HCC cells
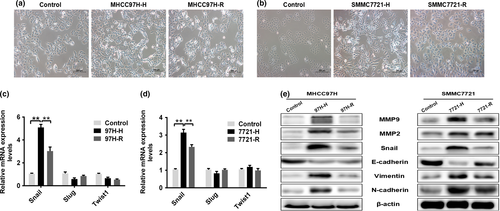
MHCC97H-H cells acquired a spindle shape with fewer cell–cell junctions as well as increased pseudopodia formation; this was distinctly different from the typical epithelial-like cobblestone appearance of cells from the control group. The morphology of MHCC97H-R cells was intermediate between the other two groups (Fig. 2a). To confirm that EMT had occurred during this process, we assessed the expression of EMT-related transcription factors (Snail, Slug, Twist1) by qPCR. The levels of Snail mRNA in MHCC97H-H cells were significantly higher than those in the control and MHCC97H-R cells (Fig. 2c). Detection of EMT markers by western blotting demonstrated a significant reduction in E-cadherin expression, but the expression levels of N-cadherin, vimentin, Snail, MMP2, and MMP9 were upregulated in MHCC97H-H cells (Fig. 2e). Evidence of the EMT process in MHCC97H-R cells was not as obvious or frequent as that in MHCC97H-H cells. Similar results could also be observed in SMMC7721, SMMC7721-H and SMMC7721-R cells (Fig. 2b,d,e). These results indicated that the subsequent hypoxic microenvironment generated by insufficient RFA could induce EMT, and alleviating the prolonged hypoxia could partially reverse the occurrence of EMT in HCC cells.
Incomplete RFA followed by the formation of a hypoxic microenvironment potentiates HCC cell chemoresistance to sorafenib
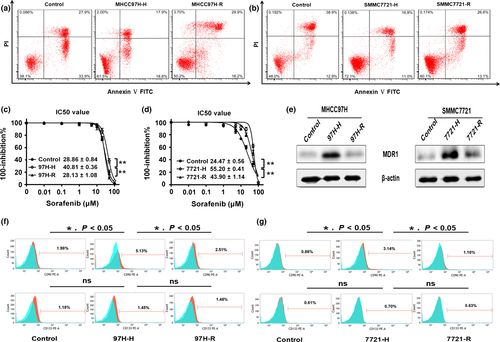
Flow cytometry analysis was performed to examine whether hypoxic conditions following insufficient RFA altered the apoptosis of HCC cells. After treatment with 25 μmol/L sorafenib, the number of apoptotic MHCC97H-H cells was significantly decreased compared with that of control cells, and the apoptotic rate of MHCC97H-R cells was higher than that of MHCC97H-H cells (Fig. 3a, P < 0.05). The effect of sorafenib on HCC cell growth was determined by assessing cell viability using the [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt; (MTS)] assay. Compared with control cells, the IC50 value of sorafenib in MHCC97H-H cells was strikingly higher, but the IC50 value of MHCC97H-R cells was significantly lower than that of MHCC97H-H cells (Fig. 3c). The multi-drug resistance gene MDR1 was highly expressed in MHCC97H-H cells, whereas MDR1 showed minimal expression in MHCC97H and MHCC97H-R cells (Fig. 3e). Similar results were also observed in SMMC7721, SMMC7721-H and SMMC7721-R cells (Fig. 3b,d,e).
A hypoxic microenvironment generated by insufficient RFA may increase the percentage of cancer stem cells (CSCs)
Because CD90 and CD133 are known liver CSC markers, we examined whether hypoxic treatment after heat intervention had any effect on the stem-like properties of our induced cell lines. As shown in Figure 3(f), the flow cytometry results show that MHCC97H-H cells had a relatively higher percentage of CD90+ cells than that of the control cells, and the percentage of cells expressing CD90 was significant lower in the MHCC97H-R group compared with that of the MHCC97H-H group. With regard to CD133, no significant expression differences among the three groups were observed (Fig. 3f). Similar results were also observed in SMMC7721, SMMC7721-H, and SMMC7721-R cells (Fig. 3g). These results indicated that the percentage of CSCs in the HCC cell population was significantly influenced by the incomplete RFA and, in particular, the subsequent formation of a hypoxic microenvironment.
HIF-1α plays a pivotal role in achieving EMT and gaining CSC characteristics in HCC cells
HIF-1α serves as a key mediator of the adaptive response to hypoxic stress. Western blotting demonstrated that compared with their respective cell groups, the protein levels of HIF-1α were elevated significantly in MHCC97H-H and SMMC7721-H cells (Fig. 4a). To identify whether HIF-1α plays a critical role in the induction of EMT, chemoresistance, and the development of CSC characteristics by the development of a hypoxic environment following insufficient RFA, we established stable cell lines with knockdown of HIF-1α and blocked HIF-1α with 10 μmol/L YC-1. The western blotting analysis showed that after heat treatment and continuous exposure to hypoxia, HIF-1α expression was reduced in both shHIF-1α cells and YC-1-treated cells compared with the shNC cells (Fig. 4b). We further investigated whether knockdown or inhibition of HIF-1α was associated with the biological activity of HCC cells. Using these approaches on MHCC97H-H and SMMC7721-H cells, the results showed that E-cadherin expression in shHIF-1α cells and YC-1-treated cells was increased in conjunction with decreases in N-cadherin, vimentin, and Snail expression (Fig. 4c). The stimulatory effect of the hypoxic microenvironment on HCC cell migration and invasion was abrogated by either knockdown or inhibition of HIF-1α (Fig. S1). Moreover, we observed that shHIF-1α cells and YC-1-treated cells were more sensitive to sorafenib (Fig. 4d,e), and the expression of MDR1 was significantly reduced compared with that of the shNC cells (Fig. 4f). Furthermore, we discovered that under insufficient RFA and hypoxic conditions, depleting HIF-1α expression could reduce the percentage of CSCs (Fig. 4g). All of these data revealed that HIF-1α might play a pivotal role in increasing the migration, invasion, and sorafenib chemoresistance by inducing EMT; moreover, HIF-1α could be involved in the acquisition of the stem cell-like traits.

Hypoxic conditions following non-lethal thermal ablation inhibited tumor growth but promoted distant metastasis in vivo
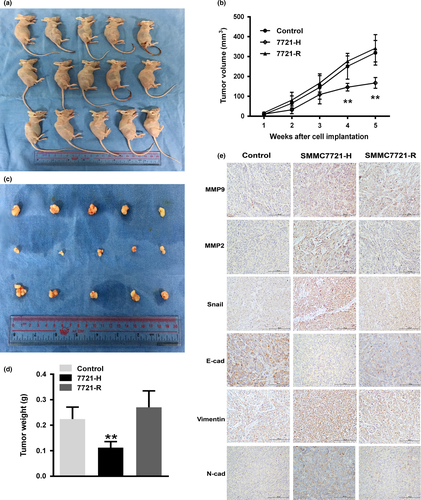
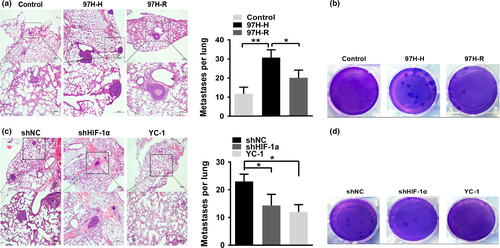
Because our in vitro studies showed that prolonged exposure to a hypoxic microenvironment after insufficient RFA significantly enhanced the migration and invasion of HCC cells, we utilized a nude mouse xenograft model to determine the effect of this process on tumorigenicity. The volumes and weights of tumors in the SMMC7721-H group were significantly reduced compared with those of the control and SMMC7721-R groups (Fig. 5a–c,e); these results suggest that persistent hypoxia after RFA attenuated the ability of HCC cells to initiate tumors in vivo. Immunohistochemistry analysis further demonstrated that a hypoxic microenvironment following insufficient RFA induced changes in the xenograft tumors consistent with EMT (Fig. 5d). A tail vein injection assay was used to evaluate the impact of sustained exposure to a hypoxic microenvironment after RFA on the in vivo metastatic ability of MHCC97H cells, which have greater metastatic potential. The number of pulmonary metastatic foci in the MHCC97H-H group was significantly higher than that in the control and MHCC97H-R groups (Fig. 6a,b). Moreover, hypoxic conditions also increased the colony forming ability of MHCC97H-H cells in soft agar (Fig. 6c). To examine whether knockdown or inhibition of HIF-1α reduces lung metastasis in vivo, we injected shNC, shHIF-1α, and YC-1-treated cells into the tail veins of nude mice and observed that both knockdown and inhibition of HIF-1α critically reduced the number of metastatic foci in the lungs (Fig. 6d,e). Furthermore, knockdown or inhibition of HIF-1α decreased the colony forming ability of MHCC97H cells in soft agar (Fig. 6f). These data implied that either altering the hypoxic conditions or depleting HIF-1α decreased the ability of HCC cells to resist anoikis.
The enhanced invasive and metastatic capabilities of HCC cells are associated with HIF-1α/TGF-β1/Snail signaling
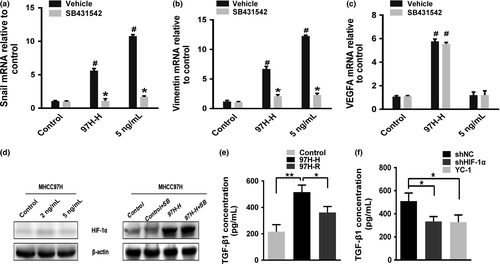
Transforming growth factor-β1 (TGF-β1) has been identified as the key driver in inducing EMT;15 therefore, we explored whether the induction of EMT due to the creation of a hypoxic microenvironment following incomplete RFA required TGF-β signaling. Increased mRNA levels of Snail and vimentin were observed in MHCC97H-H cells (Fig. 7a,b); also, TGF-β1 has been shown to an increase the mRNA levels of Snail and vimentin in HCC cells. Upregulation of Snail and vimentin by either heat and hypoxic intervention or TGF-β1 could be completely prevented by pretreatment with SB431542. However, upregulation of vascular endothelial growth factor A (VEGFA) in MHCC97H-H cells was unaffected by pretreatment with SB431542 (Fig. 7c), and TGF-β1 did not increase VEGFA mRNA levels, suggesting that TGF-β signaling occurs downstream of HIF activation and is specific to EMT-related genes. Additionally, TGF-β1 did not activate HIF-1α in HCC cells cultured under normal conditions; consistent with this, HIF-1α activation in MHCC97H-H cells was not prevented by SB431542 (Fig. 7d). In accordance with the above observations, the concentration of TGF-β1 in conditioned medium from MHCC97H-H cell cultures was significantly higher than that in the control and MHCC97H-R media (Fig. 7e). Likewise, inhibiting the expression of HIF-1α could reduce the TGF-β1 concentration in the cultured medium (Fig. 7f). Similar results were also observed in the SMMC7721 cell line (Fig. S2).
Discussion
Locally thermal ablation therapies maintain an important role in the treatment of liver tumors in patients with low volume of disease. The types of thermal ablation available are RFA, microwave ablation, laser ablation, and high-intensity-focused ultrasound ablation. The operating principle of them are similar, both of them are utilized to destroy tumors by producing heat. The reason why we only mentioned RFA is that RFA is more frequently used in our hospital and our follow-up experiments are also based on insufficient RFA model. Previous studies have demonstrated that insufficient RFA promoted the growth and the migratory of HCC cells,16 as well as resulting a higher frequency of portal invasion and less tumor differentiation17 and that EMT may occur during this progression.18 Another study elucidated that hyperthermia may play a pivotal role in the rapid growth of residual cells after RFA by inducing angiogenesis near residual HCC through HIF-1α/VEGFA signaling.19 A chronically hypoxic tissue zone can be generated following thermal ablation, where the microenvironment of any residual viable cells may be altered.20 The development of RFA-induced hepatic parenchymal hypoxia is associated with an increased risk of recurrence.21 Moreover RFA could accelerate the outgrowth of colorectal liver metastases in a hypoxia-driven manner.22 However, the mechanism by which hypoxia affects the invasive and metastatic ability of the residual cells after RFA treatment is poorly understood.
Growing evidence has identified EMT as a pivotal mechanism of cancer invasion and metastasis, with epithelial cells losing their cell polarity and acquiring the mobility of mesenchymal cells.23 In our present work, we found that hypoxic conditions following insufficient RFA enhanced the migratory, invasive, and chemoresistant potential of HCC cells in vitro; all of these characteristics are associated with EMT. Further evidence provided by a xenograft nude mouse model showed alterations in the protein levels of these EMT markers. Additionally, the enhanced metastatic potential of MHCC97H-H cells in vivo further confirms the in vitro results. Therefore, our results suggest that EMT is of great importance in enhancing the invasiveness and metastatic potential of HCC cells subjected to a hypoxic microenvironment after non-lethal thermal ablation. EMT is also tightly linked with resistance to anoikis.11 Cells undergo a natural apoptotic process known as anoikis, a type of cellular death due to the loss of contact with the extracellular matrix, as a barrier to metastasis. Cancer cells acquire resistance to anoikis after detaching from the primary tumor site, thereby traveling through the circulatory system to disseminate throughout the body.24 Zhang et al. reported that the anoikis-resistant process of HCC cells was also accompanied by the EMT process, whereas the miRNA miR-424-5p could prevent HCC progression by reversing these two processes that are critically involved in metastasis.25 In this study, MHCC97H-H cells formed more colonies in soft agar; coupled with their promoted metastatic ability in vivo, these data suggested that EMT-like cells are more resistant to anoikis.
HIFs form heterodimers from various α-subunits and a common β-subunit. The α-subunits are scarcely detectable at the protein level under normoxic conditions because they are rapidly degraded in an oxygen-dependent manner.26 Stabilizing and activating the HIF-1α/HIF-1β transcription complex trigger the expression of its target genes, which correlates with many different cellular processes such as angiogenesis, anticancer drug resistance, and EMT.27 In our study, overexpression of HIF-1α in cells under hypoxic conditions was observed; however, when the hypoxic environment was altered, the levels of HIF-1α decreased. Furthermore, altering hypoxia also impaired the elevated migratory, invasive, and chemoresistant capabilities of the cells and partially reversed EMT. Consequently, we hypothesized that altering the hypoxia conditions could suppress EMT and its related characteristics via degradation of HIF-1α. To verify our hypothesis, we established cell lines with stable knockdown of HIF-1α and treated cells with YC-1, and our data indicated that the promotive effects of the hypoxic environment following insufficient RFA on promoting EMT was abolished by silencing HIF-1α. Additionally, MDR1 expression is regulated by HIF-1α, which implies that HIF-1α-mediated MDR1 expression is a possible mechanism for sorafenib resistance in HCC cells. Therefore, HIF-1α expression is highly detrimental to the prognosis of patients with HCC who experienced suboptimal RFA.
Sorafenib has been approved as the first-line systemic drug for advanced HCC. Previous studies indicated that sorafenib suppresses the transition of residual HCC cells from epithelial phenotype to the mesenchymal phenotype after insufficient RFA.28 Another study suggested that RFA combined with sorafenib exerts a better curative effect in terms of tumor suppression than RFA alone, probably through inhibiting HIF-1α and vascular endothelial growth factor expression.29 However, Zhao D et al. revealed that sorafenib upregulates HIF-2α by switching the hypoxia response from HIF-1α to HIF-2α-dependent pathways, resulting in the activation of the TGF-α/EGFR pathway, and subsequently the phosphorylation of STAT3, AKT and ERK, which contributes to the resistance of HCC cells to sorafenib. While the HIF-1α expression is downregulated, the expression of the HIF-2α increased via a compensatory mechanism.30 In our study, however, sorafenib is merely employed as a chemotherapeutic to examine the impact of hypoxia on the chemosensitivity of HCC cells. Compared with normoxia cultured cells, the hypoxic residual cells are more resistant to sorafenib and the apoptosis rate is lower. The variate is hypoxia rather than sorafenib or RFA, which is different from previous studies, and we are just trying to state the phenomenon instead of discussing the specific mechanism underlying the resistance of hypoxic HCC cells to sorafenib.
Cancer stem cells, also known as cancer initiating cells, are defined as a subpopulation of cells that possess the properties of self-renewal, differentiation, and tumorigenesis.31 These cells govern the chemoresistance, recurrence, and metastasis of various tumor types.32 A number of molecular markers, including CD90 and CD133, have been identified for therapeutic treatment, and the expression of these markers may be upregulated by HIF-1a.17 According to results from Fan and colleagues,33 there is a positive correlation between CD90 expression and the tumorigenic and metastatic potential. As few as 5000 CD90+ cells, which were isolated from the circulating blood of HCC patients, were able to initiate tumors with similar histological features as the primary tumors. Furthermore, CD133+ liver CSCs were demonstrated to both promote angiogenesis through the mitogen-activated protein kinase and IL-8 pathways and increase cell invasiveness through MMP2.34 Inhibiting CD133 expression in HCC cells also suppressed metastasis.35 The EMT has been reported to play an essential role in regulating CSCs, which often exhibit an EMT phenotype in HCC.36 In our study, we observed that a chronic hypoxic microenvironment due to insufficient RFA generated more CD90+ cells without impacting CD133 expression and that down-regulation of HIF-1α reduced the percentage of CD90+ cells. This suggested that the HIF-1α pathway is involved in CSC regulation. However, we observed that hypoxic conditions after incomplete RFA plays a suppressive role in tumor growth – this discrepancy might be interpreted as EMT-induced inhibition of mitochondrial respiration, which is usually associated with some degree of growth inhibition.37
Reportedly, EMT can be induced by TGF-β1 within the tumor microenvironment, and TGF-β signaling also plays a major role in regulating cancer stemness.38 Researchers have noticed that the expression levels of CD90, CD133, and Oct4 were significantly upregulated in TGF-β1 treated cells,39 and our work was consistent with these studies to some extent. Other reports stated that TGF-β signaling was both necessary for the hypoxia-induced activation of HIFs and capable of activating HIFs independent of hypoxia. However, our study suggested that TGF-β signaling is not required for HIF activation in HCC cells by hypoxia and that TGF-β1 does not activate HIFs; rather, we clearly demonstrated that TGF-β signaling occurs downstream of HIF activation. The results of the ELISA assays show that hypoxia promoted TGF-β1-dependent EMT in HCC cells by increasing TGF-β1 secretion.
Collectively, continuous hypoxia after incomplete RFA may have a suppressive impact on tumor proliferation; however, the hypoxic microenvironment significantly enhanced the invasive and migratory abilities as well as the anti-apoptotic characteristics of CSCs in vitro and the metastatic abilities in vivo, possibly through HIF-1α/TGF-β1/Snail signaling. Consequently, either reversing the hypoxic microenvironment or silencing HIF-1α expression and/or activity may provide new insight into post-RFA clinical therapies. However, our study only simulated the residual cells in vitro, and further studies concerning the hypoxic microenvironment following insufficient RFA in animal models should be conducted to continue to elucidate this phenomenon.
Acknowledgments
This work was supported by the Nature Science Foundation of China (81671704); the Science Foundation of Guangdong Province (2016A030313306); the Doctoral Fund of Ministry of Education (20120171110080); a grant from the Guangdong Science and Technology Department (2015B050501004); Grant [2013] 163 from the Key Laboratory of Malignant Tumor Molecular Mechanisms and Translational Medicine of the Guangzhou Bureau of Science and Information Technology; and Grant KLB09001 from the Key Laboratory of Malignant Tumor Gene Regulation and Target Therapy of the Guangdong Higher Education Institute.
Disclosure Statement
The authors have no conflicts of interest to declare.




