Predictive and prognostic role of serum neopterin and tryptophan breakdown in prostate cancer
Funding Information
No funding received.
Abstract
The γ-interferon-induced enzymes indoleamine 2,3-dioxygenase and GTP-cyclohydrolase are key players in tumor immune escape mechanisms. We quantified serum levels of neopterin and tryptophan breakdown (tryptophan, kynurenine, and kynurenine-to-tryptophan ratio) in addition to prostate-specific antigen (PSA) in newly diagnosed prostate cancer (PCa) patients (n = 100) before radical prostatectomy (RP) as well as at time of biochemical recurrence (BCR) after RP (n = 50) in comparison to healthy men (n = 49). Effects of biomarkers on the risk of PCa diagnosis on transrectal biopsy, worse histopathological characteristics of the RP specimens, and cancer-specific survival (CSS) after BCR were investigated. Neopterin (hazard ratio [HR], 2.46; 95% confidence interval [CI], 1.08–5.61; P = 0.032) and kynurenine (HR, 2.93; 95% CI, 1.26–6.79; P = 0.012) levels were univariately associated with CSS. When adjusted for other biomarkers, only neopterin remained an independent predictor of CSS (HR, 2.56; 95% CI, 1.07–6.12; P = 0.035). Only PSA was associated with an increased risk of PCa diagnosis on biopsy, univariately (odds ratio, 3.14; 95% CI, 1.68–5.88; P < 0.001) as well when adjusted for other biomarkers (odds ratio, 3.29; 95% CI, 1.70–6.35; P < 0.001). Moreover, only preoperative PSA was able to predict positive surgical margin (area under the receiver operating characteristic curve [AUC] = 0.71; 95% CI, 0.59–0.82; P = 0.001), higher Gleason score (AUC = 0.75; 95% CI, 0.66–0.85; P < 0.001) and extraprostatic involvement (AUC = 0.79; 95% CI, 0.69–0.88; P < 0.001) at RP specimens, respectively. Although serum neopterin and tryptophan breakdown cannot be considered as biomarkers in detecting PCa or in predicting worse final pathological findings, neopterin levels are useful for stratifying patients into different prognostic groups after BCR.
Chronic inflammation plays a crucial role in the development of PCa. Infiltrating immune cells are the major reasons for pro-inflammatory changes in the prostate microenvironment.1 Moreover, increased production of pro-inflammatory cytokines like IL-8 and IL-6 contributes to PCa progression and to the onset of castration resistance in metastatic disease.2 This could be explained by an activation of different pathways like JAK–STAT3, RAS–RAF–MAPK, and PI3K–AKT3 being responsible for cell proliferation, survival, invasion, and metastasis.1 Therefore, a network of cytokines is essential in the interaction between microenvironment and cancer cells.
γ-Interferon was identified as the most important pro-inflammatory stimulus for the enzyme GTP-cyclohydrolase in human monocyte-derived macrophages and dendritic cells, which induces neopterin production reflecting cellular immune activation.4, 5
As IFN-γ stimulates several antiproliferative biochemical pathways,6 neopterin turned out to be useful as a potential biomarker of cancer diagnosis and prognosis,7 depending on tumor entitity and stage.8 In parallel, IFN-γ activates the enzyme IDO, which converts tryptophan to kynurenine, resulting in increased tryptophan breakdown, and subsequently elevated kynurenine-to-tryptophan ratio (KTR). Accelerated breakdown of tryptophan and increased production of neopterin were found to parallel the course of different malignant diseases and predicting poor prognosis.9 Moreover, elevated baseline plasma neopterin levels and KTR were associated with a significantly higher risk of developing cancer in the general population.10
However, the diagnostic and prognostic role of IFN-γ-induced inflammatory markers focusing on PCa is still unclear. The aim of this pilot study was to determine serum levels of neopterin and tryptophan breakdown in newly diagnosed PCa patients before RP and to test their relation to BCR after RP and prognosis (CSS).
Materials and Methods
This retrospective pilot study was approved by the local ethical committee (study number UN-3174; AM3174) of the Medical University Innsbruck (Innsbruck, Austria). Informed consent was obtained from all patients included in the study. From a review of several charts from PCa patients treated at our department, 199 patients were enrolled in the study population and separated into three different patient groups to assess the predictive as well as the prognostic role of serum neopterin and tryptophan breakdown. All descriptive and histopathological data of patients were assessed using our prostate biobank database. A flow chart of the study design is shown in Figure 1.
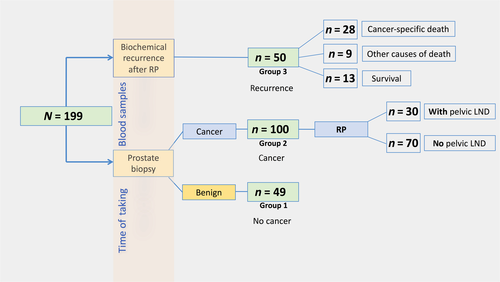
Groups 1 and 2 consisted of 149 men in total undergoing transrectal needle prostate biopsy on the basis of elevated age adjusted PSA (ng/mL) levels and fPSA% of <18% or suspicious digital rectal examinations.11 A PSA cut-off of 1.25, 1.75, 2.25, and 3.25 ng/mL was used as criteria for biopsy in men aged 40–49 years, 50–59 years, 60–69 years, and 70–75 years, respectively, as published previously.11 Ten systematically transrectal ultrasound-guided prostate biopsy cores were taken in a standard spatial distribution. No template-based perineal biopsies or MRI-fusion biopsies were carried out. Benign needle biopsy was confirmed in 49 (32.9%) men (group 1, no cancer), while 100 (67.1%) men showed tumor-positive prostate biopsy (group 2, newly diagnosed PCa). All men with PCa consecutively received RP, and patients with an intermediate- or high-risk PCa based on the D'amico risk group classification system12 in addition to simultaneous pelvic LND (n = 30/100).
Finally, group 3 included another 50 men with verified BCR (defined by two consecutive PSA values ≥ 0.2 ng/mL) 13 at a median (range) time of 1.8 (0.2–10.3) years after RP (group 3, recurrence).
Biochemical analyses
After obtaining informed consent from every patient, serum samples were collected during routine blood examination at two different time points: 10 days (mean; range, 5–16 days) prior to transrectal biopsy (group 1 and group 2) and 31 days (mean; range, 0–56 days) after verified BCR with previous RP (group 3). All the samples were frozen at −80°C until analysis. We undertook HPLC on reversed phase for tryptophan breakdown measurements, as described previously.14, 15 In brief, serum samples were diluted with potassium phosphate buffer (0.05 mol/L, pH 6.0) containing the internal calibrator 3-nitro-l-tyrosine (100 μmol/L). Protein was then precipitated with 50 μL trichloroacetic acid (2 mol/L) and removed by centrifugation. For separation, reversed-phase cartridges LiChroCART RP18 columns (244 mm length, 5 μm grain size; Merck, Darmstadt, Germany) with sodium acetate buffer (pH 4.0, 15 mmol/L) and 5% methanol were used. Analyses were carried out at a flow rate of 0.9 mL/min and a temperature of 25°C by a Varian ProStar liquid chromatography system with autosampler Model 400 (Varian, Palo Alto, CA, USA). l-Kynurenine was measured by a Jasco UV 975 detector (Jasco, Tokyo, Japan) at 360 nm; tryptophan was detected by a fluorescence detector (Model ProStar 360; Varian) at an excitation wavelength of 286 nm and an emission wavelength of 366 nm. Peaks were identified by comparing their retention time to those of an albumin-based calibrator containing 10 μmol/L l-kynurenine and 100 μmol/L l-tryptophan prepared like serum samples. To estimate IDO activity, KTR was calculated. Serum neopterin was determined by a commercially available ELISA (BRAHMS Diagnostics, Henningsdorf, Germany).16
Statistics
All demographic and baseline characteristics were analyzed descriptively (absolute and relative frequency for qualitative data, and mean and SD for quantitative data), overall and stratified by group (no cancer/newly diagnosed PCa/BCR). As data were not normally distributed, Mann–Whitney U-tests and Kruskal–Wallis tests were used as a non-parametric method for comparing groups for different variables (PSA, fPSA, neopterin, tryptophan, kynurenine, and KTR). Pairwise comparisons were carried out according to the Dunn–Bonferroni method accounting for the type 1 error rate inflation in multiple testing. Correlations between ordinal variables were assessed by non-parametric Spearman's rank correlation coefficient (rs). Receiver operating characteristic curves and the AUC were calculated to predict PCa diagnosis as well as positive surgical margins, higher Gleason score, and extraprostatic involvement at RP specimens.17, 18 Univariate and multivariate (adjusted for serum biomarkers like neopterin, tryptophan, kynurenine, KTR, and PSA) logistic regression models were used to estimate unadjusted and adjusted ORs and 95% CIs to identify predictive factors for PCa diagnosis. Cancer-specific survival curves were obtained using Kaplan–Meier product-limit estimation and groups (≤3rd quartile vs >3rd quartile) for each prognostic variable were compared with the log–rank test. Univariate and multivariate (adjusted for serum biomarkers such as neopterin, tryptophan, kynurenine, KTR, and PSA) Cox proportional hazard regression models were used to estimate unadjusted and adjusted HRs and 95% CIs and to identify prognostic factors for CSS. A significance level of α = 0.05 (two-tailed) was applied. Statistical analyses were carried out in R, version 3.1.1 (www.r-project.org) and spss version 22.0 (SPPS, Chicago, IL, USA). Graphics were generated using GraphPad Prism 6 (GraphPad Software, La Jolla, CA, USA).
Results
Serum levels of neopterin and tryptophan breakdown have no diagnostic accuracy in detecting PCa on transrectal biopsy
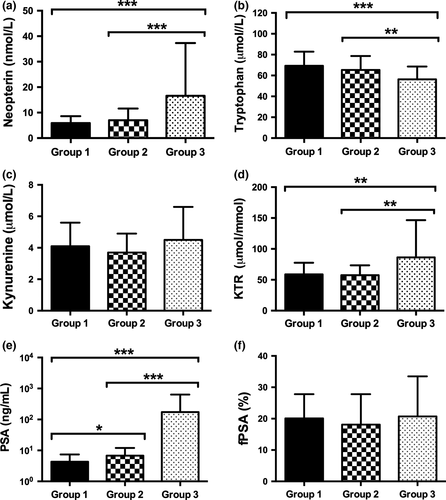
One hundred and forty-nine men (groups 1 and 2) with a mean ± SD (range) age of 62.8 ± 8.2 years (44–77 years) and elevated bisected age-specific PSA levels (mean ± SD, 6.0 ± 4.8 ng/mL) underwent consecutive transrectal prostate biopsy. Positive and negative prostate biopsy was confirmed in 100 (67.1%) and 49 (32.9%) patients, respectively. Comparing both subgroups (group 1, no cancer versus group 2, cancer) with each other, neopterin (P = 0.146), tryptophan (P = 0.313), kynurenine (P = 0.411), KTR (P = 0.870), and fPSA (P = 0.213) levels did not differ significantly. Prostate-specific antigen was the only parameter showing significantly higher levels in patients with positive biopsy compared to those with negative biopsy (mean ± SD, 6.8 ± 5.3 vs 4.3 ± 3.1, P = 0.012) (Fig. 2).
Moreover, a 1 ng/mL increase in PSA was associated with a more than three-fold increased risk of detecting PCa on univariate analysis (OR, 3.14; 95% CI, 1.68–5.88; P < 0.001) and remained an independent predictive factor for PCa diagnosis when adjusted for all other serum biomarkers (OR, 3.29; 95% CI, 1.70–6.35; P < 0.001], respectively (Table 1).
| Predictor† | AUC (95% CI)‡ | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P-value | OR | 95% CI | P-value§ | ||
| Neopterin, ng/mL | 0.61 (0.50–0.72) | 2.436 | 0.935–6.339 | 0.068 | 2.558 | 0.781–8.377 | 0.121 |
| Tryptophan, μmol/L | 0.58 (0.49–0.68) | 0.216 | 0.037–1.249 | 0.087 | 0.614 | 0.054–6.924 | 0.693 |
| Kynurenine, μmol/L | 0.56 (0.46–0.67) | 0.524 | 0.190–1.444 | 0.212 | 0.474 | 0.119–1.887 | 0.289 |
| KTR, μmol/mmol | 0.51 (0.40–0.61) | 0.850 | 0.268–2.695 | 0.782 | 0.371 | 0.108–1.681 | 0.381 |
| PSA, ng/mL | 0.69 (0.60–0.78) | 3.144 | 1.681–5.880 | <0.001 | 3.292 | 1.706–6.352 | <0.001 |
| fPSA, % | 0.60 (0.51–0.70) | 0.574 | 0.268–1.228 | 0.152 | NA | NA | NA |
- †Predictors were log-transformed. ‡Obtained according to the DeLong method. §Multivariate models adjusted for neopterin, tryptophan, kynurenine, kynurenine-to-tryptophan ratio (KTR), and prostate-specific antigen (PSA). CI, confidence interval; fPSA, free PSA; HR, hazard ratio; NA, not adopted due to missing values.
Significant differences in neopterin, tryptophan, KTR, and PSA levels comparing healthy men, newly diagnosed PCa patients, and those with BCR after RP
The PSA concentrations (mean ± SD) were lowest in benign, non-cancer patients (4.3 ± 3.1 ng/mL), higher in first-diagnosed PCa patients (6.8 ± 5.2 ng/mL), and highest in those men with newly diagnosed BCR (173.8 ± 462.4 ng/mL, P < 0.001).
Similar to PSA, neopterin (P < 0.001) and KTR (P = 0.001) levels confirmed a significant increase with the highest levels in cases of advanced tumor disease with BCR (16.6 ± 20.7 nmol/L neopterin and 86.2 ± 60.4 μmol/mmol KTR). Conversely, serum tryptophan levels were lowest in PCa patients with BCR after RP (56.3 ± 12.3 μmol/L, P < 0.001) (Fig. 2a–d).
Focusing on PCa patients (n = 150, groups 2 and 3), BCR after RP was associated with higher neopterin (P < 0.001), PSA (P < 0.001), and KTR (P = 0.001) and lower tryptophan (P = 0.001) levels compared to patients with first-diagnosed PCa before RP (Fig. 2a–d).
There was a positive correlation between serum neopterin concentrations and KTR (rs = 0.512; P < 0.001), whereas neopterin correlated inversely with tryptophan levels (rs = −0.369, P < 0.001) and positively with kynurenine (rs = 0.252, P < 0.001).
Preoperative neopterin levels and tryptophan breakdown rates do not allow prediction of histopathological factors of RP specimens
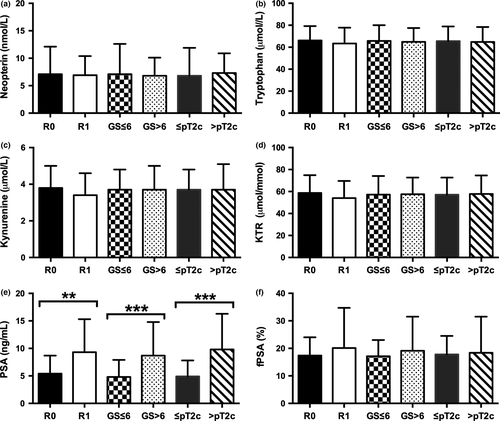
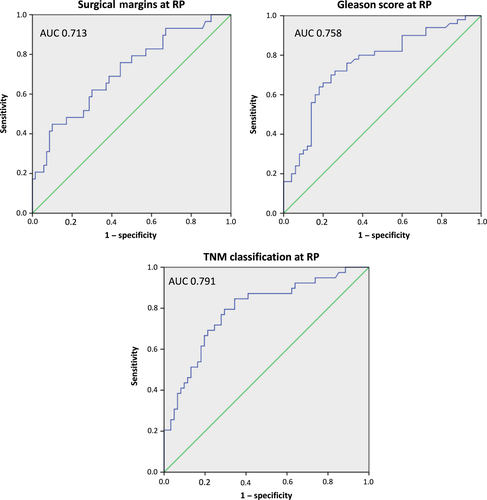
All 100 men with newly diagnosed PCa (group 2) underwent consecutive RP with simultaneous bilateral pelvic LND in 30% due to intermediate- or high-risk potential. In those men with LND, positive lymph node status (>2) was confirmed in 5 (16.7%) of 30 men with the necessity of consecutive androgen deprivation therapy. Positive surgical margins (R1) at RP were seen in 29%, while extracapsular extension was identified in 39% of 100 patients. Neopterin, tryptophan, kynurenine, KTR, and fPSA levels showed no significant differences concerning histopathological factors of RP specimens (Fig. 3a–d,f). Prostate-specific antigen was the only parameter showing significant differences in mean values (mean ± SD) concerning surgical margins at RP (R0 vs R1 status, 5.4 ± 3.3 vs 9.3 ± 6.5; P = 0.001), Gleason score (GS ≤ 6 vs GS > 6, 4.8 ± 3.1 vs 8.7 ± 6.1; P < 0.001) and pathological tumor stage (≤pT2c vs >pT2c, 4.9 ± 2.9 vs 9.8 ± 6.5; P < 0.001) at RP specimens (Fig. 3e). Moreover, R1 status (AUC = 0.713; 95% CI, 0.599–0.826; P = 0.001), higher GS (AUC = 0.758; 95% CI, 0.662–0.853; P < 0.001), and advanced tumor stage with extraprostatic involvement (AUC = 0.791; 95% CI, 0.699–0.883; P < 0.001) was predictable only by preoperative PSA levels, as shown in the receiver operating curve analysis (Fig. 4).
Increased neopterin levels at BCR after RP as an independent prognostic factor of CSS
Of the 50 patients with BCR, 13 (26%) men were alive after a median (range) survival time of 1 year (0–12 years). Twenty-eight (56%) patients died of tumor progression after a median (range) follow-up of 0.9 years (0–4.4 years) after cancer recurrence. In addition, 9 (18%) patients died of other non-cancer-specific causes. Tumor grading at RP specimens confirmed GS 6, GS 7, and GS 8–10 in 8 (16%), 16 (32%), and 26 (52%) men, respectively. Regarding pathological staging, pT2a, pT2b, pT2c, and pT3–4 was reported in 2 (4%), 3 (6%), 5 (10%), and 40 (80%) patients, respectively.
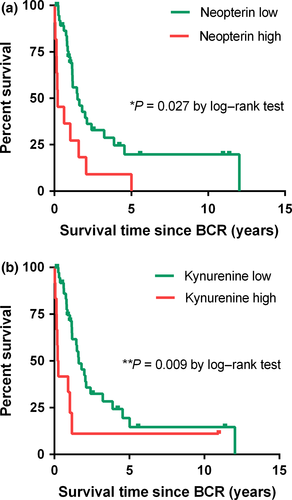
As described previously, serum levels were dichotomized for Kaplan–Meier survival analysis on the basis of the cut-off point of 15.7 nmol/L neopterin, 63.3 μmol/L tryptophan, 5.5 μmol/L kynurenine, 92.7 μmol/mmol KTR, 100.0 ng/mL PSA, and 33% fPSA, representing the third quartile of the observed distributions. Kaplan–Meier survival analysis revealed a significant negative association between neopterin levels and kynurenine levels at BCR and CSS (P = 0.027 for neopterin, P = 0.009 for kynurenine; log–rank test) (Fig. 5). Specifically, median CSS was 0.6 vs 1.8 years for high (>15.7 nmol/L) versus low (≤15.7 nmol/L) neopterin and 0.2 vs 1.9 years for high (>5.5 μmol/L) vs low (≤5.5 μmol/L) kynurenine, respectively. On univariate Cox proportional hazard analysis, neopterin (HR, 2.46; 95% CI, 1.08–5.61; P = 0.032) and kynurenine (HR, 2.93; 95% CI, 1.26–6.79; P = 0.012) were associated with a more than two-fold increased risk of cancer-specific death. When adjusting for other biomarkers, only neopterin was found to be an independent prognostic factor for CSS (HR, 2.56; 95% CI, 1.07–6.12; P = 0.035) (Table 2).
| Predictor† | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value‡ | |
| Neopterin, >15.7 vs ≤15.7 | 2.459 | 1.078–5.608 | 0.032 | 2.558 | 1.068–6.124 | 0.035 |
| Tryptophan, ≤63.3 vs >63.3 | 1.797 | 0.776–4.161 | 0.171 | 1.279 | 0.502–3.255 | 0.606 |
| Kynurenine, >5.5 vs ≤5.5 | 2.933 | 1.265–6.798 | 0.012 | 3.466 | 0.746–16.106 | 0.113 |
| KTR, >92.7 vs ≤92.7 | 1.985 | 0.837–4.707 | 0.119 | 0.673 | 0.145–3.133 | 0.614 |
| PSA, >100.0 vs ≤100.0 | 1.701 | 0.684–4.228 | 0.253 | 1.940 | 0.752–5.007 | 0.171 |
| fPSA%, ≤33 vs >33 | 1.362 | 0.290–6.388 | 0.695 | NA | NA | NA |
- †Predictors were dichotomized on the basis of the 3rd quartile of the observed distribution. ‡Multivariate models adjusted for neopterin, tryptophan, kynurenine, kynurenine-to-tryptophan ratio (KTR), and prostate-specific antigen (PSA). CI, confidence interval; HR, hazard ratio; NA, not adopted due to missing values.
Discussion
Neopterin levels reflect the stage of activation of the cellular immune reaction, playing an important role in the pathogenesis and prognosis of infections, rejection episodes after organ transplantation, and certain malignant diseases.19 Moreover, tryptophan metabolism is essential for cell proliferation, inflammation, and immunoregulation.20 Regarding the immunological aspect, IFN-γ was identified as the most relevant cytokine responsible for neopterin production by GTP-cyclohydrolase and for tryptophan breakdown by IDO.21, 22
Therefore, IDO activity coincides with increased neopterin production, confirming their common trigger for release,21, 23 although only neopterin formation is specific for monocyte-derived cells, whereas tryptophan breakdown is also inducible in a variety of other cells, like endothelial cells and fibroblasts and in various tumor cells.4, 23, 24 Concerning PCa, our data confirmed a statistically significant correlation between tryptophan metabolic changes and serum neopterin concentrations, suggesting IDO activity is responsible for tryptophan breakdown.
Due to the fact that tryptophan deprivation through IDO activation can also block the proliferation of T cells, IDO seems to also play an essential role regarding immune escape mechanisms for tumor cells and is one well-known molecule that contributes as an immunosuppressive effector mechanism of regulatory T cells.25, 26 Recently, a new subset of human CD14+ regulatory dendritic cells was identified, which suppressed antitumor immune response through CTLA-4-dependent IL-10 and IDO production in hepatocellular carcinoma patients, showing the importance of IDO in the mediation of suppressive effect of CD14+ dendritic cells.27 Consequently, blocking CTLA-4 in transgenic mice resulted in a significant reduction in PCa incidence, and those mice with PCa had a lower tumor grade.28 Lower tryptophan levels were closely related to rapid disease progression and shorter survival in melanoma patients.29 In leukemia patients, the IDO activity correlated with the tumor burden in the course of therapy.30 This data supports the view that a higher degree of IDO expression plays a crucial role in tumor progression and worse survival.31
Significant serum neopterin increase depends on the primary localization and the type of tumor, and mostly correlates with advanced tumor disease.32 Similar to the accelerated tryptophan degradation, cancer recurrence, progression, and tumor growth or incomplete resection of the tumor is associated with increasing neopterin levels in different cancer entities.33-35
Although IDO gene expression was more frequently detectable and quantitatively higher in PCa tissues compared to benign prostatic hyperplasia36 and IDO activity influenced tumor growth using a transgenic PCa animal model,37 only a few published clinical studies with a limited number of enrolled patients have discussed neopterin production and tryptophan degradation as a diagnostic and prognostic indicator in humans. For example, serum neopterin seemed to be closely related to the clinical course of disease in 102 PCa patients.38 Another study concluded that elevated serum neopterin (>10 mmol/L) at the time of cancer diagnosis had a higher risk of developing BCR (27% vs 7%) on follow-up with lower survival rates (P < 0.001).39 Therefore, serum neopterin levels may be considered in follow-up as well as in PCa prognosis. Our data add to earlier studies affirming the highest levels of neopterin and tryptophan breakdown in those PCa patients with BCR compared to healthy men and newly diagnosed PCa patients.
We confirmed no diagnostic value of serum neopterin and tryptophan degradation in predicting PCa on transrectal biopsy, neither on univariate nor on multivariate analysis. Our results are in line with other data confirming that high neopterin levels were not associated with a higher probability of PCa in biopsy.40 In addition, the kynurenine pathway cannot be considered as a novel serum biomarker for detecting PCa. These findings are in accordance with other results showing that tryptophan, kynurenine, and KTR levels were not statistically different in patients with diagnosed PCa compared to the control group,41 in contrast to other cancer entities.9, 29-31
Increased serum neopterin levels were reported to relate to advanced tumor stage.42 However, our results did not support this theory as to whether preoperative neopterin or tryptophan degradation concentrations correlated with histopathological aspects in RP specimens. Increased concentrations of neopterin and tryptophan breakdown indicate a poor prognosis and survival in different cancer entities.7, 29, 31, 34, 35 This fact is strongly supported by our results, confirming that increased serum neopterin at the time of biochemical recurrence was independently associated with a higher cancer-specific mortality. The obvious limitation of this pilot study is the heterogeneous study population and the limited sample size of 199 patients, with retrospectively evaluated oncological results. The small sample size of the biochemical recurrence group, resulting in limitation of statistical power, has to be considered particularly regarding the role of neopterin and tryptophan breakdown in survival analysis.
In conclusion, accelerated neopterin and tryptophan breakdown was associated with BCR after RP. In contrast to PSA, neither neopterin nor tryptophan breakdown were predictive determinants for histopathological factors at RP. Despite the absent predictive value in detecting PCa on biopsy, neopterin was the only independent prognostic factor for cancer-specific survival after BCR. These data may support the recommendation of neopterin measurement in patients with biochemical recurrence being helpful in making decision for further oncologic treatment strategies and in monitoring patients following BCR. These encouraging preliminary findings, especially in patients with BCR, call for further prospective trials with sufficient statistical power prior to drawing a final conclusion.
Acknowledgment
We thank Christian Murr for his support and helpful contribution to this work.
Disclosure Statement
The authors have no conflict of interest.
Abbrevations
-
- AUC
-
- area under the receiver operating characteristic curve
-
- BCR
-
- biochemical recurrence
-
- CI
-
- confidence interval
-
- CSS
-
- cancer-specific survival
-
- CTLA-4
-
- cytotoxic T-lymphocyte-associated protein 4
-
- fPSA%
-
- percentage of free prostate-specific antigen
-
- GS
-
- Gleason score
-
- HR
-
- hazard ratio
-
- IDO
-
- indoleamine 2,3-dioxygenase-1
-
- IFN-γ
-
- γ-interferon
-
- IL
-
- interleukin
-
- KTR
-
- kynurenine-to-tryptophan ratio
-
- LND
-
- lymph node dissection
-
- OR
-
- odds ratio
-
- PCa
-
- prostate cancer
-
- PSA
-
- prostate-specific antigen
-
- RP
-
- radical prostatectomy




