BRCA/Fanconi anemia pathway implicates chemoresistance to gemcitabine in biliary tract cancer
Funding Information:
Mitsui Life Social Welfare Foundation and Grant-in-Aid for Scientific Research(C).
Abstract
The BRCA/Fanconi anemia (FA) pathway plays a key role in the repair of DNA double strand breaks. We focused on this pathway to clarify chemoresistance mechanisms in biliary tract cancer (BTC). We also investigated changes in the CD24+/44+ population that may be involved in chemoresistance, as this population likely includes cancer stem cells. We used three BTC cell lines to establish gemcitabine (GEM)-resistant (GR) cells and evaluated the expression of BRCA/FA pathway components, chemoresistance, and the effect of BRCA/FA pathway inhibition on the CD24+/44+ population. FANCD2 and CD24 expression were evaluated in 108 resected BTC specimens. GR cells highly expressed the BRCA/FA components. The BRCA/FA pathway was upregulated by GEM and cisplatin (CDDP) exposure. Inhibition using siRNA and RAD51 inhibitor sensitized GR cells to GEM or CDDP. The CD24+/44+ population was increased in GR and parent BTC cells treated with GEM or CDDP and highly expressed BRCA/FA genes. FANCD2 was related to CD24 expression in resected BTC specimens. Inhibition of the BRCA/FA pathway under GEM reduced the CD24+/44+ population in MzChA1-GR cells. Thus, high expression of the BRCA/FA pathway is one mechanism of chemoresistance against GEM and/or CDDP and is related to the CD24+/44+ population in BTC.
Biliary tract cancer (BTC) is a malignancy with a poor prognosis and increasing incidence worldwide.1 Complete surgical resection is the only potentially curative therapy for patients with BTC;2-4 however, no effective chemotherapy is available for patients with unresectable BTC. Gemcitabine (GEM)-based chemotherapy is an standard treatment, and cisplatin (CDDP) plus GEM can prolong survival.1, 2, 5 However, no other first-line or second-line chemotherapies are available.6-9 Thus, it is important to explore the mechanism of GEM resistance in BTC.
In the present study, we focused on the DNA damage response (DDR) in several potential mechanisms of chemoresistance. Chemoresistance was recently reported to be associated with the DNA repair genes ERCC1, RAD21, MGMT, PARP1, PARP2, BRCA1, BRCA2, MSH2, MSH6, FAN1 and XRCC1 in various cancers, including lung, colon, breast, ovary and stomach cancer.10-16 GEM is a nucleoside analogue that inhibits DNA elongation and ribonucleotide reductase.17 In addition, GEM may contribute to DNA damage18 and cells may be sensitized by the inhibition of checkpoint kinase 1 (CHK1), which coordinates the DDR,19 suggesting that other DDR proteins are involved in chemoresistance.
The DDR generally protects against genomic instability, which enables cancer development.20, 21 Among the DDR-related genes, the BRCA/Fanconi anemia (FA) pathway genes play a role in homologous recombination repair (HRR), particularly the repair of fatal DNA double strand breaks,22 and are related to the development of several cancers. BRCA/FA pathway genes are well known tumor suppressors,21-23 but the percentage of mutations is limited.23 However, the downregulation of BRCA2 may cause radio-sensitization20 and chemosensitization.24, 25 We hypothesized that upregulation of BRCA/FA pathway components caused chemoresistance in BTC.
Our objective was to investigate the role of the BRCA/FA pathway in BTC, focusing on several key molecules in the BRCA/FA pathway. In addition to BRCA2, FANCD2 is a central gene in this pathway and associated with cell cycle control at the S/G2 checkpoint. RAD51c is a final factor in this pathway and directly repairs DNA damage in combination with other components.22 We also investigated the relationship between the DDR and CD24+/44+ population, which was reported as a candidate marker for extracting cancer stem cells (CSC) in BTC.26 Enrichment of CSC is a well-known mechanism of chemoresistance.27 The DDR works in stem cells28 and may contribute to the CSC-like population in several cancers.29, 30 Our results demonstrate that inhibition of the BRCA/FA pathway not only sensitizes BTC cells to GEM or CDDP, but also reduces the CD24+/44+ population in BTC.
Materials and Methods
Establishment of gemcitabine-resistant biliary tract cancer cells (MzChA1-GR, CCLP1-GR and KMCH1-GR)
Human BTC cell lines (MzChA1, CCLP1 and KMCH1) were kindly provided by Dr Gregory J. Gores of the Mayo Clinic, Rochester, MN, USA.31-34 The GEM-resistant MzChA1 cell line (MzChA1-GR) was recently established in our department.35 The primary MzChA1-GR cell line was developed through exposure to increasing concentrations of GEM (0.2–2.0 ng/mL) with repeated subculturing until the cells became fully resistant. Primary MzChA1-GR cells were cultured in GEM-free medium for 3 weeks prior to the next limiting dilution. After the primary MzChA1-GR cells were confirmed to be significantly more resistant to GEM than the parent cells, a single MzChA1-GR cell was seeded in a 96-well microplate by limiting dilution. Eight MzChA1-GR clones were established from the primary MzChA1-GR cell. To reduce the risk of contamination, we cultured each cell line separately with 6 months interval and each GR cell was also established by different two scientists. The MzChA1-GR cells were cultured under the same conditions as other cell lines, without GEM.35 The concordances of short tandem repeat were 21% in MzChA1 and MzChA1-GR, 81% in CCLP1 and CCLP1-GR, and 90% in KMCH1 and KMCH1-GR (BEX, Tokyo, Japan) like as the previous report included the data of STR changes by DNA damage drug treatment.36
The GEM-resistant CCLP1 cell line (CCLP1-GR) and the GEM-resistant KMCH1 cell line (KMCH1-GR) were developed through exposure to increasing concentrations of GEM (CCLP1, from 5 to 300 ng/mL; KMCH1, from 0.3 to 100 ng/mL) and established using the same method as MzChA1-GR.
Microarray analysis
DNA microarray analysis was performed using a 3D-Gene Human Oligo chip 25k (Toray Industries, Tokyo, Japan). We compared the MzChA1-parent and three MzChA1-GR clones. We determined that RRM1 and dCK mRNA levels were generally upregulated in all GR cells. The normalized data were used to identify genes whose expression appeared to be upregulated or downregulated.
Immunocytochemistry
Immunocytochemistry studies of γH2AX were performed using BTC cells. As a positive control, we also assessed BTC cells 4 h after 6 Gray irradiation using a Gamma Cell 40 Exactor (Nordion International, Ottawa, ON, Canada). Briefly, cells were cultured on six-well chamber slides, fixed with 4% paraformaldehyde, and permeabilized. The cells were then incubated with monoclonal mouse anti-γH2AX (diluted 1:500 [Millipore, Billerica, MA, USA]), followed by Alexa Fluor anti-mouse IgG conjugated to Alexa Fluor 488 (diluted 1:500 [Cell Signaling Technology, Danvers, MA, USA]). The slides were viewed by fluorescence microscopy (BZ-8000 [Keyence, Osaka, Japan]).
Flow cytometry for CD24, CD44 and CD133
The isolation of only CSC is difficult; therefore, we evaluated several populations that have been reported to contain a high concentration of CSC. In BTC, CD24, CD44 and CD133 have been reported to be markers for the selection of CSC-like populations.26, 37-40 To analyze the cell surface markers by flow cytometry, cells were resuspended in PBS with 1% FBS at a concentration of 106 cells/100 μL and incubated for 30 min at room temperature with 100-fold dilutions of the following antibodies: anti-CD24-phycoerythrin (BD Biosciences, Mississauga, ON, Canada, 555428), anti-CD44-fluorescein isothiocyanate (BD Biosciences, 559942) and anti-CD133/1-allophycocyanin (Miltenyi Biotec, Bergisch Gladbach, Germany 130-090-826). After incubation, the samples were washed twice with PBS containing 1% FBS and resuspended in PBS containing 1% FBS. Dead cells were eliminated by adding 4′,6-diamidino-2-phenylindole (final concentration 1 μg/mL; Sigma, Tokyo, Japan). Flow cytometric analysis was performed using a FACSAria (BD Immunocytometry System, Franklin Lakes, NJ, USA) after collecting and staining the cells as described earlier. The cells were routinely sorted twice and re-analyzed for purity, which was typically >90%.
Clinical samples
Biliary tract cancer samples (n = 108) were obtained from patients who underwent resection surgery at Osaka University Hospital, Japan between 2004 and 2012. All of the patients were diagnosed with BTC based on clinicopathological findings. Patient characteristics were collected prospectively by the Cancer Board and confirmed by the Clinico-Pathological Conference. The mean patient age was 63 ± 13 years, and the male-to-female ratio was 3:2. The main tumor locations were intrahepatic bile duct (n = 21), extrahepatic bile duct (n = 55), gallbladder (n = 16) and papilla of Vater (n = 16). Fifty-two (48%) patients had pathological lymph node metastasis. Resected specimens were formalin-fixed and preserved in paraffin blocks before immunohistochemistry. The use of resected samples was approved by the Human Ethics Review Committee of the Graduate School of Medicine, Osaka University. Written informed consent was obtained from all patients included in the study.
Classification of immunohistochemistry
FANCD2 and CD24 immunostaining were categorized as described previously.41, 42 Briefly, we considered samples to be FANCD2-negative when the staining area in the nucleus was <1% or FANCD2-positive if the staining area was 1–25% (weak), 25–50% (moderate) or ≥50% (strong). We considered samples as CD24-negative when the staining area was 0% or CD24-positive if the staining area was <20% (weak), 20–50% (moderate) or ≥50%. FANCD2 and CD24 immunostaining was classified by two authors (S. N and S. K) with agreement in all cases.
Statistical analysis
All data were expressed as the mean ± standard deviation of at least three independent experiments. Statistical analyses were performed using Student's t-test or Fisher's exact test for categorical data. The unpaired Student's t-test was used to examine differences in the growth inhibitory effects in vitro. P-values < 0.05 were considered significant.
Results
Establishment and characteristics of gemcitabine-resistant biliary tract cancer cells
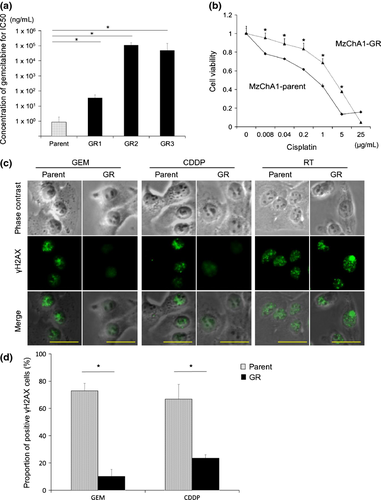
We confirmed that MzChA1-GR cells maintained GEM-resistance compared to MzChA1-parent cells. Three MzChA1-GR clones were resistant to GEM (IC50 >100 ng/mL; P < 0.001; Fig. 1a). MzChA1-GR cells were also resistant to CDDP (IC50, 3.18 μg/mL; P < 0.001; Fig. 1b). We evaluated γH2AX expression, a critical event in the mammalian DDR, 72 h after GEM (1.0 ng/mL) or CDDP (0.03 μg/mL) exposure. The positive control for γH2AX expression was irradiated cells. γH2AX was expressed at higher levels, and more cells formed nuclear foci in MzChA1-parent cells compared to GR cells (Fig. 1c,d). Thus, DNA damage was reduced in GR cells. Proliferation was more rapid in GR cells (Fig. S1a, P < 0.01) and the cell cycle distribution was not different between MzChA1-parent and GR cells (Fig. S1b). Five CCLP1-GR clones and three KMCH1-GR clones were established and resistant to GEM, as well as CDDP (Fig. S2, P < 0.001). The morphologies of MzChA1-GR, KMCH1-GR and CCLP1-GR cells were spindle-shaped and disconnected (Fig. 3).
Microarray analysis of MzChA1-parent and gemcitabine-resistant cells
In a microarray analysis of MzChA1-parent and MzChA1-GR cells, we evaluated the expression of genes involved in the major mechanisms of the DNA repair pathway (homologous recombination repair, non-homologous end joining, direct reversal repair, mismatch repair, base excision repair and nucleotide excision repair).43 Almost all components of the BRCA/FA pathway were upregulated in MzChA1-GR cells (Table 1).
| Parent | GR1 | GR2 | GR3 | Fold change | |
|---|---|---|---|---|---|
| FANCA | 11 | 20 | 19 | 16 | 1.64 |
| FANCB | 8 | 32 | 24 | 31 | 3.53 |
| FANCC | 30 | 40 | 44 | 41 | 1.40 |
| FANCD1 (BRCA2) | 14 | 48 | 37 | 41 | 3.03 |
| FANCD2 | 3 | 10 | 9 | 9 | 3.55 |
| FANCE | 8 | 12 | 12 | 15 | 1.71 |
| FANCF | 95 | 93 | 116 | 125 | 1.17 |
| FANCG | 18 | 55 | 45 | 53 | 2.80 |
| FANCI | 76 | 534 | 401 | 504 | 6.35 |
| FANCJ (BRIP1) | 3 | 11 | 11 | 7 | 3.50 |
| FANCL | 157 | 305 | 239 | 193 | 1.57 |
| FANCM | 2 | 7 | 5 | 5 | 3.54 |
| FANCN (PALB2) | 126 | 167 | 155 | 171 | 1.31 |
| FANCP (SLX4) | 41 | 36 | 34 | 38 | 0.87 |
| FANCO (RAD51c) | 44 | 77 | 74 | 80 | 1.75 |
Expression of BRCA/Fanconi anemia pathway mRNA and proteins in gemcitabine-resistant biliary tract cancer cells
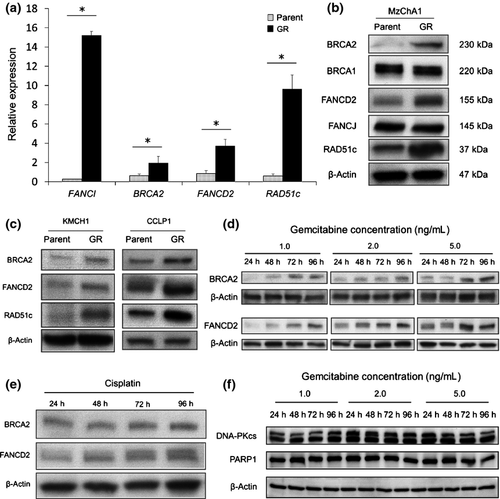
We confirmed the expression of BRCA2, FANCD2, FANCI and RAD51c mRNA in MzChA1-parent and MzChA1-GR cells. We used the gene-specific oligonucleotide primers provided in Table S1. All mRNA were expressed at significantly higher levels in MzChA1-GR cells than MzChA1-parent cells (P < 0.001; Fig. 2a). Western blot analysis revealed that the expression of BRCA2, FANCD2 and RAD51c proteins was elevated in MzChA1-GR cells (Fig. 2b). We confirmed that these proteins were also elevated in CCLP1-GR and KMCH1-GR cells (Fig. 2c). Accordingly, long-term GEM exposure (i.e. in resistant cells) elevated mRNA and protein levels in the BRCA/FA pathway.
Expression of BRCA/Fanconi anemia pathway components in parent biliary tract cancer cells after gemcitabine or cisplatin exposure
We evaluated changes in BRCA/FA proteins in MzChA1 cells after GEM exposure. The MzChA1 cells were treated with concentrated GEM (1.0, 2.0 or 5.0 ng/mL) for 24, 48, 72 and 96 h. Total proteins were extracted for immunoblotting of BRCA2 and FANCD2. The expression of BRCA2 and FANCD2 protein increased in a time-dependent and dose-dependent manner (Fig. 2d). The expression of these two proteins also increased after CDDP treatment (0.03 μg/mL) (Fig. 2e). However, we did not observe gradual higher expression of PARP1 and DNA-dependent protein kinase catalytic subunit (DNA-PKcs), which are involved in other DNA repair pathways (Fig. 2f). Taken together, the results indicate that short-term GEM or CDDP exposure increases the expression of the BRCA/FA pathway in the DNA repair system.
Effect of inhibiting the BRCA/Fanconi anemia pathway on chemoresistance
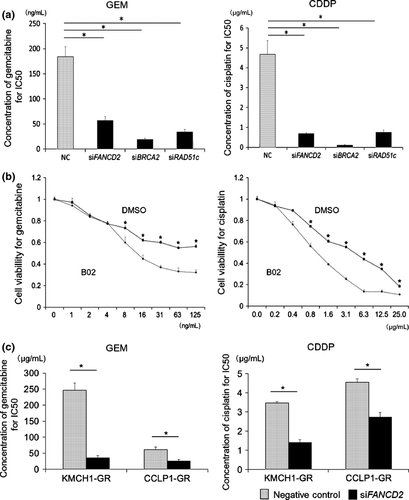
Next, we assessed BRCA2, FANCD2 and RAD51c mRNA and protein expression using MzChA1-GR cells transfected with siRNA (siFANCD2, siBRCA2, siRAD51c). The expression of all mRNA and proteins was inhibited in transfected GR cells (Fig. S4). Inhibition of the BRCA/FA pathway sensitized cells to not only GEM but also CDDP (Fig. S5), and reduced the IC50 for GEM (NC, 183.8 ng/mL; siFANCD2, 57.1 ng/mL, P < 0.001; siBRCA2, 38.4 ng/mL, P < 0.001; siRAD51c, 48.8 ng/mL, P < 0.001; Fig. 3a, left) and CDDP (NC, 4.67 μg/mL; siFANCD2, 0.69 μg/mL, P < 0.001; siBRCA2, 0.11 μg/mL, P < 0.001; siRAD51c; 0.77 μg/mL, P < 0.001; Fig. 3a, right). Inhibition of RAD51c using B02, a specific RAD51 inhibitor binding nucleoprotein filament at the site of damaged DNA,44 also reduced the IC50 for GEM and CDDP (13.0 ng/mL and 1.05 μg/mL, respectively, P < 0.001; Fig. 3b). The concentration of the inhibitor was determined as the maximum dose that did not influence proliferation (Fig. S6). We confirmed that inhibition of FANCD2 by siRNA (Fig. S7) reduced the IC50 for GEM and CDDP in KMCH1-GR and CCLP1-GR cells (KMCH1-GR, 35.8 and 1.40 μg/mL, P < 0.001; CCLP1-GR, 25.6 and 2.73 μg/mL, P < 0.001; Fig. 3c). Thus, silencing the BRCA/FA pathway sensitized GR cells to GEM or CDDP.
CD24+/44+ population in parent and gemcitabine-resistant cells
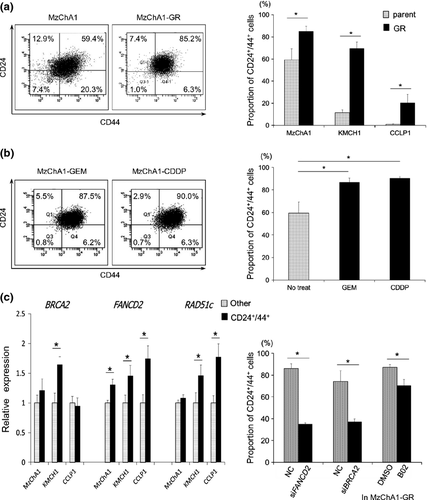
Using FACS, the proportion of CD24+/44+ cells was higher in MzChA1-GR cells than MzChA1-parent cells (P = 0.035; Fig. 4a). The same results were observed in KMCH1-GR and CCLP1-GR cells (KMCH1-GR, P < 0.001; CCLP1-GR, P = 0.048; Fig. 4a, right). CD133+ cells were not detected in MzChA1-parent and MzChA1-GR cells, and few were detected in KMCH1 (0.2%) and KMCH1-GR cells (1.4%) (Fig. S8). The CD24+/44+ population was increased in MzChA1 cells after GEM or CDDP exposure for 72 h compared to no treatment (GEM, P = 0.030; CDDP, P = 0.031; Fig. 4b). With short-term and long-term exposure to GEM, the CD24+/44+ population was increased in BTC cells.
BRCA/Fanconi anemia pathway in CD24+/44+ biliary tract cancer cells
Next, MzChA1, CCLP1 and KMCH1 cells were sorted by CD24+/44+ expression. BRCA2, FANCD2 and RAD51c mRNA levels were determined in the CD24+/44+ population and other populations (CD24− or CD44−) by qRT-PCR. BRCA2 mRNA was expressed at significantly higher levels in the CD24+/44+ population of KMCH1 cells (P = 0.013; Fig. 4c). FANCD2 mRNA levels were also higher in the CD24+/44+ populations of MzChA1 (P = 0.030), CCLP1 (P = 0.027) and KMCH1 (P = 0.045; Fig. 4c) cells. RAD51c mRNA levels were higher in the CD24+/44+ population of CCLP1 (P = 0.026) and KMCH1 (P = 0.047; Fig. 4c) cells. Therefore, BRCA/FA genes were expressed at higher levels in the CD24+/44+ population compared to the other population.
Effect of inhibiting the BRCA/Fanconi anemia pathway on the CD24+/44+ population
The proportion of CD24+/44+ cells decreased in MzChA1-GR cells transfected with siRNA and treated with GEM compared to MzChA1-GR cells transfected with scrambled oligonucleotide siRNA (siFANCD2, P = 0.001; siBRCA2, P = 0.018; Fig. 4d). The CD24+/44+ population was also decreased in MzChA1-GR cells treated with GEM and B02 (P = 0.019; Fig. 4d).
Expression of FANCD2 and CD24 in resected specimens
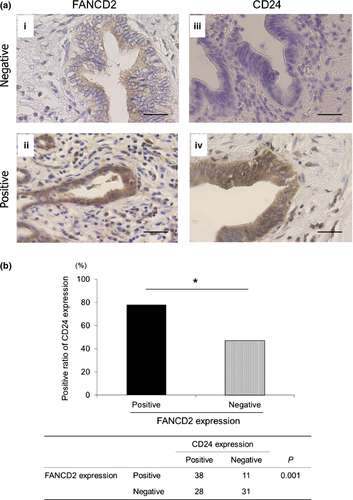
We evaluated FANCD2 staining in the nucleus and CD24 staining in the cytoplasm. Positive reactivity for FANCD2 was observed in 49 (45%) BTC specimens (Fig. 5a[i,ii]), whereas 66 (61%) BTC specimens were positive for CD24 (Fig. 5a[iii,iv], c and d). FANCD2 expression corresponded with CD24 expression (P = 0.001; Fig. 5b).
Discussion
Our data show that the BRCA/FA pathway is upregulated by short-term and long-term exposure to GEM, which contributes to GEM and/or CDDP resistance, and is closely related to the CD24+/44+ population. The main mechanism of action of GEM is inhibition of DNA elongation and ribonucleotide reductase,17 and GEM has been reported to cause DNA double strand breaks.18 DNA double strand breaks are also thought to be caused by radiation, topoisomerase inhibitors and DNA interstrand cross-linking (ICL) agents, such as CDDP or mitomycin C.21 Our data on γH2AX expression confirm that GEM, and CDDP, induces DNA double strand breaks.45, 46 A recent report showed that CHK1, which coordinates the DDR, is essential for GEM sensitization,19 and our data support GEM being influenced by the DDR system. Two mechanisms are known for the repair of DNA double strand breaks: HRR and non-homologous end joining. The former involves the BRCA/FA pathway and specifically repairs “fatal DNA double strand breaks.”22 As BRCA-silenced cells were reported to be sensitized to not only CDDP, but also GEM,47 the BRCA/FA pathway would be necessary for the DDR after GEM or CDDP exposure, and this was supported by our results of silencing BRCA2, FANCD2 and RAD51c. In contrast, short-term and long-term exposure to GEM induced temporal and/or sustained elevated expression of BRCA/FA pathway genes, which could induce co-resistance against CDDP as in previous reports of lung cancer and pancreatic cancer.48-50 Notably, all of our BTC cells expressed BRCA/FA pathway genes. In BRCA2-mutated cancer cells, our speculation would be inappropriate. Thus, we need to address drug selection for first-line and second-line chemotherapies in cases of GEM-refractory BTC.
In this study, we also investigated the relationship between the BRCA/FA pathway and the CD24+/44+ population in BTC. The DDR should work in stem cells to escape “fatal DNA damage.”28 To address whether the DDR would work in CSC in BTC, we investigated the expression of BRCA/FA pathway genes in the CD24+/44+ population and how silencing BRCA/FA pathway genes affects these cells, as recent reports support the presence of highly concentrated CSC in the CD24+/44+ population in BTC.26, 27, 39 Our data show that the expression of BRCA/FA pathway components was elevated in the CD24+/44+ population and silencing of the BRCA/FA pathway contributed to a decrease in the CD24+/44+ population. The DDR may contribute to CSC-like populations in several cancers;29, 30 thus, HRR may work in the CD24+/44+ population in BTC. Taken together, our results indicate that GEM exposure leads to enrichment of the CD24+/44+ population highly expressing BRCA/FA genes in BTC, which would cause co-resistance to GEM and CDDP.
Does regulation of the DDR contribute to a new strategy for treating BTC? Several phase I studies have started evaluating the DDR.21 Recent reports show that inhibition of the BRCA/FA pathway by siRNA (e.g. FANCF, BRCA1, and BRCA2) is effective in treating breast and ovarian cancers.51, 52 Inhibiting the BRCA/FA pathway and decreasing the CD24+/44+ population in GEM-resistant BTC cells could be a new treatment for BTC, although we need to consider the influence on somatic cells and the drug delivery system. In conclusion, high expression of the components of the BRCA/FA pathway is one of the mechanisms of chemoresistance to GEM and/or CDDP and is related to the CD24+/44+ population in BTC.
Acknowledgments
This work was supported by the Mitsui Life Social Welfare Foundation and a Grant-in-Aid for Scientific Research (C).
Disclosure Statement
The authors have no conflict of interest to declare.




