Nuclear export signal within CALM is necessary for CALM-AF10-induced leukemia
Abstract
The CALM–AF10 fusion gene, which results from a t(10;11) translocation, is found in a variety of hematopoietic malignancies. Certain HOXA cluster genes and MEIS1 genes are upregulated in patients and mouse models that express CALM-AF10. Wild-type clathrin assembly lymphoid myeloid leukemia protein (CALM) primarily localizes in a diffuse pattern within the cytoplasm, whereas AF10 localizes in the nucleus; however, it is not clear where CALM-AF10 acts to induce leukemia. To investigate the influence of localization on leukemogenesis involving CALM-AF10, we determined the nuclear export signal (NES) within CALM that is necessary and sufficient for cytoplasmic localization of CALM-AF10. Mutations in the NES eliminated the capacity of CALM-AF10 to immortalize murine bone-marrow cells in vitro and to promote development of acute myeloid leukemia in mouse models. Furthermore, a fusion of AF10 with the minimal NES can immortalize bone-marrow cells and induce leukemia in mice. These results suggest that during leukemogenesis, CALM-AF10 plays its critical roles in the cytoplasm.
The chromosome translocation t(10;11)(p13;q14), found in T-cell acute lymphoblastic leukemia (T-ALL), acute myeloid leukemia (AML) and malignant lymphomas, results in the fusion of the clathrin assembly lymphoid myeloid leukemia protein (CALM) and AF10 1, 2. The CALM–AF10 fusion protein consists of almost all CALM and AF10 proteins, with the exception of one or two plant homeodomain (PHD).1, 3 AF10, also known as MLLT10, interacts with the transcription factor Ikaros and H3K4me3 through its octapeptide motif–leucine zipper (OM-LZ) region and PHD domains, respectively.4-6 In both mice and humans, CALM-AF10 upregulates certain HOXA cluster genes (HOXA5, HOXA7, HOXA9 and HOXA10) and MEIS1.7, 8 Hoxa5 upregulation, which is critical for CALM-AF10-induced leukemogenesis,9, 10 is mediated by an interaction between the AF10 OM-LZ region and the histone methyltransferase DOT1L, resulting in H3K79 hypermethylation at the Hoxa5 locus.9 These findings suggest that CALM-AF10 might function in the nucleus.
The CALM protein shuttles between the cytoplasm and the nucleus under the control of a CRM1-dependent nuclear export signal (NES).11 In contrast to AF10, which localizes in the nucleus,4 CALM-AF10 primarily localizes in the cytoplasm.6, 9 Other fusion partners of AF10 in acute myeloid and lymphoid leukemias include MLL, DDX3 and HNRNPH1.12, 13 MLL and hnRNPH1 primarily localize in the nucleus,14, 15 whereas DDX3, like CALM, is mostly distributed throughout the cytoplasm.16, 17 These observations prompted us to investigate whether CALM-AF10 exerts its function in the nucleus or the cytoplasm. We found that mutant CALM-AF10 lacking the NES localized in the nucleus and lost its ability to induce leukemia in mice. Conversely, a fusion consisting of the minimal NES and AF10 localized in the cytoplasm and induced leukemia. These results indicate that the cytoplasmic location of CALM-AF10 is critical for its role in leukemogenesis.
Materials and Methods
Generation of CALM-AF10 mutant constructs
Plasmid encoding pcDNA3β-FLAG-CALM-AF10 was a gift from Y. Zhang (Department of Biochemistry and Biophysics, University of North Carolina).9 Plasmid encoding the NES-deficient mutant FLAG-CALMNES4A-AF10 and FLAG-CALMNES4A were generated by introducing four point mutations into the CALM NES sequence by inverse PCR using a site-specific mutagenesis kit (Toyobo, Osaka, Japan).18 Specifically, leucine (L)-544, L-547, L-551 and isoleucine (I)-553 in the putative NES sequence within CALM-AF10 were replaced by alanine (A), using the following primers: L544A, 5′-GCAGCCAACCTTGTGGGCAATCTTGGC-3′, and 5′-AGATGAATCCAAGTCATCAGATACT-3′; L547A, 5′-GCTGTGGGCAATCTTGGCATCGGAAAT-3′, and 5-′GTTGGCTGCAGATGAATCCAAGTCA-3′; L551A, 5′-GCTGGCATCGGAAATGGAACCACTAAG-3′, and 5′-ATTGCCCACAGCGTTGGCTGCAGAT-3′; I553A, 5′-GCCGGAAATGGAACCACTAAGAATGAT-3′, and 5′-GCCAGCATTGCCCACAGCGTTGGCT-3′. All mutations were confirmed by DNA sequencing. The AF10 sequence encoding amino acids 81–1027 (mAF10) was amplified by PCR and cloned into pcDNA3β-FLAG. To generate the fusion of the minimal NES with AF10, NES1 (amino acids 543–554) or NES2 (amino acids 539–558) within CALM was generated by PCR amplification using FLAG-CALM-AF10 as a template, and then cloned into pcDNA3β-FLAG-mAF10. The pMY-IG-FLAG-CALM-AF10-IRES-GFP, pMY-IG-FLAG-CALMNES4A-AF10-IRES-GFP, pMY-IG-FLAG-mAF10-IRES-GFP and pMY-IG-FLAG-NES2-AF10-IRES-GFP constructs were generated by inserting the corresponding cDNA into the pMY-IG/IRES-GFP vector.
Cell culture and transfection
COS-7 cells were maintained in DMEM supplemented with 10% FBS, 100 U/mL penicillin, 100 μg/mL streptomycin and 2 mM l-glutamine at 37°C in a humidified 5% CO2 incubator. COS-7 cells were transfected with pcDNA3β-FLAG constructs using the Effectene Transfection Reagent (Qiagen, Hilden, Germany).
Immunofluorescence analysis
Forty-eight hours after transfection, COS-7 cells transfected with pcDNA3β-FLAG constructs were fixed with 3.7% formaldehyde in PBS and examined by immunofluorescence staining with anti-FLAG M2 monoclonal antibody (Sigma-Aldrich, St. Louis, MO, USA), followed by secondary Alexa Fluor 488-conjugated goat anti-mouse IgG (Invitrogen, Carlsbad, CA, USA). Stained cells were mounted in VECTASHIELD mounting medium and observed using a BX50 fluorescence microscope (Olympus, Tokyo, Japan). Cytospins of murine bone-marrow cells transduced with pMY-IG/IRES-GFP viral constructs encoding FLAG-CALM-AF10, FLAG-CALMNES4A-AF10, FLAG-NES2-AF10 or FLAG-mAF10 were fixed with 4% paraformaldehyde and stained with anti-FLAG M2 monoclonal antibody (Sigma-Aldrich) and anti-KMT4/DOT1L polyclonal rabbit antibody (Abcam, Cambridge, MA, USA), followed by secondary Alexa Fluor 568-conjugated goat anti-mouse IgG (Invitrogen) and Alexa Fluor 488-conjugated goat anti-rabbit IgG (Invitrogen), respectively. Stained bone-marrow cells were mounted in VECTASHIELD mounting medium with DAPI (Vector Laboratories, Burlingame, CA, USA) or Prolong Gold (Invitrogen) and observed under a BZ-9000 fluorescence microscope (Keyence Corporation, Osaka, Japan) or a FluoView FV10i confocal laser scanning microscopy (Olympus).
Retroviral infection and bone-marrow transplantation
C57BL/6J mice were purchased from CLEA Japan (Tokyo, Japan). All mouse experiments were approved by the National Cancer Center Animal Ethics Committee and performed in accordance with the institutional guidelines. The pMY-IG/IRES-GFP constructs encoding FLAG-CALM-AF10, FLAG-CALMNES4A-AF10, FLAG-NES2-AF10 or FLAG-mAF10 were transfected into PLAT-E cells using the GeneJuice transfection reagent (Novagen, Nottingham, UK), and retrovirus supernatants were collected 48 h after transfection. c-kit+ cells (1 × 105 cells), selected from murine total bone-marrow cells using CD117 MicroBeads (Miltenyi Biotec, Bergisch Gladbach, Germany), were incubated with the retrovirus and RetroNectin (Takara Bio, Madison, WI, USA) for 24 h in StemPro-34 SFM medium (Invitrogen) containing cytokines (20 ng/mL SCF, 10 ng/mL IL-6 and 10 ng/mL IL-3). The transduced donor bone-marrow cells were then transplanted into lethally irradiated (9.5 Gy) 7–8-week-old female C57BL/6J recipient mice by intravenous injection. For secondary transplants, bone-marrow cells from the primary leukemia mice were intravenously injected into sublethally irradiated (6 Gy) female C57BL/6J mice.
Serial-replating assay
Bone-marrow cells transduced with pMY-IG/IRES-GFP constructs encoding FLAG-CALM-AF10, FLAG-CALMNES4A-AF10, FLAG-NES2-AF10 or FLAG-mAF10 were cultured for 3 days in methylcellulose medium (MethoCult M3234; StemCell Technologies, Vancouver, Canada) supplemented with murine SCF, IL-3 and GM-CSF. The GFP+ cells in methylcellulose medium were then sorted using a JSAN cell sorter (Bay Bioscience, Kobe, Japan) and replated every 3–4 days in methylcellulose medium; colonies and cells were counted at each passage. Cells from the second-round and fifth-round colonies were harvested and analyzed by real-time PCR (RT-PCR).
Real time-PCR analysis
Total RNA from replating colonies was purified using an RNeasy Mini Kit (Qiagen). Purified RNA were reverse-transcribed into cDNA using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA). Real-time PCR was performed using the 7500 Fast Real-time PCR System (Applied Biosystems) using the FastStart Universal Probe Master with ROX (Roche, Basel, Switzerland) and the following TaqMan probes (Applied Biosystems): Hoxa5 (Mm04213381_s1), Hoxa7 (Mm00657963_m1), Hoxa9 (Mm00439364_m1), Hoxa10 (Mm00433966_m1) and Meis1 (Mm00487664_m1). The relative expression levels of these genes were normalized against the level of Actb (Mm00607939_s1).
Flow-cytometry analysis
Bone-marrow cells from leukemic mice were pre-incubated with rat IgG (Sigma-Aldrich), and then incubated on ice with the appropriate staining reagents: anti-CD115(CSF1R)-PE (eBioscience, San Diego, CA, USA), anti-Mac-1(M1/70)-PE-Cy7 (eBioscience), anti-Gr-1(RB6-8C5)-APC (BD Pharmingen, San Diego, CA, USA) and anti-c-Kit(2B8)-APC-eF780 (eBioscience). FACS analysis and cell sorting were performed using the JSAN cell sorter and the results were analyzed using the flowjo software (Tree Star, Standford, CA, USA).
Results
The nuclear export signal within CALM is required for cytoplasmic localization of CALM-AF10
To investigate the role of subcellular localization of CALM-AF10 in leukemogenesis, we focused on the NES within the CALM portion of the fusion protein (amino acids 543–554 of CALM).9 We generated NES-deficient mutants CALMNES4A-AF10 and CALMNES4A, in which leucine-544, leucine-547, leucine-551 and isoleucine-553 in the putative NES region within CALM were substituted with alanines (NES4A) (Fig. 1a). Expression vectors for FLAG-tagged CALM-AF10, CALM, CALMNES4A-AF10, CALMNES4A and mAF10 (the AF10 portion of CALM-AF10) were transiently transfected into COS-7 cells. Immunofluorescence analysis revealed that CALM and CALM-AF10 primarily localized in the cytoplasm, whereas mAF10 and the NES mutants CALMNES4A-AF10 and CALMNES4A localized in the nucleus (Fig. 1b,c).
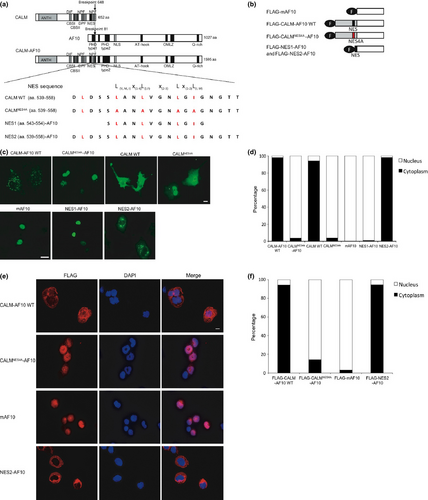
To determine the minimal NES, two sequences, NES1 (aa. 543–554) and NES2 (aa. 539–558), were fused to AF10 (Fig. 1a,b). As with mAF10, NES1-AF10 was in the nucleus; by contrast, NES2-AF10 was in the cytoplasm (Fig. 1d). The same results were obtained when these fusion proteins were transduced into murine hematopoietic progenitor cells by retroviral infection (Fig. 1e). These results indicate that NES1 is not sufficient, but its flanking regions including leucine-540 are necessary for cytoplasmic localization of CALM-AF10. Thus, we concluded that the NES2 region is the minimal NES that mediates cytoplasmic localization of CALM-AF10.
The nuclear export signal within CALM is necessary for CALM-AF10-induced immortalization of cells in vitro
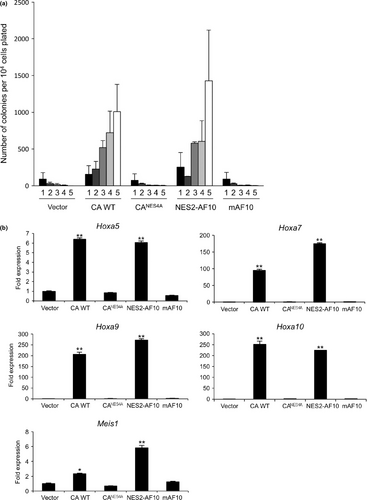
We next investigated whether the NES within CALM-AF10 is required for leukemogenesis. To this end, primary murine bone-marrow stem/progenitor cells (HSPC) were infected with retrovirus encoding CALM-AF10, CALMNES4A-AF10, NES2-AF10 and mAF10. Serial-replating assays revealed that both CALM-AF10 and NES2-AF10 immortalized HSPC, and that the cells formed colonies for at least five rounds of replating (Fig. 2a). By contrast, neither mAF10 nor CALMNES4A-AF10, which lacks a functional CALM NES, could immortalize cells. Transduced cells with elevated colony-forming abilities also exhibited upregulation of the Hoxa cluster (Hoxa5, Hoxa7, Hoxa9 and Hoxa10) and Meis1 genes (Fig. 2b)7, 9. These results indicated that the CALM NES is necessary for CALM-AF10 to immortalize hematopoietic stem/progenitor cells.
The nuclear export signal within CALM-AF10 is necessary to induce leukemia in vivo
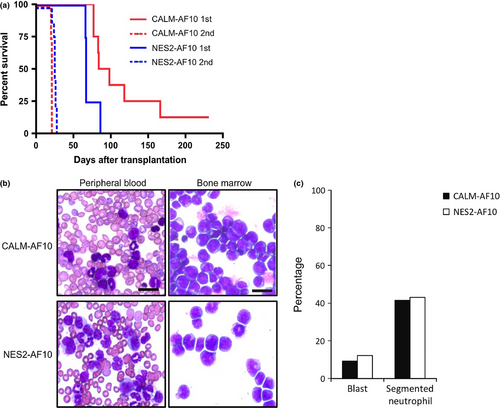
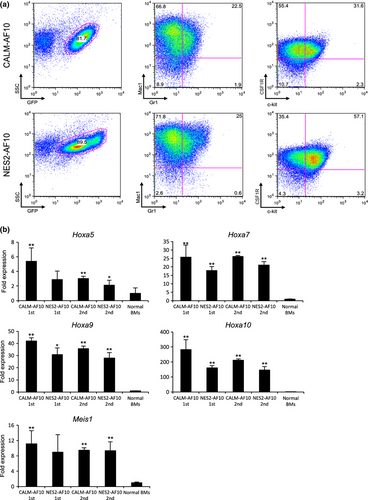
To determine whether CALM-AF10 and NES2-AF10 can induce leukemia in mice, we injected bone-marrow progenitor cells transduced with CALM-AF10 and NES2-AF10 into lethally irradiated mice. Seven out of eight mice transplanted with cells expressing CALM-AF10 developed leukemia within 6 months after transplantation (Fig. 3a), and all mice transplanted with cells expressing NES2-AF10 developed leukemia within 3 months after transplantation. When cells prepared from bone marrow of these leukemic mice were transplanted into secondary recipient mice, all recipients promptly developed leukemia (medians: CALM-AF10 donors, 21 days [n = 4]; NES2-AF10 donors, 25 days [n = 9]). Morphological analysis revealed large populations of blast cells in leukemic mice receiving cells transduced with either CALM-AF10 or NES2-AF10 (Fig. 3b,c). Flow cytometry analysis showed that cells expressing CALM-AF10 and NES2-AF10 in the bone marrow cells of primary recipient mice were Mac1+, CSF1R+ and c-kit+ (Fig. 4a). Moreover, as shown in Figure 4b, Hoxa5, Hoxa7, Hoxa9, Hoxa10 and Meis1 expression levels were upregulated in cells expressing CALM-AF10 and NES2-AF10 compared with normal bone marrow cells, although upregulation of Hoxa5 and Meis1 in primary recipient mice harboring NES2-AF10 was not significant (P = 0.084 and P = 0.093, respectively). These data demonstrate that the NES within CALM-AF10 is a critical element for induction of leukemia.
It has been reported that CALM-AF10 interacts with the histone methyltransferase DOT1L to mediate H3K79 hypermethylation at the Hoxa5 locus.9 To determine whether Dot1L colocalizes with CALM-AF10 and NES2-AF10 in the leukemia cells, we performed immunofluorescence analysis. Dot1L mainly localized in the nucleus while CALM-AF10 and NES2-AF10 mainly localized in the cytoplasm (Fig. 5a,b). Dot1L partially colocalized with both CALM-AF10 and NES2-AF10, but neither CALM-AF10 nor NES2-AF10 altered the localization of Dot1L (Fig. 5a).
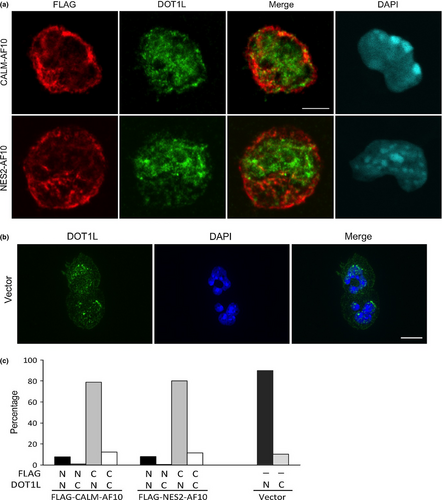
Discussion
AF10 and CALM localize diffusely in the nucleus and cytoplasm, respectively, whereas CALM-AF10 primarily localizes in cytoplasmic speckle domains (see Fig. 1c,d). The fact that CALM-AF10 regulates histone methylation at the Hoxa5 locus suggests that CALM-AF10 is likely to function in the nucleus.9 However, the results described here indicate that the CALM NES is essential for CALM-AF10-induced leukemogenesis, suggesting that cytoplasmic localization (or shuttling between nucleus and cytoplasm) is critical for the function of CALM-AF10. During the preparation of the manuscript, another group reported similar findings,19 indicating that the results are reproducible and the conclusions can be validated using alternative experimental systems.
The molecular mechanism by which CALM-AF10 exerts its function in the cytoplasm remains unclear, but two possibilities are consistent with the existing data: CALM-AF10 may affect cytoplasmic signaling pathways that regulate expression of its target genes, including HoxA cluster genes; alternatively, CALM-AF10 may affect the functions of transcriptional regulators by changing their localization from the nucleus to the cytoplasm. DOT1L, a candidate mediator of CALM-AF10-induced leukemia, interacts with AF10 and induces H3K79 hypermethylation at Hoxa5.9 However, our present data suggest that CALM-AF10 and NES2-AF10 did not affect the localization of Dot1L (see Fig. 5a). It is possible that CALM-AF10 squelches DOT1L inhibitors by exporting them to the cytoplasm.
CALM plays an important role in clathrin-mediated endocytosis.17, 20, 21 It and other endocytosis-related genes, such as EPS15, EEN, CLTC and HIP1, are involved in multiple types of leukemia-associated chromosomal translocations (e.g. MLL-CALM, MLL-EPS15, MLL-EEN, CLTC-ALK and HIP1-PDGFBR),22-26 suggesting that these leukemia-associated fusions might affect endocytosis in a manner that contributes to leukemogenesis. However, recent reports have shown that the clathrin-binding domain of CALM is not essential for CALM-AF10-mediated leukemogenesis.27, 28 Here, we show that nuclear export of CAL-AF10 is critical for the leukemogenesis. Because the endocytosis-related proteins mentioned above are also exported from the nucleus to the cytoplasm, as in the case of CALM,11, 23, 29 it is possible that changes in the localization of fusions involving endocytosis-related proteins have some shared consequence that is important for leukemogenesis.
Molecular exchange between the nucleus and cytoplasm takes place through nuclear pore complexes (NPC). Fusion proteins containing NUP98 and NUP214, which are components of the NPC, have been found in AML and T-ALL;30, 31 as in cells expressing CALM-AF10, a set of Hoxa and Meis1 genes are upregulated in leukemia cells expressing these NUP98 fusions and NUP214 fusions.32, 33 In addition, the NPC-component fusions interact with CRM1, the major receptor for the nuclear export of protein.33, 34 These observations suggest that alteration of the localization of certain factors by NUP98 fusions and NUP214 fusions might be important for leukemogenesis, and that a common mechanism may underlie leukemias induced by CALM and NUP fusions.
Acknowledgments
We thank Dr Y. Zhang for pcDNA3β-FLAG-CALM-AF10 plasmid. This work was supported in part by Grants-in-Aid for Scientific Research from the Ministry of Health, Labor, and Welfare and from the Ministry of Education, Culture, Sports, Science, and Technology; the National Cancer Center Research and Development Fund; the Naito Foundation; Cosmetology Research Foundation; and Nara Women's University Intramural Grant for Project Research. MS is a Research Fellow for Yong Scientist of Japan Society for the Promotion of Science.
Disclosure Statement
The authors have no conflict of interest.




