Bleeding risk with concurrent use of anticoagulants and ibrutinib: A population-based nested case-control study
Corresponding Author
Marion Allouchery
Pharmacologie Clinique et Vigilances, CHU de Poitiers, Poitiers, France
Faculté de Médecine, Université de Poitiers, Poitiers, France
Univ. Bordeaux, INSERM, BPH, U1219, Team AHeaD, Bordeaux, France
Correspondence
Marion Allouchery, Pharmacologie Clinique et Vigilances, CHU de Poitiers, 2 rue de la Milétrie, 86021 Poitiers Cedex, France.
Email: [email protected]
Search for more papers by this authorCécile Tomowiak
Onco-Hématologie et Thérapie Cellulaire, CHU de Poitiers, Poitiers, France
INSERM CIC 1402, CHU de Poitiers, Poitiers, France
Search for more papers by this authorAllison Singier
Univ. Bordeaux, INSERM, BPH, U1219, Team AHeaD, Bordeaux, France
Search for more papers by this authorMathieu Puyade
INSERM CIC 1402, CHU de Poitiers, Poitiers, France
Médecine Interne et Maladies Infectieuses, CHU de Poitiers, Poitiers, France
Search for more papers by this authorLoubna Dari
Univ. Bordeaux, INSERM, BPH, U1219, Team AHeaD, Bordeaux, France
Médecine Vasculaire, CHU de Bordeaux, Bordeaux, France
Search for more papers by this authorElodie Pambrun
Univ. Bordeaux, INSERM, BPH, U1219, Team AHeaD, Bordeaux, France
Search for more papers by this authorAntoine Pariente
Univ. Bordeaux, INSERM, BPH, U1219, Team AHeaD, Bordeaux, France
Search for more papers by this authorJulien Bezin
Univ. Bordeaux, INSERM, BPH, U1219, Team AHeaD, Bordeaux, France
CHU de Bordeaux, Pôle de Santé Publique, Service de Pharmacologie Médicale, Bordeaux, France
Search for more papers by this authorMarie-Christine Pérault-Pochat
Pharmacologie Clinique et Vigilances, CHU de Poitiers, Poitiers, France
Laboratoire de Neurosciences Expérimentales et Cliniques, INSERM, UMR1084, Université de Poitiers, Poitiers, France
Search for more papers by this authorFrancesco Salvo
Univ. Bordeaux, INSERM, BPH, U1219, Team AHeaD, Bordeaux, France
CHU de Bordeaux, Pôle de Santé Publique, Service de Pharmacologie Médicale, Bordeaux, France
Search for more papers by this authorCorresponding Author
Marion Allouchery
Pharmacologie Clinique et Vigilances, CHU de Poitiers, Poitiers, France
Faculté de Médecine, Université de Poitiers, Poitiers, France
Univ. Bordeaux, INSERM, BPH, U1219, Team AHeaD, Bordeaux, France
Correspondence
Marion Allouchery, Pharmacologie Clinique et Vigilances, CHU de Poitiers, 2 rue de la Milétrie, 86021 Poitiers Cedex, France.
Email: [email protected]
Search for more papers by this authorCécile Tomowiak
Onco-Hématologie et Thérapie Cellulaire, CHU de Poitiers, Poitiers, France
INSERM CIC 1402, CHU de Poitiers, Poitiers, France
Search for more papers by this authorAllison Singier
Univ. Bordeaux, INSERM, BPH, U1219, Team AHeaD, Bordeaux, France
Search for more papers by this authorMathieu Puyade
INSERM CIC 1402, CHU de Poitiers, Poitiers, France
Médecine Interne et Maladies Infectieuses, CHU de Poitiers, Poitiers, France
Search for more papers by this authorLoubna Dari
Univ. Bordeaux, INSERM, BPH, U1219, Team AHeaD, Bordeaux, France
Médecine Vasculaire, CHU de Bordeaux, Bordeaux, France
Search for more papers by this authorElodie Pambrun
Univ. Bordeaux, INSERM, BPH, U1219, Team AHeaD, Bordeaux, France
Search for more papers by this authorAntoine Pariente
Univ. Bordeaux, INSERM, BPH, U1219, Team AHeaD, Bordeaux, France
Search for more papers by this authorJulien Bezin
Univ. Bordeaux, INSERM, BPH, U1219, Team AHeaD, Bordeaux, France
CHU de Bordeaux, Pôle de Santé Publique, Service de Pharmacologie Médicale, Bordeaux, France
Search for more papers by this authorMarie-Christine Pérault-Pochat
Pharmacologie Clinique et Vigilances, CHU de Poitiers, Poitiers, France
Laboratoire de Neurosciences Expérimentales et Cliniques, INSERM, UMR1084, Université de Poitiers, Poitiers, France
Search for more papers by this authorFrancesco Salvo
Univ. Bordeaux, INSERM, BPH, U1219, Team AHeaD, Bordeaux, France
CHU de Bordeaux, Pôle de Santé Publique, Service de Pharmacologie Médicale, Bordeaux, France
Search for more papers by this authorFrancesco Salvo and Marie-Christine Pérault-Pochat contributed equally to this study.
Summary
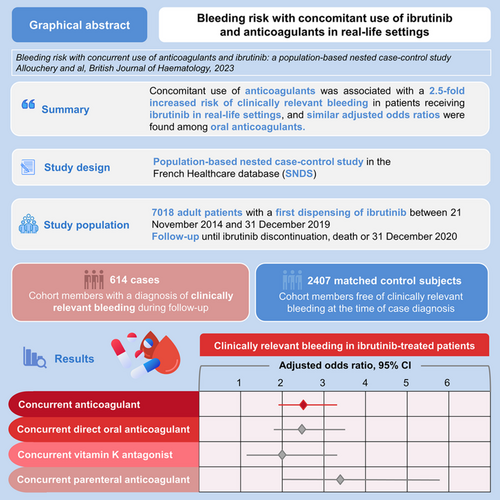
Data regarding the safety of co-administration of ibrutinib with anticoagulants in real-life settings are scarce. Using a nationwide database, we conducted a nested case-control study in a cohort of new users of ibrutinib to assess the risk of clinically relevant bleeding (CRB) associated with anticoagulation. Cases were patients with a diagnosis of CRB, defined as hospitalization with a diagnosis of bleeding. The date of CRB constituted the index date. Up to four controls were matched on sex, age at index date and duration of follow-up. The risk of CRB associated with anticoagulation in patients receiving ibrutinib was estimated using conditional logistic regression models, providing odds ratios (OR) adjusted for risk factors of bleeding. Among 614 cases and 2407 matched controls, the risk of CRB was significantly higher in patients receiving both ibrutinib and anticoagulants (adjusted OR [aOR] 2.54, confidence interval [CI] 95% [1.94; 3.32]). When considering anticoagulant class, aOR was 1.99 (CI 95% [1.19; 3.33]) for VKA, 2.48 (CI 95% [1.76; 3.47]) for direct oral anticoagulants and 3.40 (CI 95% [2.01; 5.75]) for parenteral anticoagulants. In conclusion, this study found a 2.5-fold increased risk of CRB in patients receiving both ibrutinib and anticoagulants in real-life settings, and similar aOR among oral anticoagulants.
Graphical Abstract
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
According to data protection and French regulations, the authors cannot publicly release the data from the French National Healthcare Database (SNDS). However, any person or organization (public or private; for-profit or non-profit) can access anonymized SNDS data to perform a study, research or an evaluation of public interest, upon authorization from an independent ethics and scientific committee (CESREES) and the French Data Protection Office (CNIL) (https://www.health-data-hub.fr/page/faq-english).
Supporting Information
| Filename | Description |
|---|---|
| bjh18995-sup-0001-DataS1.docxWord 2007 document , 44.3 KB |
Data S1. |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
REFERENCES
- 1Treon SP, Tripsas CK, Meid K, Warren D, Varma G, Green R, et al. Ibrutinib in previously treated Waldenström's macroglobulinemia. N Engl J Med. 2015; 372(15): 1430–1440.
- 2Chanan-Khan A, Cramer P, Demirkan F, Fraser G, Silva RS, Grosicki S, et al. Ibrutinib combined with bendamustine and rituximab compared with placebo, bendamustine, and rituximab for previously treated chronic lymphocytic leukaemia or small lymphocytic lymphoma (HELIOS): a randomised, double-blind, phase 3 study. Lancet Oncol. 2016; 17(2): 200–211.
- 3Miklos D, Cutler CS, Arora M, Waller EK, Jagasia M, Pusic I, et al. Ibrutinib for chronic graft-versus-host disease after failure of prior therapy. Blood. 2017; 130(21): 2243–2250.
- 4Dimopoulos MA, Tedeschi A, Trotman J, García-Sanz R, Macdonald D, Leblond V, et al. Phase 3 trial of ibrutinib plus rituximab in Waldenström's macroglobulinemia. N Engl J Med. 2018; 378: 2399–2410.
- 5Burger JA, Barr PM, Robak T, Owen C, Ghia P, Tedeschi A, et al. Long-term efficacy and safety of first-line ibrutinib treatment for patients with CLL/SLL: 5 years of follow-up from the phase 3 RESONATE-2 study. Leukemia. 2019; 34(3): 787–798.
- 6Byrd JC, Hillmen P, O'Brien S, Barrientos JC, Reddy NM, Coutre S, et al. Long-term follow-up of the RESONATE phase 3 trial of ibrutinib vs ofatumumab. Blood. 2019; 133(19): 2031–2042.
- 7Moreno C, Greil R, Demirkan F, Tedeschi A, Anz B, Larratt L, et al. Ibrutinib plus obinutuzumab versus chlorambucil plus obinutuzumab in first-line treatment of chronic lymphocytic leukaemia (iLLUMINATE): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019; 20(1): 43–56.
- 8Shanafelt TD, Wang XV, Kay NE, Hanson CA, O'Brien S, Barrientos J, et al. Ibrutinib-rituximab or chemoimmunotherapy for chronic lymphocytic leukemia. N Engl J Med. 2019; 381(5): 432–443.
- 9Herman SEM, Gordon AL, Hertlein E, Ramanunni A, Zhang X, Jaglowski S, et al. Bruton tyrosine kinase represents a promising therapeutic target for treatment of chronic lymphocytic leukemia and is effectively targeted by PCI-32765. Blood. 2011; 117(23): 6287–6296.
- 10Ponader S, Chen S-S, Buggy JJ, Balakrishnan K, Gandhi V, Wierda WG, et al. The Bruton tyrosine kinase inhibitor PCI-32765 thwarts chronic lymphocytic leukemia cell survival and tissue homing in vitro and in vivo. Blood. 2012; 119(5): 1182–1189.
- 11Byrd JC, Brown JR, O'Brien S, Barrientos JC, Kay NE, Reddy NM, et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med. 2014; 371(3): 213–223.
- 12Burger JA, Tedeschi A, Barr PM, Robak T, Owen C, Ghia P, et al. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med. 2015; 373(25): 2425–2437.
- 13Levade M, David E, Garcia C, Laurent PA, Cadot S, Michallet AS, et al. Ibrutinib treatment affects collagen and von Willebrand factor-dependent platelet functions. Blood. 2014; 124(26): 3991–3995.
- 14Bye AP, Unsworth AJ, Vaiyapuri S, Stainer AR, Fry MJ, Gibbins JM. Ibrutinib inhibits platelet integrin αIIb β3 outside-in signaling and thrombus stability but not adhesion to collagen significance. Arterioscler Thromb Vasc Biol. 2015; 35(11): 2326–2335.
- 15Kamel S, Horton L, Ysebaert L, Levade M, Burbury K, Tan S, et al. Ibrutinib inhibits collagen-mediated but not ADP-mediated platelet aggregation. Leukemia. 2015; 29(4): 783–787.
- 16Lipsky AH, Farooqui MZH, Tian X, Martyr S, Cullinane AM, Nghiem K, et al. Incidence and risk factors of bleeding-related adverse events in patients with chronic lymphocytic leukemia treated with ibrutinib. Haematologica. 2015; 100(12): 1571–1578.
- 17Dobie G, Kuriri FA, Omar MMA, Alanazi F, Gazwani AM, Tang CPS, et al. Ibrutinib, but not zanubrutinib, induces platelet receptor shedding of GPIb-IX-V complex and integrin αIIbβ3 in mice and humans. Blood Adv. 2019; 3(24): 4298–4311.
- 18O'Brien S, Hillmen P, Coutre S, Barr PM, Fraser G, Tedeschi A, et al. Safety analysis of four randomized controlled studies of ibrutinib in patients with chronic lymphocytic leukemia/small lymphocytic lymphoma or mantle cell lymphoma. Clin Lymphoma Myeloma Leuk. 2018; 18(10): 648–657.e15.
- 19Mock J, Kunk PR, Palkimas S, Sen JM, Devitt M, Horton B, et al. Risk of major bleeding with ibrutinib. Clin Lymphoma Myeloma Leuk. 2018; 18(11): 755–761.
- 20Tuppin P, Rudant J, Constantinou P, Gastaldi-Ménager C, Rachas A, de Roquefeuil L, et al. Value of a national administrative database to guide public decisions: from the système national d'information interrégimes de l'Assurance Maladie (SNIIRAM) to the système national des données de santé (SNDS) in France. Rev Epidémiol Sante Publique. 2017; 65: S149–S167.
- 21Bezin J, Duong M, Lassalle R, Droz C, Pariente A, Blin P, et al. The national healthcare system claims databases in France, SNIIRAM and EGB: powerful tools for pharmacoepidemiology. Pharmacoepidemiol Drug Saf. 2017; 26(8): 954–962.
- 22Essebag V, Platt RW, Abrahamowicz M, Pilote L. Comparison of nested case-control and survival analysis methodologies for analysis of time-dependent exposure. BMC Med Res Methodol. 2005; 5(1): 5.
- 23Schulman S, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients: definitions of major bleeding in clinical studies. J Thromb Haemost. 2005; 3(4): 692–694.
- 24Brown JR, Moslehi J, Ewer MS, O'Brien SM, Ghia P, Cymbalista F, et al. Incidence of and risk factors for major haemorrhage in patients treated with ibrutinib: an integrated analysis. Br J Haematol. 2019; 184(4): 558–569.
- 25van Walraven C, Hart RG, Singer DE, Laupacis A, Connolly S, Petersen P, et al. Oral anticoagulants vs aspirin in nonvalvular atrial fibrillation: an individual patient meta-analysis. JAMA. 2002; 288(19): 2441.
- 26Jones JA, Hillmen P, Coutre S, Tam C, Furman RR, Barr PM, et al. Use of anticoagulants and antiplatelet in patients with chronic lymphocytic leukaemia treated with single-agent ibrutinib. Br J Haematol. 2017; 178(2): 286–291.
- 27Boriani G, Corradini P, Cuneo A, Falanga A, Foà R, Gaidano G, et al. Practical management of ibrutinib in the real life: focus on atrial fibrillation and bleeding. Hematol Oncol. 2018; 36(4): 624–632.
- 28Raz MA, Arnason J, Bairey O, Shvidel L, Aviv A, Ben Baruch S, et al. The risk of bleeding in patients receiving ibrutinib combined with novel direct oral anticoagulants. Br J Haematol. 2020; 189(2): e31–e33.
- 29Quintavalla R, Lombardi M, Prandoni P, Manotti C, Tadonio I, Re F, et al. Increased dabigatran plasma concentration during ibrutinib treatment: a case of cerebral hemorrhage and successful dabigatran reversal by idarucizumab. Aging Clin Exp Res. 2018; 30(1): 93–95.
- 30 European Medicines Agency. Imbruvica: EPAR - Product Information [Internet]. 2023. Available from: https://www.ema.europa.eu/en/documents/product-information/imbruvica-epar-product-information_en.pdf. Accessed 20 Jun 2023.
- 31 U.S. Food and Drug Administration. Imbruvica: FDA – Label [Internet]. 2023. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/205552s040210563s017lbl.pdf. Accessed 20 Jun 2023.
- 32Streiff MB, Abutalib SA, Farge D, Murphy M, Connors JM, Piazza G. Update on guidelines for the management of cancer-associated thrombosis. Oncologist. 2021; 26(1): e24–e40.
- 33Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2021; 42(5): 373–498.
- 34Maura G, Blotière P-O, Bouillon K, Billionnet C, Ricordeau P, Alla F, et al. Comparison of the short-term risk of bleeding and arterial thromboembolic events in nonvalvular atrial fibrillation patients newly treated with dabigatran or rivaroxaban versus vitamin K antagonists: a French nationwide propensity-matched cohort study. Circulation. 2015; 132(13): 1252–1260.
- 35Blin P, Dureau-Pournin C, Lassalle R, Abouelfath A, Droz-Perroteau C, Moore N. A population database study of outcomes associated with vitamin K antagonists in atrial fibrillation before DOAC. Br J Clin Pharmacol. 2016; 81(3): 569–578.
- 36Blin P, Dureau-Pournin C, Cottin Y, Bénichou J, Mismetti P, Abouelfath A, et al. Comparative effectiveness and safety of standard or reduced dose dabigatran vs. rivaroxaban in nonvalvular atrial fibrillation. Clin Pharmacol Ther. 2019; 105(6): 1439–1455.
- 37Byrd JC, Hillmen P, Ghia P, Kater AP, Chanan-Khan A, Furman RR, et al. Acalabrutinib versus ibrutinib in previously treated chronic lymphocytic leukemia: results of the first randomized phase III trial. J Clin Oncol. 2021; 39(31): 3441–3452.
- 38Tam CS, Opat S, D'Sa S, Jurczak W, Lee HP, Cull G, et al. A randomized phase 3 trial of zanubrutinib vs ibrutinib in symptomatic Waldenström macroglobulinemia: the ASPEN study. Blood. 2020; 136(18): 2038–2050.
- 39 European Medicines Agency. Calquence: EPAR - Product Information [Internet]. 2023. Available from: https://www.ema.europa.eu/en/documents/product-information/calquence-epar-product-information_en.pdf. Accessed 20 Jun 2023.
- 40 European Medicines Agency. Brukinsa: EPAR - Product Information [Internet]. 2023. Available from: https://www.ema.europa.eu/en/documents/product-information/brukinsa-epar-product-information_en.pdf. Accessed 20 Jun 2023.