Safety of tralokinumab in adult patients with moderate-to-severe atopic dermatitis: pooled analysis of five randomized, double-blind, placebo-controlled phase II and phase III trials*
Plain language summary available online
Abstract
Background
Tralokinumab is a fully human monoclonal antibody that neutralizes the activity of interleukin-13, a key pathogenic driver of atopic dermatitis (AD). Clinical trials including adults with moderate-to-severe AD, of up to 52 weeks’ duration, showed tralokinumab was efficacious and well tolerated.
Objectives
To characterize the safety profile of tralokinumab for the treatment of moderate-to-severe AD.
Methods
Safety and laboratory measures were assessed in pooled analyses of phase II and III placebo-controlled clinical trials of tralokinumab in moderate-to-severe AD (NCT02347176, NCT03562377, NCT03131648, NCT03160885, NCT03363854).
Results
In total, 2285 patients were randomized in the initial treatment periods up to 16 weeks (1605 tralokinumab, 680 placebo). The frequencies of any adverse event (AE) were 65·7% for tralokinumab and 67·2% for placebo. The respective rates were 640 and 678 events per 100 patient-years of exposure (ep100PYE); rate ratio 1·0, 95% confidence interval (CI) 0·9–1·1. Serious AEs occurred in 2·1% of patients with tralokinumab and 2·8% with placebo (7·4 and 11·9 ep100PYE; rate ratio 0·7, 95% CI 0·4–1·2). The most common AEs occurring at a higher frequency and rate with tralokinumab vs. placebo were: viral upper respiratory tract infection (15·7% vs. 12·2%; 65·1 vs. 53·5 ep100PYE); upper respiratory tract infection (5·6% vs. 4·8%; 20·8 vs. 18·5 ep100PYE); conjunctivitis (5·4% vs. 1·9%; 21·0 vs. 6·9 ep100PYE); and injection-site reaction (3·5% vs. 0·3%; 22·9 vs. 4·0 ep100PYE). Some events in safety areas of interest occurred at a lower frequency and rate with tralokinumab vs. placebo: skin infections requiring systemic treatment (2·6% vs. 5·5%; 9·7 vs. 22·8 ep100PYE), eczema herpeticum (0·3% vs. 1·5%; 1·2 vs. 5·2 ep100PYE), opportunistic infections (3·4% vs. 4·9%; 13·0 vs. 21·3 ep100PYE) and serious infections (0·4% vs. 1·1%; 1·3 vs. 3·7 ep100PYE). AEs did not increase with continued maintenance and open-label treatment, including rates of common or serious AEs and AEs leading to study drug discontinuation. No clinically meaningful changes in mean laboratory measures were observed with treatment up to 1 year.
Conclusions
Across the AD population pool from five clinical trials, tralokinumab was well tolerated, with consistent safety findings during treatment of patients with moderate-to-severe AD. The safety profile during prolonged tralokinumab treatment was consistent with that during the initial treatment period; the frequency of events did not increase over time.
- Tralokinumab is a fully human monoclonal antibody that specifically neutralizes interleukin-13, a key cytokine driving skin inflammation and epidermal barrier dysfunction in atopic dermatitis (AD).
- In clinical trials in moderate-to-severe AD, tralokinumab provided significant and early improvements in the extent and severity of AD and was well tolerated, with an overall safety profile comparable with placebo over 52 weeks.
- We report the frequency and rate of adverse events (AEs) from pooled observations of over 2000 patients from five phase II and phase III placebo-controlled clinical trials of tralokinumab in moderate-to-severe AD.
- During initial treatment up to 16 weeks, the frequencies of any AE and of serious AEs were similar for tralokinumab and placebo. AE rates did not increase with continued treatment up to 52 weeks.
- Common AEs occurring more frequently with tralokinumab vs. placebo were viral and upper respiratory tract infection, conjunctivitis and injection-site reaction. Some events occurred at a lower frequency and rate with tralokinumab vs. placebo, such as skin infections requiring systemic treatment, eczema herpeticum and opportunistic and serious infections.
- No clinically meaningful changes in mean laboratory measures were observed.
Atopic dermatitis (AD) is a chronic skin disease resulting from type 2 skin inflammation and skin barrier dysfunction.1 Intense itch carries a high burden for patients due to sleep disturbance, anxiety and depression, which impact work and social activity.1-3 More safe and effective treatment options are needed for long-term use, particularly for patients with moderate-to-severe disease. Traditional systemic immunosuppressants used to treat moderate-to-severe AD have safety concerns and requirements for continuous monitoring, limiting long-term use.4, 5 Biologic treatments targeting cytokines driving underlying AD immune processes are expected to have fewer safety concerns (such as off-target effects) and be suitable for longer-term use. Nonetheless, it is important to have a full understanding of their safety profile.
Tralokinumab is a fully human immunoglobulin G4 monoclonal antibody that specifically binds to interleukin (IL)-13 with high affinity. Blocking interaction with the IL-13 receptor inhibits downstream IL-13 signalling.6 IL-13 is a key cytokine driving skin inflammation and epidermal barrier dysfunction.7, 8 This is supported by trials demonstrating the efficacy and safety of tralokinumab in adults with moderate-to-severe AD,9-11 including significant improvements in AD severity, early improvements in itch and sleep interference9-11 and no effect on immune responses to commonly used vaccines.12 The objective of this analysis is to further characterize the safety profile of tralokinumab by analysing safety data from five completed phase II and III trials in adult patients with moderate-to-severe AD.
Patients and methods
Studies
Pooled analyses included five completed double-blind, randomized, placebo-controlled tralokinumab trials in AD (the AD pool) consisting of one phase IIb dose-finding trial (NCT02347176); one phase II vaccine response trial, ECZTRA 5 (NCT03562377); and three phase III trials, ECZTRA 1 (NCT03131648), ECZTRA 2 (NCT03160885) and ECZTRA 3 (NCT03363854) (Figure S1; see Supporting Information). The full trial designs were reported previously.10-13 All trials were conducted in accordance with the ethical principles derived from the Declaration of Helsinki and Good Clinical Practice guidelines and were approved by an appropriate local institutional review board or independent ethics committee for each centre.
The safety of initial tralokinumab treatment with or without a topical corticosteroid (TCS) was assessed in the AD pool over 16 weeks in the ECZTRA trials and 12 weeks in the dose-finding trial. The safety of maintenance tralokinumab treatment and open-label treatment with tralokinumab plus optional TCS was assessed in the ECZTRA 1 and ECZTRA 2 monotherapy trials (the monotherapy pool).
Patients
All trials enrolled adults aged ≥18 years with AD for ≥1 year and Investigator’s Global Assessment (IGA) score ≥ 3 (Table S1; see Supporting Information). All patients who received at least one dose of subcutaneous tralokinumab 300 mg or placebo with or without TCS were included in the pooled analyses. Patients were categorized by treatment group according to treatment received (safety analysis set).
Endpoints
All adverse events (AEs) were treatment emergent, reported after the first dose of study drug. Investigators recorded AEs during the treatment period, which were coded or recoded (phase IIb dose-finding trial) using the Medical Dictionary for Regulatory Activities (MedDRA) version 20·0.
AEs of special interest (AESIs) were predefined in the ECZTRA trials based on areas of safety interest for monoclonal antibodies in AD – skin infections requiring systemic treatment, eczema herpeticum and eye disorders – and were captured retrospectively for the dose-finding trial with MedDRA searches. Malignancies were assessed using a standardized MedDRA query across all periods and safety follow-up in the AD pool. Other safety areas of interest were captured retrospectively using MedDRA searches of all AEs in all trials based on known risks of the mechanism of action of tralokinumab, AEs reported for other monoclonal antibodies, or AEs of regulatory interest such as injection-site reactions and severe or serious infections. MedDRA searches are listed in Appendix S1 (see Supporting Information). Clinical laboratory evaluations are reported for the ECZTRA trials.
Statistical methods
Analyses in the manuscript are based on pooled safety information from individual trials; no sample calculation has been performed.
Frequency was defined as the percentage of patients with at least one event. AE rates were calculated as the number of events per 100 patient-years of exposure (PYE), with PYE calculated as the time from the start (date and time of first dose) until the end of exposure (end of treatment visit if existing, otherwise date of permanent discontinuation of investigational medicinal product). For analyses based on the first event only, PYE was calculated as the time up to the first event. Cochran–Mantel–Haenszel weights were applied to calculate adjusted AE frequencies and rates to account for different randomization ratios across trials (adjusted rates)14 (Figure S1 and Table S1). Simple pooling was used to combine identically designed monotherapy trials to assess safety beyond week 16. Rate ratios (RRs) were estimated if at least one patient in both treatment groups experienced event(s), using Poisson regression with treatment as the fixed effect, and log values of PYE as the offset variable. Model assumptions were not checked.
Additional details are presented in Appendix S2 (see Supporting Information).
Results
Patients
Figure S2 (see Supporting Information) describes the patient disposition and exposure. Demographics and disease characteristics were balanced between treatment groups in the AD pool (Table 1). The mean age was approximately 38 years, the mean AD duration was 28 years, 47% of patients had an IGA score of 4, and the mean Eczema Area and Severity Index score was 31 (Table 1). More than half of patients had a history of systemic steroid use, more than one-third received systemic immunosuppressants (Table S2; see Supporting Information), and current medical history was similar between the treatment groups (Table S3; see Supporting Information). Seasonal allergy, asthma and food allergy were the most frequent atopic conditions in the AD pool.15 Characteristics in the monotherapy pool were similar to those in the AD pool (Table S4; see Supporting Information).
| Placebo (N = 680) | Tralokinumab q2w (N = 1605) | |
|---|---|---|
| Age (years), mean (SD) | 37·0 (14·3) | 37·9 (14·3) |
| Male, n (%) | 375 (55·1) | 921 (57·4) |
| Ethnicity, n (%) | ||
| White | 430 (63·2) | 1089 (67·9) |
| Black | 80 (11·8) | 139 (8·7) |
| Asian | 143 (21·0) | 324 (20·2) |
| Other or missing data | 27 (4·0) | 53 (3·3) |
| Duration of AD (years), mean (SD) | 27·7 (15·2) | 27·7 (15·4) |
| Age of AD onset (years), median (IQR) | 3·0 (1·0–12·0) | 3·0 (1·0–14·0) |
| Affected BSA (%), mean (SD) | 50·2 (24·7) | 51·0 (24·4) |
| EASI, mean (SD) | 30·9 (13·4) | 31·1 (13·6) |
| IGA, n (%) | ||
| IGA 3 (moderate) | 362 (53·2) | 840 (52·3) |
| IGA 4 (severe) | 318 (46·8) | 762 (47·5) |
| IGA 5 (very severe)a | 0 | 3 (0·2) |
- BSA, body surface area; EASI, Eczema Area and Severity Index; IGA, Investigator’s Global Assessment; IQR, interquartile range; q2w, every 2 weeks. aOnly the dose-finding study allowed for the inclusion of patients with very severe disease (IGA 5) at baseline.
Initial treatment up to 16 weeks (atopic dermatitis pool)
Summary of adverse events
The frequencies of any AE were 65·7% and 67·2% with tralokinumab and placebo, respectively, corresponding to AE rates of 419 and 480 patients per 100 PYE [rate ratio (RR) 0·9, 95% confidence interval (CI) 0·8–1·0] and 640 and 678 events per 100 PYE (RR 1·0, 95% CI 0·9–1·1), respectively (Table 2). Most AEs were classified as mild or moderate; severe events occurred in 4·6% and 6·3% of patients with tralokinumab and placebo, respectively (Table S5; see Supporting Information). Events leading to permanent discontinuation of study drug occurred in 2·3% (n = 38) and 2·8% (n = 20) of patients with tralokinumab and placebo, respectively, most commonly AD (preferred term: dermatitis atopic), injection-site reaction, eosinophilia and conjunctivitis (Table S6; see Supporting Information). Most events resolved during the trial (Table 2).
| Placebo (N = 680; PYE: 193) | Tralokinumab q2w (N = 1605; PYE: 473) | RR vs. placebo (95% CI) | |||
|---|---|---|---|---|---|
| Patients (adj. %) | Adj. ratea | Patients (adj. %) | Adj. ratea | ||
| ≥ 1 AE, n | 449 (67·2) | 480 | 1080 (65·7) | 419 | 0·9 (0·8–1·0) |
| Events | Adj. rateb | Events | Adj. rateb | ||
| Total number of AEs | 1276 | 678 | 3148 | 640 | 1·0 (0·9–1·1) |
| Severity | |||||
| Mild | 738 | 391 | 2127 | 430 | 1·2 (1·1–1·3) |
| Moderate | 478 | 254 | 917 | 190 | 0·8 (0·7–0·9) |
| Severe | 60 | 33·0 | 104 | 20·2 | 0·7 (0·5–1·0) |
| Leading to permanent discontinuation of study drug | 25 | 13·3 | 47 | 9·9 | 0·8 (0·5–1·2) |
| Outcome | |||||
| Fatal | 0 | 0 | 1 | 0·4 | NC |
| Not recovered or not resolved | 126 | 65·2 | 312 | 65·4 | 1·0 (0·8–1·2) |
| Recovering or resolving | 45 | 22·7 | 87 | 18·9 | 0·8 (0·6–1·1) |
| Recovered or resolved | 1096 | 585 | 2699 | 545 | 1·0 (0·9–1·1) |
| Recovered or resolved with sequelae | 3 | 1·7 | 18 | 3·5 | 2·4 (0·7–8·3) |
| Unknown | 6 | 3·3 | 31 | 7·0 | 2·1 (0·9–5·1) |
- CI, confidence interval; NC, not calculable; PYE, patient-years of exposure; q2w, every 2 weeks; RR, rate ratio. AEs collected during the exposure time in the initial treatment period are shown. aRate calculated as number of patients divided by PYE calculated up until first event, multiplied by 100. bRate calculated as number of events divided by PYE multiplied by 100. Adjusted frequencies and rates were calculated using Cochran–Mantel–Haenszel weights. RRs and 95% CIs are estimated from Poisson regression with treatment as fixed effect; log values of patient-years of exposure were used as offset variables.
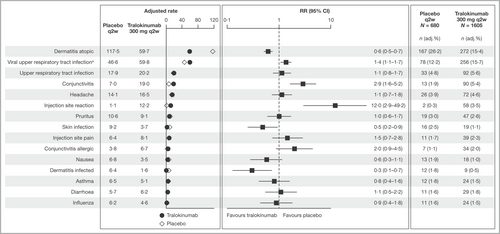
Most frequent adverse events
The most common AE was AD and the frequency was lower in patients receiving tralokinumab compared with placebo: 15·4% vs. 26·2% of patients (68·0 vs. 140 events per 100 PYE), respectively (Figure 1; and Table S7; see Supporting Information). Common AEs occurring more frequently with tralokinumab than with placebo were viral upper respiratory tract infection (15·7% vs. 12·2%; 65·1 vs. 53·5 events per 100 PYE), upper respiratory tract infection (5·6% vs. 4·8%; 20·8 vs. 18·5 events per 100 PYE), conjunctivitis (5·4% vs. 1·9%; 21·0 vs. 6·9 events per 100 PYE) and injection-site reaction (3·5% vs. 0·3%; 22·9 vs. 4·0 events per 100 PYE) (Figure 1 and Table S7). Most events reported as viral upper respiratory tract infection were symptoms related to common cold. Skin infections and dermatitis infected occurred at lower rates with tralokinumab vs. placebo (Figure 1 and Table S7).
Serious adverse events
In the initial treatment period, 2·1% and 2·8% of patients experienced a serious adverse event (SAE) in the tralokinumab and placebo groups, corresponding to SAE rates of 7·3 and 9·7 patients per 100 PYE (RR 0·8, 95% CI 0·5–1·5) and 7·4 and 11·9 events per 100 PYE (RR 0·7, 95% CI 0·4–1·2), respectively (Table 3). Fewer than 1% of patients in either group experienced SAEs leading to permanent discontinuation of study drug; most patients recovered or the events were resolved (Table S8; see Supporting Information).
| Placebo (n = 680; PYE: 193) | Tralokinumab q2w (n = 1605; PYE: 473) | RR vs. placebo (95% CI) | |||
|---|---|---|---|---|---|
| Patients (adj. %) | Adj. ratea | Patients (adj. %) | Adj. ratea | ||
| ≥ 1 SAE, n (%) | 18 (2·8) | 9·7 | 37 (2·1) | 7·3 | 0·8 (0·5–1·5) |
| Events | Adj. rateb | Events | Adj. rateb | ||
| Total number of SAEs | 22 | 11·9 | 38 | 7·4 | 0·7 (0·4–1·2) |
| Severity | |||||
| Mild | 0 | 0 | 2 | 0·4 | NC |
| Moderate | 8 | 4·2 | 10 | 1·9 | 0·5 (0·2–1·3) |
| Severe | 14 | 7·6 | 26 | 5·1 | 0·8 (0·4–1·5) |
| Leading to permanent discontinuation of study drug | 1 | 0·6 | 6 | 1·1 | 2·4 (0·3–20·3) |
| Outcome | |||||
| Fatal | 0 | 0 | 1 | 0·4 | NC |
| Not recovered or not resolved | 4 | 2·1 | 0 | 0 | NC |
| Recovering or resolving | 0 | 0 | 2 | 0·4 | NC |
| Recovered or resolved | 16 | 8·7 | 32 | 5·9 | 0·8 (0·4–1·5) |
| Recovered or resolved with sequelae | 1 | 0·6 | 2 | 0·4 | 0·8 (0·1–9·0) |
| Unknown | 1 | 0·6 | 1 | 0·2 | 0·4 (0·0–6·5) |
- CI, confidence interval; NC, not calculable; PYE, patient-years of exposure; RR, rate ratio. Adverse events collected during the exposure time in the initial treatment period are shown. aRate calculated as number of patients divided by PYE calculated up until first event, multiplied by 100. bRate calculated as number of events divided by PYE multiplied by 100. Adjusted frequencies and rates were calculated using Cochran–Mantel–Haenszel weights. RRs and 95% CIs were estimated from Poisson regression with treatment as fixed effect; log values of patient-years of exposure were used as offset variables.
Most frequent serious adverse events
The most frequent class of SAE was infections and infestations, which occurred at a lower frequency with tralokinumab vs. placebo, in 0·4% and 1·1% of patients (1·3 vs. 3·7 events per 100 PYE; RR 0·3, 95% CI 0·1–1·0), respectively (Table S9; see Supporting Information). Dermatitis atopic (skin and subcutaneous tissue disorders) was the most frequent SAE in the tralokinumab group (Table S10; see Supporting Information). All other SAEs were single events within each treatment group, with no apparent clustering of class or event.
Safety areas of interest
Skin infections requiring systemic treatment and eczema herpeticum occurred less frequently with tralokinumab vs. placebo, in 2·6% vs. 5·5% (9·7 vs. 22·8 events per 100 PYE; RR 0·4, 95% CI 0·3–0·7) and 0·3% vs. 1·5% (1·2 vs. 5·2 events per 100 PYE; RR 0·2, 95% CI 0·1–0·7), respectively (Figure 2; and Tables S11 and S12; see Supporting Information).
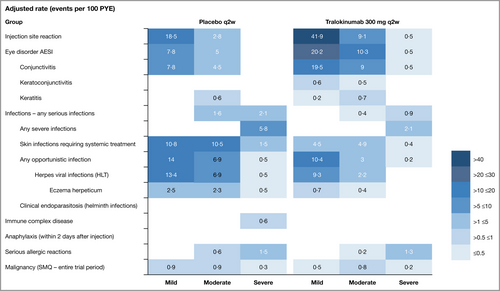
Severe infection; serious infection; any infection requiring parenteral antibiotics; any infection requiring oral antibiotics, antivirals or antifungals >2 weeks; and any opportunistic infection occurred at lower rates with tralokinumab vs. placebo (Table S13; see Supporting Information). Most opportunistic infections in both treatment groups were herpes virus infections, which occurred in fewer patients in the tralokinumab vs. placebo groups (Table S14; see Supporting Information).
Eye disorder AESIs classified by the investigator as conjunctivitis, keratoconjunctivitis or keratitis occurred in more patients receiving tralokinumab than placebo, in 7·9 and 3·4%, respectively (31·1 and 12·9 events per 100 PYE; RR 2·6, 95% CI 1·7–4·1) (Tables S11 and S15; see Supporting Information). The most frequent eye disorder AESI overall was conjunctivitis. Most eye disorder AESIs (98%) were mild or moderate (Figure 2), none were serious and the majority resolved during the study; however, two eye disorder events led to permanent discontinuation of tralokinumab (Table S11). Previous literature presents a detailed analysis of conjunctivitis events.16
Injection-site reactions occurred more frequently with tralokinumab vs. placebo, in 7·2% vs. 3·0%, respectively (51·5 vs. 21·3 events per 100 PYE; RR 2·7, 95% CI, 1·9–3·9) (Tables S11 and S16; see Supporting Information). They were transient and most (99%) were mild or moderate. Approximately 76% of the events resolved within 1–5 days, and all recovered or resolved during the trial (Table S11).
Entire trial period (atopic dermatitis pool)
The frequency of malignancy was similar for tralokinumab and placebo, occurring in 0·9% (n = 17) and 0·7% (n = 5) patients, respectively (1·4 vs. 2·2 events per 100 PYE; RR 0·6, 95% CI, 0·3–1·5). Few events were serious or severe, with no difference noted between treatment groups. The majority were skin malignancies; the remaining events occurred across organ systems with no observed clustering over time (Figure 2; and Table S17; see Supporting Information). A summary of deaths occurring during the pooled trials is presented in Appendix S3 (see Supporting Information).
Monotherapy pool
Initial treatment up to 16 weeks in the monotherapy pool
The frequency of any AE in the initial treatment period of the monotherapy pool was similar for tralokinumab and placebo, 69·0% and 71·5%, respectively. Overall event rates during this time were similar to those in the initial treatment period of the AD pool, based on AEs, SAEs and frequent AEs (Figure S3 and Table S18; see Supporting Information).
Maintenance and open-label tralokinumab treatment up to 52 weeks in the monotherapy pool
In total, 324 patients received tralokinumab in the maintenance treatment period of the monotherapy pool and 1121 patients received open-label tralokinumab plus an optional TCS (Figure S2). AE frequency in these periods was similar to those in the initial treatment period and corresponding AE rates did not increase with continued treatment (Table 4; and Table S19; see Supporting Information). Occurrences of SAEs were similar for the group dosed every 4 weeks and the open-label group (3·6% and 3·8%; 9·1 and 7·4 events per PYE), compared with the group dosed every 2 weeks (0·6% and 1·2 events per 100 PYE) (Table 4). The rate of events leading to permanent discontinuation of the study drug varied slightly across each group, with 3·5, 2·3 and 5·0 events per 100 PYE in the groups dosed every 2 weeks, every 4 weeks and open label, respectively (Table 4).
| Tralokinumab q2w (N = 159) | Tralokinumab q4w (N = 165) | Open-label tralokinumab q2w + optional TCS (N = 1121) | |||||
|---|---|---|---|---|---|---|---|
| Patients (%) | Ratea | Patients (%) | Ratea | Patients (%) | Ratea | ||
| Any AE, n (%) | |||||||
| ≥ 1 AE | 116 (73·0) | 309 | 109 (66·1) | 249 | 814 (72·6) | 279 | |
| ≥ 1 SAE | 1 (0·6) | 1·2 | 6 (3·6) | 7·0 | 43 (3·8) | 6·6 | |
| Events | Rateb | Events | Rateb | Events | Rateb | ||
| AEs | |||||||
| Total number of AEs | 423 | 499 | 363 | 414 | 2870 | 432 | |
| Serious | 1 | 1·2 | 8 | 9·1 | 49 | 7·4 | |
| Severity | |||||||
| Mild | 280 | 331 | 284 | 324 | 1907 | 287 | |
| Moderate | 138 | 163 | 74 | 84·4 | 888 | 134 | |
| Severe | 5 | 5·9 | 5 | 5·7 | 75 | 11·3 | |
| Leading to permanent discontinuation of study drug | 3 | 3·5 | 2 | 2·3 | 33 | 5·0 | |
| Outcome | |||||||
| Fatal | 0 | – | 0 | – | 0 | – | |
| Not recovered or not resolved | 37 | 43·7 | 45 | 51·3 | 375 | 56·4 | |
| Recovering or resolving | 25 | 29·5 | 21 | 24·0 | 138 | 20·8 | |
| Recovered or resolved | 359 | 424 | 293 | 334 | 2319 | 349 | |
| Recovered or resolved with sequelae | 0 | – | 1 | 1·1 | 9 | 1·4 | |
| Unknown | 2 | 2·4 | 3 | 3·4 | 29 | 4·4 | |
- PYE, patient-years of exposure; q2w, every 2 weeks; q4w, every 4 weeks; TCS, topical corticosteroid. AEs collected during the exposure time in the maintenance and open-label treatment periods in the monotherapy pool are shown. aRate calculated as number of patients divided by PYE calculated up until first event, multiplied by 100. bRate calculated as number of events divided by PYE multiplied by 100.
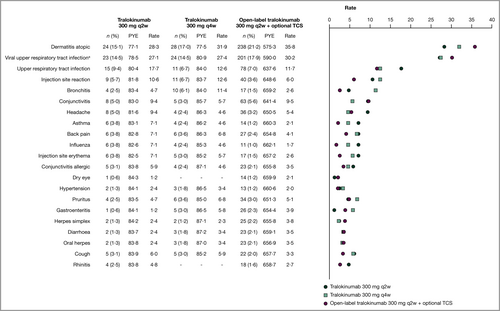
The most common events during maintenance and open-label treatment were generally consistent with the initial treatment and did not increase with continued treatment (Figure 3). Most viral upper respiratory tract infections up to week 52 were the common cold; > 80% of cases were mild, none were severe or serious or led to permanent discontinuation and > 95% resolved by the end of the trial. Safety areas of interest, including injection-site reactions and eye disorder AESIs, occurred at similar rates as seen in the initial treatment period (Tables S11–S17). Other safety areas of interest occurred infrequently (Table S20; see Supporting Information).
Laboratory measures, disease markers and immunogenicity
No clinically relevant changes were observed from baseline to the end of the initial treatment period for haematology values and biochemistry parameters in the AD pool (ECZTRA trials only; percentages of patients reported in Table S21–S23; see Supporting Information) or in the initial, maintenance or open-label periods of the monotherapy pool. Immunoglobulin E levels were reduced in patients receiving tralokinumab compared with placebo.
Eosinophil levels
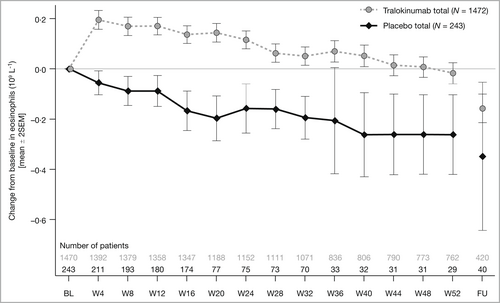
At baseline in the AD pool (ECZTRA trials only), mean eosinophil levels were above the upper limit of normal (ULN) (> 0·5 × 109 cells L−1) in both treatment groups, and 44·6% and 41·8% of patients had baseline eosinophils above the upper limit of normal in the tralokinumab and placebo groups. Mean eosinophils decreased with placebo and increased with tralokinumab to week 4, then decreased similarly in both arms, returning to near baseline levels by week 28 with tralokinumab. At safety follow-up, mean eosinophils had decreased further in both groups (Figure 4). The safety profile of tralokinumab in patients with eosinophilia was comparable with that of all patients (AD pool): 1·2% (n = 19) of tralokinumab-treated patients and 0·3% (n = 2) of placebo-treated patients had eosinophil counts >5 × 109 cells L−1 in the initial treatment period. Most levels declined over time, with no indication of permanently high counts and no reports of SAEs related to eosinophils.
Immunogenicity
The frequencies of antidrug antibodies and neutralizing antibodies were low and similar for tralokinumab and placebo after 16 weeks in the ECZTRA trials (antidrug antibodies: tralokinumab 1·4%, placebo 1·3%; neutralizing antibodies: tralokinumab 0·1%, placebo 0·2%). Antidrug antibodies and neutralizing antibodies occurred in more patients receiving tralokinumab than placebo up to 52 weeks in the ECZTRA trials, in 4·4% and 0·9%, respectively. All treatment-emergent antidrug antibodies were low titre (< 1000), with no reports of immunogenicity-related AEs, immune complex disease, serum sickness or serum sickness-like reactions, or anaphylaxis within 2 days after tralokinumab administration across all trial periods, and no increase in serious allergic reactions. There was no indication of antidrug antibodies or neutralizing antibodies impacting pharmacokinetics, efficacy or safety across all treatment periods.
Discussion
This set of data analyses is the most comprehensive safety analysis of tralokinumab clinical data to date. During initial, maintenance and open-label treatment, tralokinumab was confirmed to be well tolerated and showed no unexpected safety findings. During initial treatment, the overall frequency and rate of AEs were similar for tralokinumab and placebo. Approximately two-thirds of patients experienced one or more AEs, and the majority were mild or moderate. Overall rates of AEs and SAEs did not increase during maintenance and open-label treatment with tralokinumab, and the pattern of most frequent events was consistent in the maintenance and open-label treatment periods compared with initial treatment. Although the study was not designed to compare tralokinumab doses, a similar pattern of events was observed regardless of dose in the maintenance period. The frequency of some events was lower with dosing every 4 weeks compared with every 2 weeks, including upper respiratory tract infection and conjunctivitis, consistent with the ECZTRA 3 study.10 The safety profile for the pooled AD data was generally similar to that observed in asthma trials, except for a higher frequency of eye disorder events.17, 18
Rates of some events differed between the tralokinumab and placebo groups. Injection-site reactions occurred more frequently with tralokinumab than with placebo, consistent with previously reported data for other biologics in AD.19, 20 Viral upper respiratory tract infection occurred at a higher rate with tralokinumab; however, > 90% of these events were common cold-related symptoms mapped to viral upper respiratory tract infection according to MedDRA version 20·0. These common cold-related symptoms are mapped to nasopharyngitis in previous and later MedDRA versions. In contrast, events that occurred at a higher rate in the placebo group included AD and, for safety areas of interest, included skin infections.
Patients with AD are susceptible to both skin and systemic infections,21-23 and adults with AD were shown to have a higher risk of death due to infections compared with those without AD.24, 25 Furthermore, several systemic immunosuppressants were associated with increased infection risk in AD.26 In this analysis, the rates of dermatitis infected, skin infections requiring systemic treatment, eczema herpeticum, opportunistic infections, and severe or serious infections were lower with tralokinumab than with placebo. The lower rate of skin infection events is consistent with the reduced Staphylococcus aureus colonization observed with tralokinumab in previous trials,11 providing further evidence of the value of targeting IL-13 towards improving the skin barrier and potentially reducing antibiotic use. Reductions in serious or severe infections were also observed in pooled analyses of dupilumab clinical trials in AD.27 In addition, the current study showed no evidence of safety concerns relating to herpes zoster observed in trials of oral Janus kinase inhibitors;28, 29 similarly, the frequency of herpes viral infections, more common with dupilumab than placebo,27 was lower with tralokinumab than with placebo in the AD pool.
Increased frequency of conjunctivitis was observed in clinical trials in AD of both tralokinumab10, 11 and dupilumab.30 In this pooled analysis, none of the eye disorder events reported as the AESIs conjunctivitis, keratoconjunctivitis or keratitis were serious, and the majority (98%) were mild or moderate. A separate analysis of conjunctivitis events in the tralokinumab AD pool showed that most conjunctivitis events resolved or were resolving within the treatment period.16 The study identified several potential predisposing factors for conjunctivitis, including more severe AD at baseline and history of allergic conjunctivitis,16 which is consistent with observations in dupilumab trials.30, 31 While the rate of conjunctivitis events in tralokinumab trials seems to be lower than that observed with dupilumab,30 more data from real-world use are needed to confirm this. Data from a recent in vitro study in human conjunctival goblet cells suggested that the functional redundancy of IL-13 and IL-4 may explain the lowered frequency of conjunctivitis with tralokinumab (anti-IL-13) compared with dupilumab, which targets both cytokines.31, 32
There were no clinically relevant changes in laboratory parameters with tralokinumab, unlike with Janus kinase inhibitors in AD.33 Eosinophilia was more frequent with tralokinumab than placebo; however, it was asymptomatic, generally transient, and associated with high baseline levels. The immunogenicity of tralokinumab was low, based on the low and transient frequency of antidrug antibodies and neutralizing antibodies.
This analysis included ECZTRA 5, a 16-week, double-blind, placebo-controlled, phase II trial of immune response to vaccines in adults with moderate-to-severe AD undergoing tralokinumab treatment.34 The results from this study suggest that inactivated or nonlive vaccines, including tetanus, diphtheria, pertussis and meningococcal vaccines, can be administered without needing to interrupt or delay tralokinumab treatment.34
TCS use was permitted in some of the trials or during specific trial periods. It is therefore difficult to assess the influence of a TCS on the safety profile of tralokinumab from the pooled analysis. However, no appreciable differences were noted between the safety profiles of tralokinumab with or without TCS in the individual trials, and inclusion of TCS more closely reflects the likely real-world use of a biologic. Use of other concomitant medications reflected the level of AD disease activity in the study population, the common comorbidities, and the pattern of AEs in the tralokinumab and placebo groups. An increase in concomitant ophthalmological medications during the initial treatment period, more apparent in the tralokinumab group, corresponded with the reported eye disorder events. Similarly, a greater increase in anti-infectives for systemic use was seen in the placebo group, reflecting the higher rate of infections.
There are some limitations. A smaller pool of patients was available after week 16 due to methodological difficulties combining trials in which patients were rerandomized. Although the monotherapy pool was smaller than the AD pool, 807 patients in the monotherapy pool had 52 weeks of tralokinumab exposure. Future data from an open-label extension study may provide additional insight into the safety profile of tralokinumab. TCS use was allowed as needed in some but not all studies. Pooling did not take into account the impact of any rescue medications used for AEs; notably, in ECZTRA 3, where TCS as needed was included in all treatment arms, AD as an AE was reported at a lower level than in the other trials in the pool.10 Overall, the safety profile of tralokinumab seems to be consistent with or without TCS use.10
The tolerability and safety of available therapeutics are important to consider when selecting AD treatments.35 This analysis from a large pool of patients exposed to tralokinumab for an extended period of time reaffirms the safety profile of tralokinumab, as it was well tolerated both in combination with TCS and as monotherapy with long-term use up to 52 weeks for moderate-to-severe AD.
Funding sources
The phase IIb dose-finding trial was sponsored by MedImmune LLC; the ECZTRA trials were sponsored by LEO Pharma. Medical writing and editorial support were provided by Kathryn Woods, PhD and Jane Beck, MA (Hons), from Complete HealthVizion, supported by LEO Pharma. R.B.W. is supported by the Manchester NIHR Biomedical Research Centre.
Conflicts of interest
E.L.S. reports grants from Galderma, Merck, Novartis, Tioga and Vanda; personal fees from Arena Pharmaceuticals, BenevolentAI, BiomX, Bluefin Biomedicine, Boehringer Ingelheim, Boston Consulting Group, California Pharmaceuticals, Collective Acumen, Coronado Biosciences, Dermira, Evidera, Excerpta Medica, Forté Bio, Janssen, Medscape, Ortho Dermatologics, Sanofi Genzyme and SPARC India; and grants and personal fees from AbbVie, Celgene/Amgen, Lilly, Incyte, Kyowa Kirin, LEO Pharma, Pfizer, Regeneron and Sanofi. J.F.M. is a consultant and/or investigator for AbbVie, Arena, Avotres, Biogen, Bristol Myers Squibb, Celgene, Dermavant, Lilly, EMD Serono, Janssen, LEO Pharma, Merck, Novartis, Pfizer, Regeneron, Sanofi, Sun and UCB. J.I.S. reports personal fees from AbbVie, AnaptysBio, Arena, Asana, Bluefin Biomedicine, Boehringer Ingelheim, Celgene, Dermavant, Dermira, DS Biopharma, Kiniksa, LEO Pharma, Lilly, Luna, Menlo, Novartis, Pfizer, Realm, Regeneron and Sanofi Genzyme; and grants and personal fees from Galderma and GlaxoSmithKline outside the submitted work. K.R. has served as an advisor and/or paid speaker for and/or participated in clinical trials sponsored by AbbVie, Almirall, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Forward Pharma, Gilead, Galderma, Janssen-Cilag, Kyowa Kirin, LEO Pharma, Lilly, Medac, Novartis, Ocean Pharma, Pfizer, Sanofi and UCB; and is cofounder of Moonlake Immunotherapeutics. R.B.W. has received research grants or consulting fees from AbbVie, Almirall, Amgen, Arena, Astellas, Avillion, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, DiCE, Lilly, GSK, Janssen, LEO Pharma, Medac, Novartis, Pfizer, Sanofi, Sun Pharma, UCB and UNION. D.S-S. has been a speaker, consultant and/or investigator for AbbVie, Almirall, Amgen, AstraZeneca, Boehringer Ingelheim, Celgene, Eli Lilly, Galderma, Janssen, LEO Pharma, Novartis, Pfizer, Sanofi Genzyme and UCB. G.G. has received personal fees from AbbVie, Abiogen, Almirall, Amgen, Biogen, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Celltrion, Fresenius Kabi, Galderma, Genzyme, Insiderma, LEO Pharma, Lilly, Menlo, Novartis, OM Pharma, Pfizer, Regeneron, Samsung, Sandoz and UCB. K.P. is an investigator, consultant, speaker or scientific officer for or has served on steering committees or advisory boards for AbbVie, Akros, Amgen, Anacor, Arcutis, Astellas, Avillion, Bausch Health/Valeant, Baxalta, Boehringer Ingelheim, Bristol Myers Squibb, Can-Fite BioPharma, Celgene, Coherus, Dermavant, Dermira, DiCE, Dow Pharmaceuticals, Evelo, Galapagos, Galderma, Gilead, GSK, Incyte, Janssen, Kyowa Kirin, LEO Pharma, Lilly, MedImmune, Meiji Seika Pharma, Merck (MSD), Merck-Serono, Mitsubishi Pharma, Moberg Pharma, Novartis, Pfizer, PRCL Research, Regeneron, Roche, Sanofi-Aventis/Genzyme, Sun Pharma, Takeda, UCB and Xencor. M.dB-W. has been a consultant, advisory board member and/or speaker for AbbVie, Almirall, Arena, Lilly, Galderma, Janssen, LEO Pharma, Pfizer, Regeneron and Sanofi Genzyme. J.P.T. reports speaker honoraria from AbbVie, Almirall, LEO Pharma, Regeneron, and Sanofi Genzyme and research grants from Regeneron and Sanofi Genzyme and has attended advisory boards for AbbVie, LEO Pharma, Lilly, Pfizer, Regeneron and Sanofi Genzyme. R.Z. and C.K.O. are employees of LEO Pharma A/S. A.W. reports honoraria for conduct of the ECZTRA 1 and ECZTRA 2 trials to Ludwig Maximilian University of Munich from LEO Pharma A/S during the conduct of the study; personal fees from AbbVie, Chugai, Galderma, LEO Pharma, Lilly, MedImmune, Novartis, Pfizer, Regeneron and Sanofi-Aventis; and grants from LEO Pharma outside the submitted work.
Author contributions
Eric Lawrence Simpson: Conceptualization (equal); investigation (equal); writing – original draft (equal); writing – review and editing (equal). Joseph Merola: Conceptualization (equal); investigation (equal); writing – original draft (equal); writing – review and editing (equal). Jonathan I Silverberg: Conceptualization (equal); investigation (equal); writing – original draft (equal); writing – review and editing (equal). Kristian Reich: Conceptualization (equal); investigation (equal); writing – original draft (equal); writing – review and editing (equal). Richard B Warren: Conceptualization (equal); investigation (equal); writing – original draft (equal); writing – review and editing (equal). D. Staumont-Salle: Conceptualization (equal); investigation (equal); writing – original draft (equal); writing – review and editing (equal). Giampiero Girolomoni: Conceptualization (equal); investigation (equal); writing – original draft (equal); writing – review and editing (equal). Kim Papp: Conceptualization (equal); investigation (equal); writing – original draft (equal); writing – review and editing (equal). Marjolein de Bruin: Conceptualization (equal); investigation (equal); writing – original draft (equal); writing – review and editing (equal). Jacob Pontoppidan Thyssen: Conceptualization (equal); investigation (equal); writing – original draft (equal); writing – review and editing (equal). Rebecca Zachariae: Conceptualization (equal); data curation (lead); formal analysis (lead); investigation (equal); methodology (equal); visualization (equal). Christiana Kurre Olsen: Conceptualization (equal); formal analysis (supporting); investigation (equal); methodology (equal); visualization (equal). Andreas Wollenberg: Conceptualization (equal); investigation (equal); writing – original draft (equal); writing – review and editing (equal).
Ethics statement
All trials were conducted in accordance with the ethical principles derived from the Declaration of Helsinki and Good Clinical Practice guidelines and were approved by an appropriate local institutional review board or independent ethics committee for each centre.
Open Research
Data availability
Data for this pooled analysis may be requested from LEO Pharma; data will be made available following review and approval by the external Patient and Scientific Review Board.




