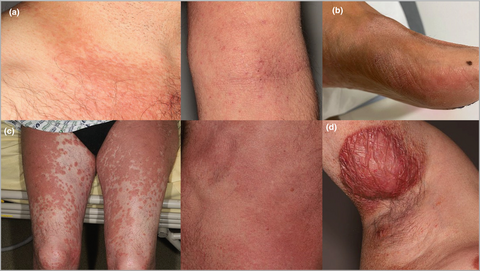
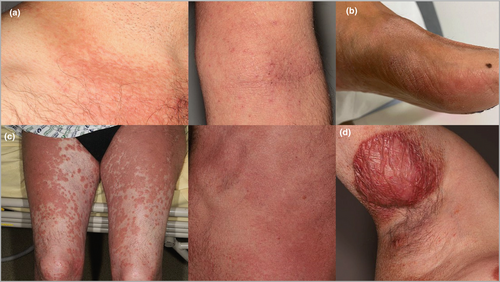
Cutaneous manifestations of lymphoid-variant hypereosinophilic syndrome
M. Battistella, M.G. and A.D.M. share last authorship.
A full list of affiliations is available in Appendix S1 (see Supporting Information).
Funding sources: none.
Conflicts of interest: the authors declare they have no conflicts of interest.
Data availability: data available upon request from the corresponding author.
Dear Editor, Hypereosinophilic syndromes (HESs) are defined by chronic blood hypereosinophilia ≥ 1·5 × 109 cells L−1 and tissue damage related to eosinophilic infiltration.1 This heterogeneous entity includes neoplastic HES (e.g. chronic eosinophilic leukaemia linked to the FIP1L1:PDGFRA fusion transcript) and reactive HES (parasitic infections, drug reactions and inflammatory and neoplastic diseases). Among the latter, the lymphoid-variant HES is linked to clonal circulating T helper 2 CD4 T cells, most commonly with a CD3− CD4+ phenotype.2 Published series of lymphoid HES remain scarce.3-5
Cutaneous T-cell lymphomas (CTCLs) – mycosis fungoides (MF) and Sézary syndrome – are characterized by skin infiltration by clonal T cells. Advanced-stage CTCLs are frequently associated with eosinophilia, linked to interleukin-5 secretion. In Sézary syndrome, the immunophenotypic abnormalities of blood tumour CD4+ T cells may include CD7 loss, and CD3 and/or CD4 downregulation. These abnormalities may be found in lymphoid HES.3, 6
We retrieved suspicious cases of lymphoid HES from the database of the French Reference Center for Hypereosinophilic Syndromes (CEREO). The definition of HES complied with the International Cooperative Working Group on Eosinophil Disorders criteria. CD3− CD4+ lymphoid HES was defined by HES and an aberrant blood CD3− CD4+ lymphoid population > 0·5% T cells, without any reactive cause of HES or chronic myeloid HES. This study was approved by the local institutional review board (approval number IRB12437, 1 February 2022) and was conducted according to the principles of the Declaration of Helsinki.
In total, 119 suspicious cases of HES were screened, among which 83 met the definition of lymphoid HES; 63 (76%) of these patients had cutaneous involvement. Thirty-four cases of lymphoid HES had sufficient information. Four had no detectable blood CD3− CD4+, but a CD3+ CD4+ CD7− population. These four patients had skin involvement (maculopapular erythema, n = 1; eczema-like lesions, n = 1; angio-oedema, n = 2); one of them later developed erythroderma and fulfilled the criteria of Sézary syndrome. Three patients had no skin involvement.
Out of 27 patients with CD3− CD4+ lymphoid HES and skin involvement, 15 were women (56%), and the median age was 48 years (range 16–83) (additional details available online: 10.6084/m9.figshare.20290704). The most frequent manifestations were angio-oedema (30%), urticaria (26%) and eczema-like lesions (26%). Three patients (11%) developed erythroderma, and three others had subcutaneous masses. A skin biopsy was taken in 15 cases, mostly showing a dermal lymphocytic infiltrate with admixed eosinophils. Skin T-cell clonality was available in eight cases, with a dominant T-cell clone in seven, identical to the blood T-cell clone.
After clinicopathological review, five cases of CD3− CD4+ lymphoid HES were found to have developed CTCL: stage I MF (n = 2), stage III MF (n = 1), Sézary syndrome (n = 1) and primary cutaneous peripheral T-cell lymphoma (PTCL), not otherwise specified (n = 1) (Figure 1). MF diagnosis was based on the presence of a dense, bandlike or interstitial, dermal infiltrate of CD3+ CD4+ small-to-medium mononuclear cells with atypical nuclei and epidermotropism, and a dominant skin T-cell clone (histological pictures available online: 10.6084/m9.figshare.20288619). The patient with Sézary syndrome had erythroderma, a skin biopsy consistent with Sézary syndrome, identical blood and skin T-cell clones, and two phenotypically aberrant T-cell populations in blood (CD3+ CD4+ CD26−, 31% and CD3− CD4+, 11% of lymphocytes). A fifth patient with subcutaneous nodules and a dense subcutaneous infiltrate of medium-size atypical lymphocytes with folliculotropism was diagnosed as having primary cutaneous PTCL, not otherwise specified. The infiltrate was CD3+ CD4+ with CD7 loss, the Ki67 index was 30–40% and a dominant T-cell clone was found in skin.
The patient with stage IA MF had complete response after treatment with topical chlormethine gel. Two patients with CTCL (one stage IB MF and one Sézary syndrome) were treated with mogamulizumab with blood complete response (disappearance of hypereosinophilia, and CD4+ CD26− T cells < 0·25 × 109 cells mL−1 in the patient with associated Sézary syndrome) but persistent skin involvement. One is now in complete remission after allogeneic stem cell transplantation (6 months of follow-up since transplant), while the remaining patient was lost to follow-up having not responded to treatment with methotrexate, and while under treatment with oral bexarotene.
This multicentre study describes the various clinical presentations of lymphoid HES. Although primary nodal PTCL has been described during the course of lymphoid HES,4 to our knowledge, primary CTCL has not been reported so far. Lymphoid HES and Sézary syndrome share a common gene expression pattern and elevated serum levels of TARC (thymus and activation-regulated chemokine),4 a CCR4 ligand, further supporting a common pathophysiology. Indeed, CCR4 is expressed by both CTCL tumour cells and CD3− CD4+ T-cell clones in lymphoid HES.7 Further molecular studies may help characterize the mutational landscape of lymphoid HES with skin involvement, although common alterations such as STAT3 (signal transducer and activator of transcription 3) mutations, present in both CTCL and lymphoid HES,8 could be expected.
This study challenges the existing paradigms of cutaneous T-cell lymphoproliferative disorders, and shows that careful skin examination and repeated skin biopsies are warranted in lymphoid HES.
Author contributions
Claire Laurent: Data curation (equal); formal analysis (equal). Guillaume Lefevre: Validation (equal); writing – review and editing (equal). Jean-Emmanuel Kahn: Validation (equal); writing – review and editing (equal). Delphine Staumont-Salle: Validation (equal); writing – original draft (equal); writing – review and editing (equal). Renaud Felten: Validation (equal); writing – review and editing (equal). Marie Puget: Validation (equal); writing – review and editing (equal). Thomas Moulinet: Validation (equal); visualization (equal). Irène Machelart: Validation (equal); writing – review and editing (equal). David Launay: Validation (equal); writing – review and editing (equal). Estelle Charvet: Visualization (equal); writing – review and editing (equal). Jean David Bouaziz: Investigation (equal); visualization (equal); writing – review and editing (equal). Marie Jachiet: Investigation (equal); validation (equal); visualization (equal). Christian Le Clec'h: Validation (equal); visualization (equal); writing – review and editing (equal). Alexandra Espitia: Validation (equal); writing – review and editing (equal). Alfred Mahr: Validation (equal); visualization (equal); writing – review and editing (equal). Marion Malphettes: Validation (equal); visualization (equal); writing – review and editing (equal). Cécile Morice: Validation (equal); writing – review and editing (equal). Samia Mourah: Investigation (equal); validation (equal); writing – review and editing (equal). Hélène Moins-Teisserenc: Investigation (equal); validation (equal); visualization (equal); writing – review and editing (equal). François Lifermann: Validation (equal); visualization (equal); writing – review and editing (equal). Karine Soulier-Guérin: Validation (equal); visualization (equal); writing – review and editing (equal). Alban Villate: Validation (equal); visualization (equal); writing – review and editing (equal). Chloé Baillou: Validation (equal); visualization (equal); writing – review and editing (equal). Aurélie Grados: Validation (equal); visualization (equal); writing – review and editing (equal). Ailsa Robbins: Validation (equal); writing – original draft (equal); writing – review and editing (equal). Noemie Abisror: Validation (equal); writing – review and editing (equal). Martine Bagot: Validation (equal); visualization (equal); writing – review and editing (equal). David Boutboul: Validation (equal); writing – review and editing (equal). Kewin Panel: Investigation (equal); project administration (equal); validation (equal); writing – review and editing (equal). Marie-Dominique Vignon-Pennamen: Visualization (equal); writing – review and editing (equal). Jacqueline Rivet: Validation (equal); visualization (equal); writing – review and editing (equal). Maxime Battistella: Formal analysis (equal); investigation (equal); validation (equal); writing – original draft (equal); writing – review and editing (equal). Matthieu Groh: Conceptualization (equal); investigation (equal); supervision (equal); visualization (equal); writing – original draft (equal); writing – review and editing (equal). Adèle De Masson: Formal analysis (equal); investigation (equal); methodology (equal); project administration (equal); supervision (equal); writing – original draft (equal); writing – review and editing (equal).